1. Introduction
With more than 88 different species, the
Orthobunyavirus is the largest genus of arthropod-transmitted viruses worldwide (ITCV) [
1]. Their amplification cycles involve vertebrate hosts and invertebrate vectors, and they are often associated with diseases in humans or livestock [
1]. The genome of orthobunyaviruses consists of three negative-sense RNA segments (L, M and S) [
1]. The S-segment encodes the non-structural protein S (NSs) and the nucleocapsid protein (N), and the L-segment encodes the RNA-dependent RNA polymerase L. Both L- and N-proteins are associated with the viral genome and form the ribonucleoprotein complexes (RNPs) [
1]. The M-segment codes for the glycoproteins Gn and Gc and the non-structural protein NSm. The glycoproteins are embedded into the lipid bilayer enveloping the viral particles. The non-structural proteins NSs and NSm are involved in the interaction with the host and probably the vector’s immune system [
1]. The genus
Orthobunyavirus is classified into 18 serologically defined arbovirus serogroups [
1]. All viruses of this genus have been classified in these groups based on complement-fixing antibodies’ serological relationships, hemagglutinating assay results and neutralising antibodies [
2]. The Bunyamwera serogroup contains over 30 viruses, including
Bunyamwera orthobunyavirus (BUNV) and
Batai orthobunyavirus (BATV) [
2]. BATV and BUNV infections in humans are primarily asymptomatic or are associated with very mild febrile symptoms [
2]. BATV was initially isolated in 1955 from
Culex gelidus mosquitoes in Malaysia [
3] and subsequently described in several countries in Europe and Asia, including Germany [
4,
5,
6]. BUNV was first discovered in Uganda [
7] and is endemic in different sub-Saharan African countries and South America [
8,
9,
10]. The above-described genome segmentation, close serological and genetic relationship and sympatric and simultaneous occurrence [
11] of BATV and BUNV provide the basis for genetic reassortment. This process was first described for the
La Crosse orthobunyavirus in mosquitoes [
12] and subsequently for many different orthobunyaviruses [
11]. Reassortment is especially frequent between viruses with a close genetic and antigenic relationship and is characterised by exchanging segments between both viruses in a co-infected cell [
11]. A natural reassortant between BATV and BUNV is the Ngari virus (NRIV) [
8,
13,
14], which was isolated from goats in Mauritania in 2010 [
15]. NRIV carries the L- and S-segments of BUNV and the M-segment from BATV and has caused at least two outbreaks of infections in humans in Central Africa, e.g., in Kenya, Tanzania and Somalia between 1998 and 1999 and in Sudan ten years before. This virus shows increased pathogenicity compared to the parental viruses associated with hemorrhagic fever [
13,
16]. Besides reassortment, it is also possible that after co-infection, a four-segmented virus occurs. Using a reverse genetic system for Rift Valley Fever virus (RVFV), Wichgers et al. (2014) showed that bunyaviruses could pack four segments into a viral particle [
17]. These four-segmented viruses were described as polyploid or diploid [
18] and might be an intermediate step for reassortment [
18].
This study aimed to analyse the capacity for reassortment of BATV and BUNV in different insect and mammalian cell lines. Furthermore, we characterised the replication of novel reassortant viruses in different cell lines to test for alteration of the viral replication due to reassortment, which might impact vector usage or pathogenesis in host animals. For this purpose, co-infections of BATV and BUNV in BHK-21 (Mesocricetus auratus), U4.4 and C6/36 (immune-competent and immune-deficient Aedes albopictus cells, respectively) were performed. The potential for reassortment in mammalian and insect cells was examined based on the frequency of isolated reassortants and diploid viruses. We developed segment-specific RT-PCRs for each virus to differentiate between the parental orthobunyaviruses and the reassortants.
2. Materials and Methods
2.1. Cells and Viruses
BHK-21 (Mesocricetus auratus; CCVL L 0179), Vero E6 (Chlorocebus sabaeus; CCVL L 0228) and PT (Ovis aris; CCVL L 0011) cells were cultivated in Minimum Essential Medium Eagle (MEM) supplemented with Hanks’ and Earle’s salts (Sigma-Aldrich, St. Louis, MO, USA), 10% fetal bovine serum (FBS), 1% non-essential amino acids and 1% sodium pyruvate solution (100 mM) (PAN Biotech GmbH, Aidenbach, Germany). SFT-R cells (Ovis aris; CCVL L 0043) were cultivated in MEM Eagle (Hank’s salts, Merck KGaA, Darmstadt, Germany) supplemented with 0.1 M sodium bicarbonate (Carl Roth, Karlsruhe, Germany) and 10% FBS. Huh7 cells (Homo sapiens; CCVL L 1079) were cultivated in Gibco™ Ham’s F-12 (Thermo Fischer Scientific, Waltham, MA, USA), with 10% FBS, Iscove’s Modified Dulbecco’s Medium (Thermo Fischer Scientific) and 0.1 M sodium bicarbonate (Carl Roth, Karlsruhe, Germany). In addition, 1% penicillin/streptomycin was added to all culture media. All mammalian cells were derived from the cell bank of the Friedrich-Loeffler-Institute (Friedrich-Loeffler-Institute, Riems, Germany) and cultivated at 37 °C with 5% CO2. FBS concentration was reduced to 5% for virus maintenance, plaque assays, TCID50, plaque-purification and co-infection experiments.
U4.4 (Aedes albopictus, CVCL_Z820) (kindly provided by Sandra Junglen, Institute for Virology, Charité, Berlin, Germany), C6/36 (Aedes albopictus; CVCL_Z230) (Friedrich-Loeffler-Institute, Riems, Germany) and Aag2 cells (Aedes aegypti; CVCL_Z617) (kindly provided by Alain Kohl, MCR-University of Glasgow Center for Virus Research, Scotland, UK) were cultivated in Schneiders’ Drosophila Medium (PAN Biotech GmbH, Aidenbach, Germany) supplemented with 10% FBS (gold(PAN Biotech GmbH, Aidenbach, Germany)), 1% l-glutamine solution (200 mM), 1% MEM NEAA (100×) without L-glutamine, 1% sodium pyruvate solution (100 mM) and 1% penicillin/streptomycin (PAN Biotech GmbH, Aidenbach, Germany—P06-07050) at 25 °C.
BUNV ATCC
® VR-87 [
7] and BATV Strain 53.2 [
6] were grown alternating on either BHK-21 cells or C6/36 cells. BHK-21 cells were seeded in T25 cm
2 tissue culture flasks (Sarstedt AG & Co., Nümbrecht, Germany) at a density of 2 × 10
6 cells per flask infected at a multiplicity of infection (MOI) of 0.01 PFU per cell. The supernatant was harvested five days post-infection (p.i.). C6/36 cells were seeded at 5 × 10
6 cells per T25 cm
2 flask and infected at an MOI of 0.1. The supernatant was harvested seven days p.i.. Viruses were concentrated using 5× Peg-it™ Virus Precipitation Solution (System Biosciences, LLC, Palo Alto, CA, USA, Cat. # LV810A-1) following manufacturer protocol. Viral titers were determined using TCID
50 and plaque assay. Viruses were stored at −80 °C.
Plaque assays were performed in 24-well plates (Sarstedt AG & Co., Nümbrecht, Germany) using 2 × 106 Vero E6 cells per plate. One day post-seeding, each well was incubated with 100 µL diluted viral supernatant (10−2–10−7) for 60 min at 37 °C and 5% CO2. Afterwards, supernatants were discarded, cells were washed with 1× PBS and layered with 1% methylcellulose solution. For staining, cells were fixed 4 to 5 days p.i. with 4% formalin solution for 30 min, washed with 1× PBS and covered with crystal-violet (10% crystal-violet and 3,7% (v/v) 37% formaldehyde) for at least 30 min or overnight.
TCID
50 were performed in 96-well tissue culture plates (Sarstedt AG & Co., Nümbrecht, Germany) using BHK-21 cells. Cells were grown in T25 cm
2 tissue culture flasks until confluency was reached, detached and split 1:4 in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 5% FBS and 1% sodium pyruvate solution (100 mM) (=ready-to-use DMEM). The cell solution was divided into four 96-well tissue culture plates (100 µL/well). Each well was supplemented with 99 µL DMEM and 11 µL/well virus-dilution (10
−1–10
−8). Plates were incubated at 37 °C and 5% CO
2 until cytopathic effects (CPE) were visible. Calculations were performed according to Reed and Muench [
19] and converted to PFU/mL (PFU/mL = 0.69 × TCID
50/
mL).
2.2. Co-Infection
For co-infections in BHK-21 cells, 2 × 106 cells per well were seeded in 6-well plates, infected at an MOI of 5 (BATV: BUNV ratio 1:1) or at an MOI of 5 and 0.1 (BATV: BUNV ratio 50:1) and incubated at 37 °C with 5% CO2. Co-infections in U4.4 and C6/36 cells were performed with 5 × 106 cells per 6-well plate, infected at an MOI of 1 (BATV: BUNV ratio 1:1) and incubated at 25 °C. Three days p.i. the supernatants of all co-infections were harvested, and viral titers determined via plaque assay.
2.3. Plaque Purification
Vero E6 cells were seeded in 6-well plates with a density of 2 × 106 cells/well. Cells were infected with viral supernatants from co-infection experiments diluted to approx. 50 PFU per well. Plates were incubated at 37 °C and 5% CO2. After 60 min, supernatants were discarded, cells were washed with 1× PBS and layered with 1% LMP agarose solution. For the LMP agarose solution, UltraPureTM LMP Agarose (Amresco, Solon, OH, USA) was solubilised in sterile water, chilled to 37 °C and supplemented with pre-heated, ready-to-use DMEM. After the LMP agarose hardened at room temperature for 60 min, the plates were incubated for at least five days at 37 °C and 5% CO2. Depending on the plaque size, the incubation time was increased to 7 days. Single plaques were picked with a 10 µL pipet tip and cultivated on 2 × 106 BHK-21 cells/well in 24-well plates at 37 °C and 5% CO2.
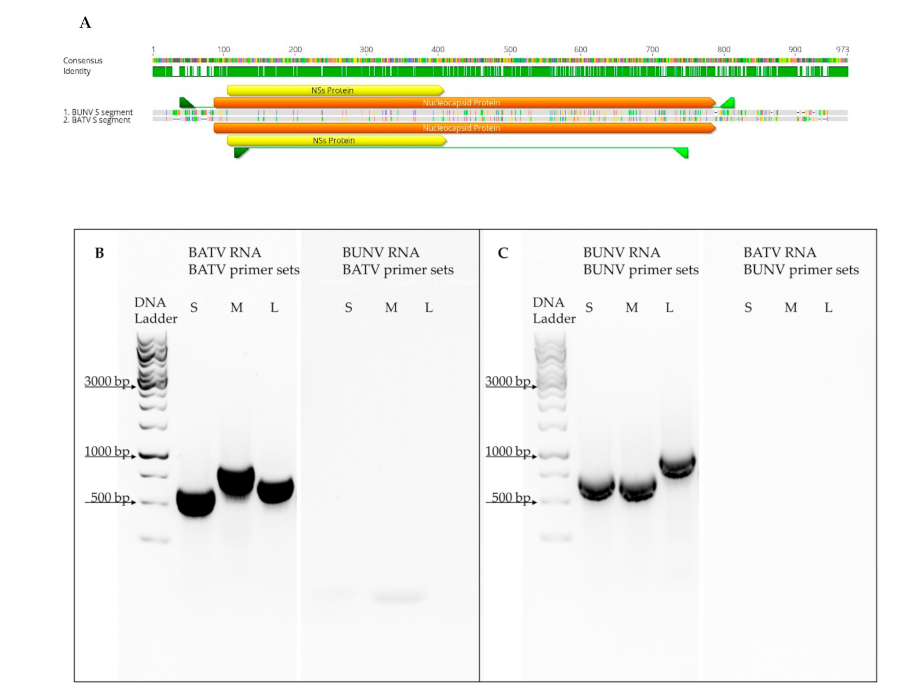
2.4. RNA Extraction, RT-PCR and NGS
To examine the genome composition of virus isolates derived from co-infection experiments, 140 µL of each plaque-purified co-infection isolate was inactivated in AVL buffer (QIAamp
® Viral RNA Mini Kit, Qiagen, Hilden, Germany). According to the QIAamp
® Viral RNA Mini Kit protocol, subsequent RNA extractions were performed. Segment-specific RT-PCRs for BATV and BUNV were performed with the QIAGEN
® One-Step RT-PCR Kit (Qiagen, Hilden, Germany) in 20 µL reactions with 8 µM forward and a reverse primer each (
Supplementary Table S1) and 2 µL RNA template. After the reverse transcription (50 °C for 30 min) and an initial denaturation step (95 °C for 15 min), a 35 cycles 3-step amplification was performed with 1 min denaturation (95 °C), 30-sec annealing at 50 °C and 2 min elongation (72 °C). PCR results were visualised on a 1% TAE-agarose gel.
Next-generation sequencing (NGS) and sequence alignments were performed at the Institute for Diagnostic Virology of the Friedrich-Loeffler Institute (Riems, Germany) by the group of Dr. Dirk Höper as previously described [
20]. Isolated RNA was transcribed into double-stranded cDNA (cDNA Synthesis System Kit, Roche, Mannheim, Germany), fragmented via ultrasonication (M220 Focused-ultrasonicator, Covaris, Woburn, MA, USA) and subsequently used for library preparation (SPRI-TE instrument, Beckman Coulter, Krefeld, Germany). Quantification of the resulting libraries was performed with KAPA Library Quant Illumina/Universal Kit (KAOA biosciences, Cape Town, South Africa) and final sequencing with a MiSeq instrument (Illumina, San Diego, CA, USA) and the Illumina MiSeq Reagent Kit v3 (600 cycle, Illumina).
2.5. Growth Kinetic Experiments
Viral growth experiments were performed in 24-well plates. With 1 × 105 cells per well, BHK-21, HuH7, PT and SFT-R cells were first incubated in a 50-mL tube as a suspension with an MOI of 0.1 for 60 min at 37 °C with 5% CO2. Cells were centrifuged at 500× g for 5 min, washed with 1× PBS (three times) and finally resuspended in ready-to-use DMEM with FBS concentration reduced to 5% and seeded in a 24-well plate. Growth kinetics in U4.4, C6/36 and Aag2 cells were performed with 1 × 106 cells/well at an MOI of 0.1. Incubation was at room temperature for 60 min. Afterwards, cells were washed with 1× PBS and resuspended with ready-to-use Schneiders’ Drosophila Medium before seeding. Incubation was at 25 °C, and at different time points, the supernatant was stored from one well for each experiment (0, 24, 48 and 72 h post-infection, 48 h p.i only for mammalian cells).
2.6. Statistical Methods
Fractions of clones from co-infection experiments were compared using Fisher’s exact test. Growth kinetics were analysed using Student’s t-test and ANOVA. Statistical analyses were performed with GraphPad Prism v9 (San Diego, CA, USA).
Raw values were first normalised for all growth kinetics to eliminate measured virus contents at time point zero. Therefore, the mean value for each virus at time point zero was calculated, and all values for this virus were divided by this mean. Next, these ratios were log-transformed for variance stabilisation and to bring values closer to a normal distribution. This step is necessary because measurements at the later time point have larger means and larger variances. Without variance stabilisation, time points would not be comparable. Finally, log-transformed ratios were shifted such that each time curve had a start value of zero.
After normalisation, time curves in each cell line were analysed by a two-way analysis of variance, including the effect of the four viruses, the four time points and the interaction between viruses and time. Studying the interaction is of particular interest to detect whether time profiles of individual viruses are parallel. An analysis of variance was performed using the lme3- and lmer test packages for the statistics software R (version 4.0.4,
www.r-project.org (accessed on 1 February 2021)) to account for the repeated measurements. Subsequent comparisons with reduced models, including one “real” and one “combined” virus, were performed for those cell lines where the interaction p-value of the full model was close to significance, i.e., reduced models refer to models applied to a subset of the data.
P-values in the full model were considered significant at a level of 5%, and
p-values in the four reduced models at a Bonferroni-corrected level of 5%/4.
For visualisation, time-specific 95% confidence intervals for each virus of the form “mean +/− z1-α/2 * standard error of the mean” were calculated, where z1-α/2 denotes the 1-α/2-quantile of the standard normal distribution.
4. Discussion
It is beneficial for each virus to replicate its genome to the highest possible number during infection. We observed a higher proportion of BUNV isolates in our co-infection studies after co-infections in BHK-21 cells (
Mesocricetus auratus) and U4.4 and C6/36 cells (
Ae. albopictus), indicating a higher fitness of BUNV in the tested cell lines. However, replication rates of BUNV and BATV were very similar in single infections in those cell lines (
Figure 3A,E,F). Furthermore, other studies showed no significant differences in the growth kinetics among BATV, BUNV and NRIV in experiments with Vero cells (
Chlorocebus aethiops) [
22]. The presumed higher fitness of BUNV in the three tested cell lines might be explained by the protein interaction with BATV, which negatively influences BATV replication in the co-infection. Alternatively, BUNV replication rates in these cell lines in a co-infection might be higher than BATV genome replication. The co-infection interference of orthobunyaviruses has been described in mammals and insects, although the mechanisms behind this interference are not revealed [
23,
24]. Further experiments would be needed to analyse this hypothesis in more detail.
The possibility for reassortment of
Orthobunyaviruses has already been demonstrated experimentally and in nature [
8,
11,
13,
25]. Our studies show that efficient reassortment was only observed in the 50:1 (BATV: BUNV) co-infection in BHK-21 cells. Furthermore, we observed reassortment via diploid viruses. The observed higher proportions of diploid viruses in the 50:1 ratio might argue that BATV genome replication is hindered by BUNV co-infection. However, other reasons, such as the inefficient packaging of BATV genome segments, might contribute to the low frequency of diploid viruses.
Interestingly, we did not observe any reassortment in U4.4 cells, although this was the only cell line in which BATV wt were isolated after co-infection (6%). Thus, it seems unlikely that an increased MOI of BATV would improve the reassortment frequency in these cells. Indeed, all tested diploid viruses from U4.4 showed a reversion to the BUNV wt after the second plaque-purification. The co-infection experiments in immune-competent (U4.4) and immune-deficient (C6/36) insect cells showed that the proportion of BUNV isolates is significantly higher in C6/36 cells than in U4.4 cells. The growth rates during single infections for both viruses in U4.4 and C6/36 cells (
Figure 3E,F) displayed a better growth of BATV in U4.4 cells but not in C6/36 cells or Aag2 cells, where both viruses showed similar growth curves. Many viruses can escape from antiviral small interfering (si) RNA pathways and other antiviral mechanisms. It has been shown that the NSs-protein of BUNV is essential for viral replication in U4.4 but not in C6/36 [
26], indicating a function necessary to escape the siRNA pathway. Potentially, BATV NSs has a similar function during replication; however, a NSs deletion mutant of BATV would be needed to show this. Furthermore, the observed BATV wt clones in U4.4. cells suggest that BATV has higher fitness in these cells than in C6/36 cells. This fitness could be attributed to the fact that our BATV isolate was derived from a mosquito in 2011 and is still well adapted to the insect vector or interaction with the insect’s immune system.
As already mentioned, we observed many isolates that did not show the classical three viral segments. The first group, the diploid viruses, harboured one additional segment: the S-segment was most frequent, followed by M-segment and L-segment diploids. Diploid viruses have been described before [
17,
18] and are speculated to be an intermediate step for reassortment [
18]. However, we observed no S-segment reassortants despite the high number of diploid isolates with two S-segments (example in
Supplementary Figure S1B). On the other side, reassortments observed after a second plaque-purification of diploid viruses were only M- and L-segment reassortants. Thus, we cannot deduce with any certainty from the data presented if these diploid viruses are a random phenomenon without any consequences for reassortment or a natural step in reassortment. Besides diploid viruses, we observed some isolates with less or equal to two segments. Since we are not aware of any description of naturally occurring two-segmented bunyaviruses that can replicate (this being a prerequisite for their isolation via plaque-purification), we assumed that these isolates are incomplete isolates, or that incomplete PCR data might have resulted from inhibition in the PCR reaction or incorrect RNA isolation.
When comparing the growth kinetics of BATV, BUNV and the two reassortant viruses I-NRIV and BAYAV, the reassortant viruses showed higher replication rates in the mammalian cell lines BHK-21 and SFT-R. However, only the virus growth curve for SFT-R was significantly different between the four viruses (
Table 1 and
Table 2). BHK-21 cells were kidney fibroblasts derived from the Syrian gold hamster (
Mesocricetus auratus), whereas SFT-R cells originated from the thymus of the domestic sheep (
Ovis aris). The other two cell lines which showed no growth differences were the human hepatoma cell line Huh7 and PT cells derived from the kidney of the domestic sheep (
Ovis aris). Huh7 and BHK-21 cells were both used to study the cell entry of the Uukuniemi virus (UUKV,
Phenuiviridae,
Bunyavirales), and BHK-21 seem to be more susceptible to UUKV than Huh7 cells. The ovine PT and SFT-R cells have not been used in comparative infections with bunyaviruses; however, growth of the Schmallenberg virus was demonstrated in SFT-R cells. SFT-R and PT cells were used in a comparative study testing the growth of different pestiviruses [
27]. Both ovine cell lines, PT and SFT-R, could be infected with all tested viruses to the same extent, whereas Huh7 cells were only infected with one of the six tested viruses. Furthermore, infection experiments in the two ovine cell lines with the bluetongue virus (BTV), serotype 1 and 8 reassortants, revealed that the reassortment phenotype (growth behaviour similar to BTV8) was more pronounced in SFT-R as compared to PT cells; however, both BTV1 and BTV 8 grew to the same titer in both cell lines [
28]. We observed very similar growth curves for BATV and BUNV in BHK-21 and Huh7 cells with identical endpoint virus titers (
Figure 3,
Table S2). However, BAYAV and I-NRIV grew to higher titers in BHK-21 cells, indicating a specific interaction of the reassortant viruses with both cell lines. A possible factor contributing to the observed differences could be the activation of interferon via the cellular receptor RIG-I. This DExD/H box RNA helicase activates interferon expression upon recognition of viral genomic RNA structures and has been demonstrated to be essential for recognising many bunyavirus infections [
29]. Whereas RIG-I dependent activation of the interferon pathway is intact in Huh7 cells [
30], severe impairment of RIG-I-dependent recognition of viral genomes has been demonstrated in BHK-21 cells [
31]. This impairment might facilitate better growth of BAYAV and I-NRIV in BHK-21 cells. However, multiple factors can influence parental and reassortant virus growth in BHK-21 and Huh7 cells since these cell lines were derived from different species and different organs; thus, species or organ-specific differences might play a role. The PT and SFT-R cells were derived from the same species, the domestic sheep, which makes the results obtained in these cells more comparable. In contrast to Huh7 and BHK-21 cells, we observed similar titers of the reassortant viruses BAYAV and I-NRIV in both cell lines (
Figure 3,
Table S2), but the parental virus growth was lower in SFT-R cells compared to PT cells. SFT-R cells were shown to be interferon-competent, and the deletion of the NSs protein of the Schmallenberg virus had a severe impact on the growth of this mutant virus in those cells [
26]. Unfortunately, nothing has been published on the interferon pathway in PT cells; thus, we can only speculate that the lower growth rates of BATV and BUNV in SFT-R cells are due to the interferon pathway-dependent inhibition of virus growth. However, a better inhibition of interferon induction can be one reason for improved viral growth (reviewed by the authors in [
32]). Furthermore, a study by Tilson et al. [
33] using minigenomes and virus-like particles to create diverse reassortants of
Oropouche orthobunyaviruses and Schmallenberg virus showed that, for example, the combination of the Oropouche M minigenome with the L- and N-proteins of Schmallenberg virus led to a 50-fold increase in minigenome activity. In contrast, the other combinations did not show increased activity. These results indicate that some new combinations of genomic segments and proteins from different viruses can enhance the transcription activity, leading to higher replication rates. The effect could be cell-type specific and contribute to our reassortant viruses’ observed enhanced growth rates in some cell lines.
Taken together, the different growth kinetics of reassortant and parental viruses in the four tested mammalian cell lines might be correlated with the different interactions with the host immune system. However, tissue and host-specific interactions independent of immune system–virus interactions or viral transcription and replication changes could be responsible. More experiments targeting specific interaction with the immune activation pathway and the use of minigenome systems to determine virus replication and transcription could help to understand the nature of differences between reassortants and parental viruses.
The replication rates for the two reassortants were lower in insect cells, with differences close to significance for C6/36 and significant differences for Aag2 cells compared to the parental viruses. In the reduced model, where we compared the reassortant viruses with each parental virus, we observed a more substantial effect for BAYAV than I-NRIV in C6/36 and Aag2 cell lines. As already mentioned above, C6/36 cells have an impaired RNA interference pathway [
21], which might lead to differences in viral growth. BAYAV carries the BATV S-segment and thus expresses the BATV NSs-protein, which might confer a similar phenotype as observed for BATV. However, since we did not observe growth differences in U4.4 but rather in C6/36, the role of NSs-Dicer-2 interaction for different growth kinetics might be neglected. The most noticeable effect of the reduced BAYAV growth was observed in the
Aedes aegypti Aag2 cells, indicating a species-specific effect. Along this line, a study using
Aedes aegypti mosquitos demonstrated that these mosquitos were moderately susceptible to BUNV, whereas the same mosquito strain was refractory to NRIV [
34]. Taken together, the potential impairment of reassortant bunyaviruses in
Aedes aegypti could be suspected.
The nature of this impairment is still enigmatic; Aag2 cells have an intact Dicer-2 and PIWI pathway [
21], making RNA interference pathway impairments unlikely to be responsible for the observed phenotype. Persistent infection with insect-specific viruses could account for different virus growth kinetics. It has been shown that the cell fusing agent virus (CFAV), which persistently infects Aag2 cells, influences the growth of the dengue virus in this cell line [
35]. With regards to our cells used in the experiment, we confirmed CFAV in our Aag2 cells. Perhaps CFAV could play a role in suppressing BAYAV and I-NRIV; however, more in-depth sequencing is needed to analyse if CFAV may play a role in growth differences.
Interestingly, we neither observed reassortment in all tested insect cells nor enhanced growth of reassortant I-NRIV and BAYAV. This observation contradicts current literature, which describes reassortment primarily in the insect vector but not in mammalian hosts. Thus far, studies to demonstrate genome reassortment in vertebrates have been unsuccessful [
12], while the reassortment of heterologous and homologous orthobunyaviruses was demonstrated in the mosquito vector [
36,
37]. However, a reassortment in mammalian hosts in nature cannot be ruled out since
Orthobunyavirus reassortants such as the Ngari virus have been isolated from both insect and mammalian hosts [
8,
38]. The observed differences between some insect vector cells (specifically Aag2) and some mammalian cell lines (BHK-21 and SFT-R) with the same novel combination of segments might point to a beneficial role of the novel combination in one system, which is disadvantageous in another system and can lead to a different adaptation of these new viruses to hosts and vectors. To further investigate the role of protein combination and adaptation processes, a reverse genetic system for BATV would be helpful. With the help of such reverse genetic systems, new reassortants could be generated using the already established BUNV reverse genetic system [
39], so the impact of diverse segment combinations could be tested.