Cross Talk between Viruses and Insect Cells Cytoskeleton
Abstract
1. Introduction
2. The Cytoskeleton in the Context of Animal–Virus Interactions
2.1. The Actin Cytoskeleton
2.2. Microtubule Cytoskeleton
3. Baculoviruses Hijack the Host Cells Cytoskeleton Actin to Replicate and Spread the Infection
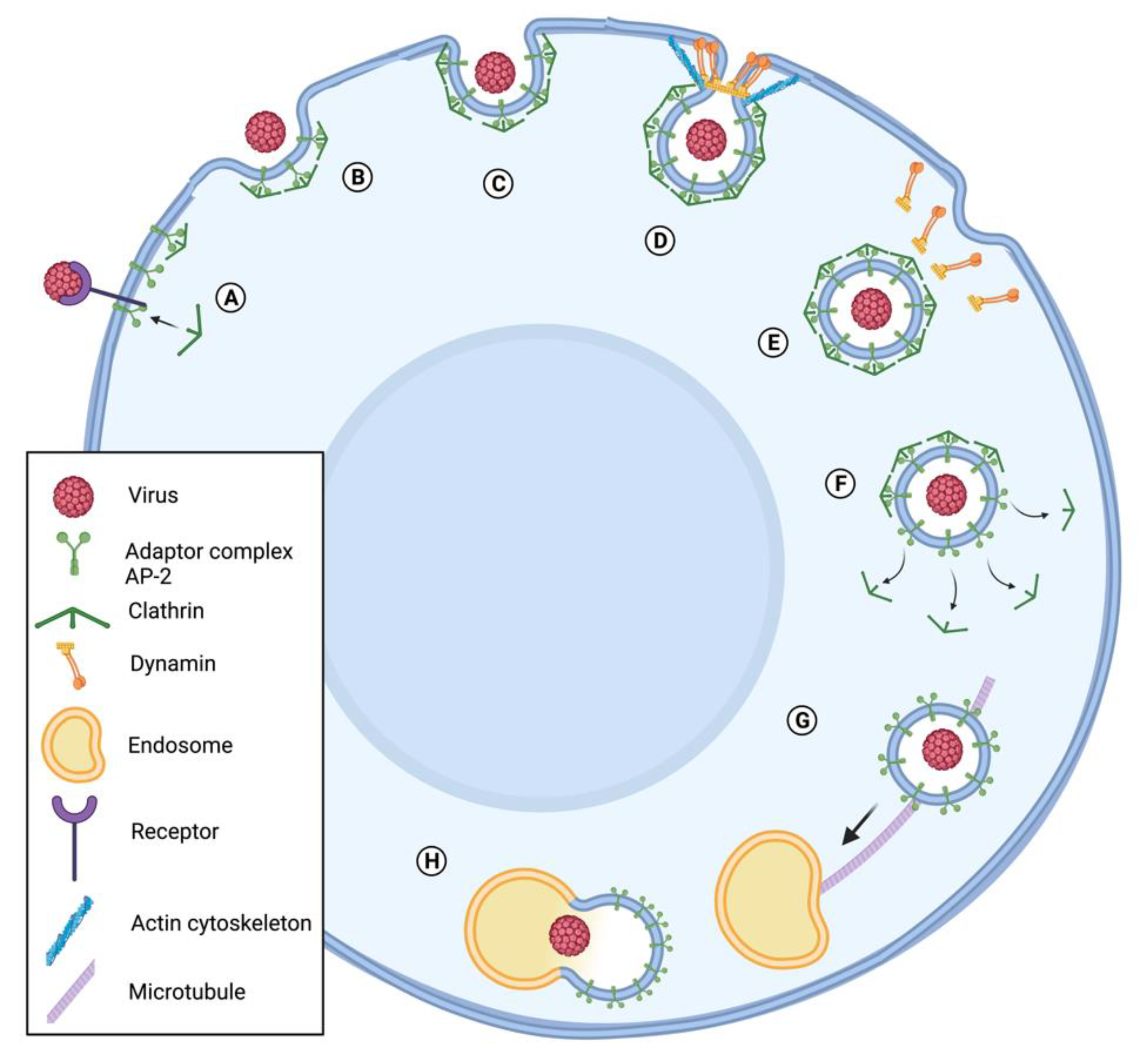
3.1. Baculovirus Entry and Transport through the Cytoplasm Depend on Re-Arrangement of Host Cell Cytoskeleton
3.2. Baculovirus Entry into the Nucleus and Viral Progeny Egression Are Mediated by Actin Re-Arrangements
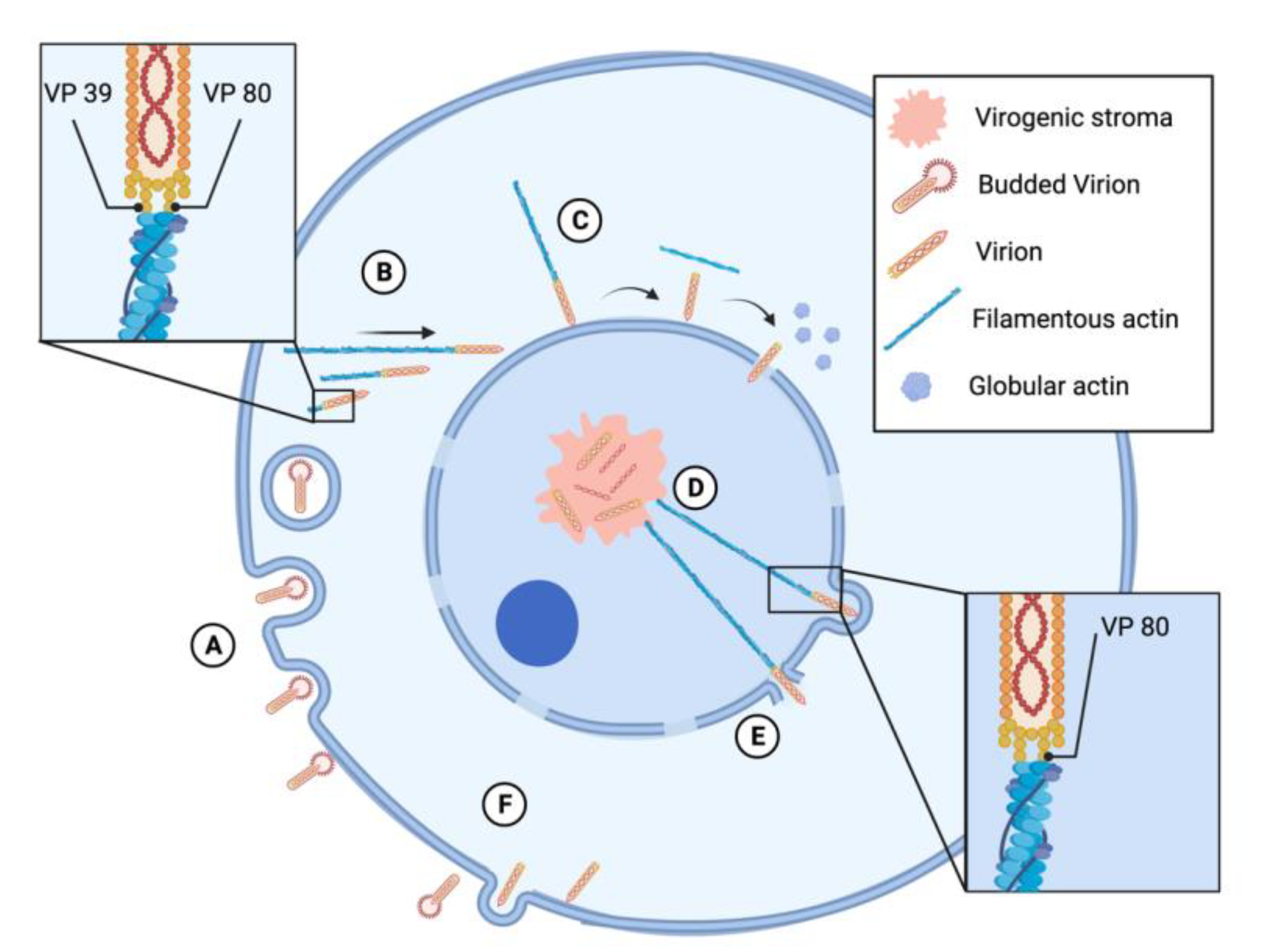
3.3. Baculovirus Interactions with Microtubules
4. Examples of Viruses Other Than Baculoviruses That Manipulate Host Cytoskeleton for Successful Transmission
4.1. Plant Viruses Transmitted by Insect Vectors
4.1.1. Plant Virus Entry, Transmission, and Breaching through the Host Intestinal Barrier Is Mediated by Viral Interaction with the Host Cytoskeleton
4.1.2. Horizontal Transmission of Plant Viruses through the Salivary Glands
4.1.3. Vertical Transmission of Plant Viruses
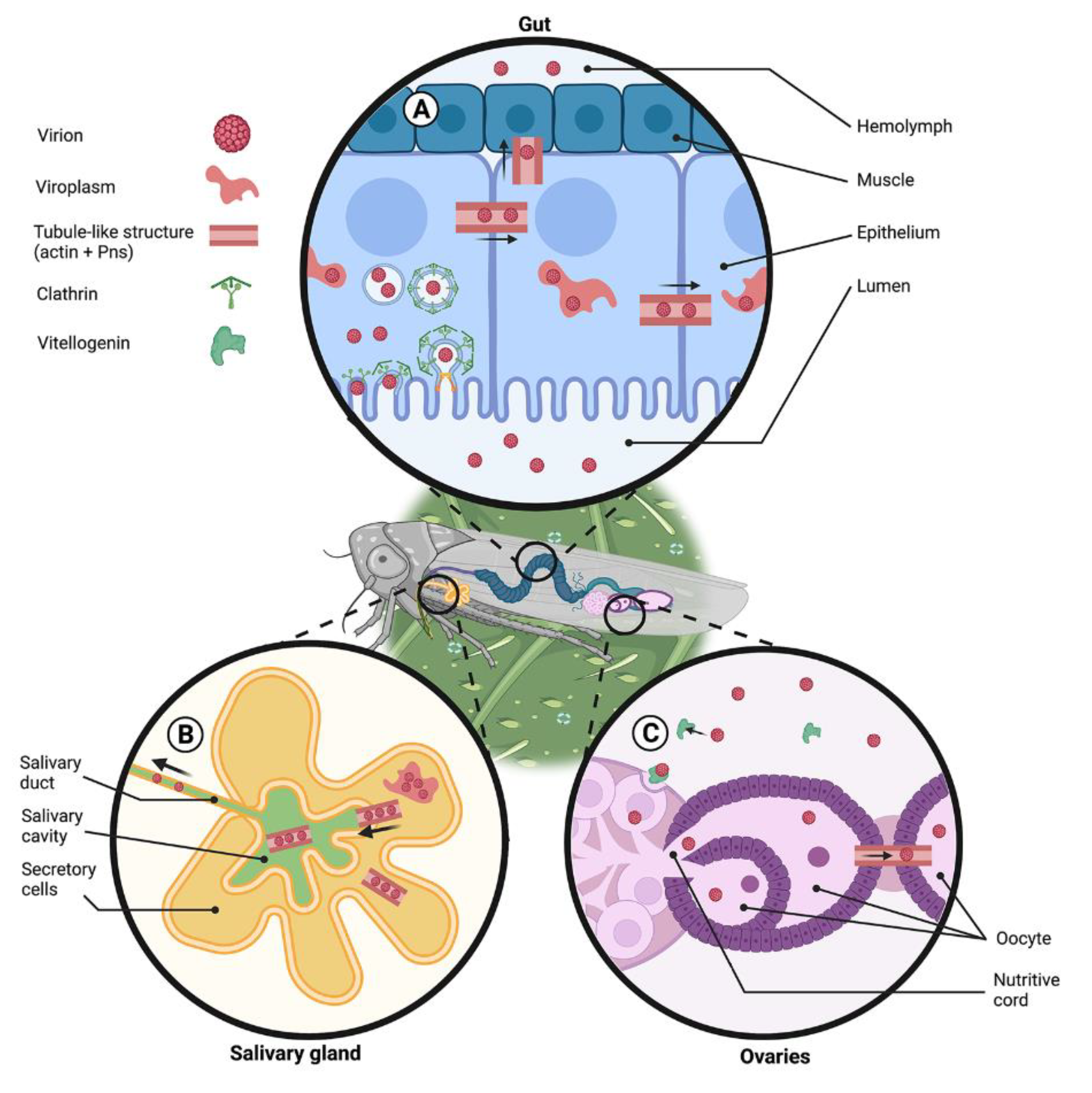
4.2. Arboviruses
4.2.1. Examples of Flavivirus Interaction with Insect Cytoskeleton
4.2.2. Examples of CHIKV Interaction with Insect Cytoskeleton
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ploubidou, A.; Way, M. Viral transport and the cytoskeleton. Curr. Opin. Cell Biol. 2001, 13, 97–105. [Google Scholar] [CrossRef]
- Wei, T.; Li, Y. Rice Reoviruses in Insect Vectors. Annu. Rev. Phytopathol. 2016, 54, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Harries, P.A.; Schoelz, J.E.; Nelson, R.S. Intracellular transport of viruses and their components: Utilizing the cytoskeleton and membrane highways. Mol. Plant-Microbe Interact. 2010, 23, 1381–1393. [Google Scholar] [CrossRef]
- Stidwill, R.P.; Greber, U.F. Lntracellular virus trafficking reveals physiological characteristics of the cytoskeleton. News Physiol. Sci. 2000, 15, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Liman, J.; Bueno, C.; Eliaz, Y.; Schafer, N.P.; Waxham, M.N.; Wolynes, P.G.; Levine, H.; Cheung, M.S. The role of the Arp2/3 complex in shaping the dynamics and structures of branched actomyosin networks. Proc. Natl. Acad. Sci. USA 2020, 117, 10825–10831. [Google Scholar] [CrossRef] [PubMed]
- Goley, E.D.; Ohkawa, T.; Mancuso, J.; Woodruff, J.B.; D’Alessio, J.A.; Cande, W.Z.; Volkman, L.E.; Welch, M.D. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science 2006, 314, 464–467. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Qin, F.; Xu, C.; Hu, J.; Lei, C.; Zheng, Z.; Peng, K.; Wang, H.; Sun, X. Dissecting the Cell Entry Pathway of Baculovirus by Single-Particle Tracking and Quantitative Electron Microscopic Analysis. J. Virol. 2019, 93, 1–25. [Google Scholar] [CrossRef]
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011, 9, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Toret, C.P.; Drubin, D.G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006, 7, 404–414. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Han, S.; Hu, X.; Zhou, Y.; Mu, J.; Pei, R.; Wu, C.; Chen, X. Identification of a novel regulatory sequence of actin nucleation promoting factor encoded by Autographa californica multiple nucleopolyhedrovirus. J. Biol. Chem. 2015, 290, 9533–9541. [Google Scholar] [CrossRef]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Welch, M.D. Baculovirus Actin-Based Motility Drives Nuclear Envelope Disruption and Nuclear Egress. Curr. Biol. 2018, 28, 2153–2159.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, L.; Zhang, Y.; Mao, Q.; Wei, T. Tubules of plant reoviruses exploit tropomodulin to regulate actin-based tubule motility in insect vector. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Seddas, P.; Boissinot, S.; Strub, J.M.; Van Dorsselaer, A.; Van Regenmortel, M.H.V.; Pattus, F. Rack-1, GAPDH3, and actin: Proteins of Myzus persicae potentially involved in the transcytosis of beet western yellows virus particles in the aphid. Virology 2004, 325, 399–412. [Google Scholar] [CrossRef]
- Naghavi, M.H.; Walsh, D. Microtubule Regulation and Function during Virus Infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Walsh, D.; Naghavi, M.H. Exploitation of Cytoskeletal Networks during Early Viral Infection. Trends Microbiol. 2019, 27, 39–50. [Google Scholar] [CrossRef]
- Simpson, C.; Yamauchi, Y. Microtubules in influenza virus entry and egress. Viruses 2020, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wei, X.; Wu, X.; Sellers, M.T.; Decker, J.M.; Moldoveanu, Z.; Orenstein, J.M.; Graham, M.F.; Kappes, J.C.; Mestecky, J.; et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 2002, 8, 150–156. [Google Scholar] [CrossRef]
- Marozin, S.; Prank, U.; Sodeik, B. Herpes simplex virus type 1 infection of polarized epithelial cells requires microtubules and access to receptors present at cell-cell contact sites. J. Gen. Virol. 2004, 85, 775–786. [Google Scholar] [CrossRef]
- Brodsky, F.M. Clathrin and Clathrin-Dependent Endocytosis. Encycl. Cell Biol. 2016, 2, 384–393. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Liu, S.; Pang, D.-W.; Xiao, G. Clathrin-Mediated Endocytosis in Living Host Cells Visualized through Quantum Dot Labeling of Infectious Hematopoietic Necrosis Virus. J. Virol. 2011, 85, 6252–6262. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; White, P.J.; Pouton, C.W. Interaction of viruses with host cell molecular motors. Curr. Opin. Biotechnol. 2010, 21, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Leopold, P.L.; Pfister, K.K. Viral strategies for intracellular trafficking: Motors and microtubules. Traffic 2006, 7, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmad, F.; Pichtel, J. Microbes and Microbial Technology: Agricultural and Environmental Applications; Springer: New York, NY, USA, 2011; Chapter 16. [Google Scholar]
- Hoopes, R.R.; Rohrmann, G.F. In vitro transcription of baculovirus immediate early genes: Accurate mRNA initiation by nuclear extracts from both insect and human cells. Proc. Natl. Acad. Sci. USA 1991, 88, 4513–4517. [Google Scholar] [CrossRef] [PubMed]
- Glocker, B.; Hoopes, R.R.; Hodges, L.; Rohrmann, G.F. In vitro transcription from baculovirus late gene promoters: Accurate mRNA initiation by nuclear extracts prepared from infected Spodoptera frugiperda cells. J. Virol. 1993, 67, 3771–3776. [Google Scholar] [CrossRef]
- Huh, N.E.; Weaver, R.F. Identifying the RNA polymerases that synthesize specific transcripts of the Autographa californica nuclear polyhedrosis virus. J. Gen. Virol. 1990, 71, 195–201. [Google Scholar] [CrossRef]
- Gasmi, L.; Jakubowska, A.K.; Herrero, S. Gasmin (BV2-5), a polydnaviral-acquired gene in Spodoptera exigua. Trade-off in the defense against bacterial and viral infections. Dev. Comp. Immunol. 2016, 56, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xu, C.; Lei, C.; Hu, J.; Sun, X. Autographa californica multiple nucleopolyhedrovirus enters host cells via clathrin-mediated endocytosis and direct fusion with the plasma membrane. Viruses 2018, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, M.; Qiu, Z.; Deng, F.; Vlak, J.M.; Hu, Z.; Wang, H. Autographa californica Multicapsid Nucleopolyhedrovirus Efficiently Infects Sf9 Cells and Transduces Mammalian Cells via Direct Fusion with the Plasma Membrane at Low pH. J. Virol. 2010, 84, 5351–5359. [Google Scholar] [CrossRef]
- Marek, M.; Merten, O.-W.; Galibert, L.; Vlak, J.M.; van Oers, M.M. Baculovirus VP80 Protein and the F-Actin Cytoskeleton Interact and Connect the Viral Replication Factory with the Nuclear Periphery. J. Virol. 2011, 85, 5350–5362. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yao, L.; Lu, S.; Qi, Y. Host filamentous actin is associated with Heliothis armigera single nucleopolyhedrosis virus (HaSNPV) nucleocapsid transport to the host nucleus. Curr. Microbiol. 2007, 54, 199–206. [Google Scholar] [CrossRef]
- Katsuma, S.; Kokusho, R. A Conserved Glycine Residue Is Required for Proper Functioning of a Baculovirus VP39 Protein. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Au, S.; Wu, W.; Zhou, L.; Theilmann, D.A.; Panté, N. A new mechanism for nuclear import by actin-based propulsion used by a baculovirus nucleocapsid. J. Cell Sci. 2016, 129, 2905–2911. [Google Scholar] [CrossRef]
- Dreschers, S.; Roncarati, R.; Knebel-Mörsdorf, D. Actin Rearrangement-Inducing Factor of Baculoviruses Is Tyrosine Phosphorylated and Colocalizes to F-Actin at the Plasma Membrane. J. Virol. 2001, 75, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Lauko, D.I.; Ohkawa, T.; Mares, S.E.; Welch, M.D. Baculovirus actin-rearrangement-inducing factor ARIF-1 induces the formation of dynamic invadosome clusters. Mol. Biol. Cell 2021, 3200. [Google Scholar] [CrossRef]
- Williams, G.V.; Faulkner, P. Cytological Changes and Viral Morphogenesis during Baculovirus Infection. Baculoviruses 1997, 61–107. [Google Scholar] [CrossRef]
- Fang, M.; Nie, Y.; Theilmann, D.A. AcMNPV EXON0 (AC141) which is required for the efficient egress of budded virus nucleocapsids interacts with β-tubulin. Virology 2009, 385, 496–504. [Google Scholar] [CrossRef]
- Danquah, J.O.; Botchway, S.; Jeshtadi, A.; King, L.A. Direct Interaction of Baculovirus Capsid Proteins VP39 and EXON0 with Kinesin-1 in Insect Cells Determined by Fluorescence Resonance Energy Transfer-Fluorescence Lifetime Imaging Microscopy. J. Virol. 2012, 86, 844–853. [Google Scholar] [CrossRef]
- van Oers, M.M.; Flipsen, J.T.; Reusken, C.B.; Vlak, J.M. Specificity of Baculovirus p10 Functions. Virology 1994, 200, 513–523. [Google Scholar] [CrossRef]
- Patmanidi, A.L.; Possee, R.D.; King, L.A. Formation of P10 tubular structures during AcMNPV infection depends on the integrity of host-cell microtubules. Virology 2003, 317, 308–320. [Google Scholar] [CrossRef]
- Carpentier, D.C.J.; Griffiths, C.M.; King, L.A. The baculovirus P10 protein of Autographa californica nucleopolyhedrovirus forms two distinct cytoskeletal-like structures and associates with polyhedral occlusion bodies during infection. Virology 2008, 371, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Bragard, C.; Caciagli, P.; Lemaire, O.; Lopez-Moya, J.J.; Macfarlane, S.; Peters, D.; Susi, P.; Torrance, L. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 2013, 51, 177–201. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Rotenberg, D. Disruption of insect transmission of plant viruses. Curr. Opin. Insect Sci. 2015, 8, 79–87. [Google Scholar] [CrossRef]
- Pirone, T.P.; Megahed, E.S. Aphid transmissibility of some purified viruses and viral RNA’s. Virology 1966, 30, 631–637. [Google Scholar] [CrossRef]
- Chen, A.Y.S.; Walker, G.P.; Carter, D.; Ng, J.C.K. A virus capsid component mediates virion retention and transmission by its insect vector. Proc. Natl. Acad. Sci. USA 2011, 108, 16777–16782. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Q.; Omura, T.; Uehara-Ichiki, T.; Wei, T. Sequential infection of Rice dwarf virus in the internal organs of its insect vector after ingestion of virus. Virus Res. 2011, 160, 389–394. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- DeRosier, D.J.; Tilney, L.G. F-actin bundles are derivatives of microvilli: What does this tell us about how bundles might form? J. Cell Biol. 2000, 148, 1–6. [Google Scholar] [CrossRef]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2017, 69, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R. A handbook of leafhopper and planthopper vectors of plant disease. Bull. Insectol. 2007, 60, 175–176. [Google Scholar]
- Wei, T.; Chen, H.; Ichiki-Uehara, T.; Hibino, H.; Omura, T. Entry of Rice Dwarf Virus into Cultured Cells of Its Insect Vector Involves Clathrin-Mediated Endocytosis. J. Virol. 2007, 81, 7811–7815. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Wei, T.; Shimizu, T.; Omura, T. Endomembranes and myosin mediate assembly into tubules of Pns10 of Rice dwarf virus and intercellular spreading of the virus in cultured insect vector cells. Virology 2008, 372, 349–356. [Google Scholar] [CrossRef][Green Version]
- Wei, T.; Kikuchi, A.; Moriyasu, Y.; Suzuki, N.; Shimizu, T.; Hagiwara, K.; Chen, H.; Takahashi, M.; Ichiki-Uehara, T.; Omura, T. The Spread of Rice Dwarf Virus among Cells of Its Insect Vector Exploits Virus-Induced Tubular Structures. J. Virol. 2006, 80, 8593–8602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Ren, T.; Xie, L.; Wei, T. Interaction between non-structural protein Pns10 of rice dwarf virus and cytoplasmic actin of leafhoppers is correlated with insect vector specificity. J. Gen. Virol. 2015, 96, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, L.; Jia, D.; Zhang, P.; Chen, Q.; Liu, Q.; Wei, T. Rice gall dwarf virus exploits tubules to facilitate viral spread among cultured insect vector cells derived from leafhopper Recilia dorsalis. Front. Microbiol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, D.; Chen, H.; Chen, Q.; Xie, L.; Wu, Z.; Wei, T. The P7-1 protein of southern rice black-streaked dwarf virus, a fijivirus, induces the formation of tubular structures in insect cells. Arch. Virol. 2011, 156, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Mao, Q.; Chen, H.; Wang, A.; Liu, Y.; Wang, H.; Xie, L.; Wei, T. Virus-Induced Tubule: A Vehicle for Rapid Spread of Virions through Basal Lamina from Midgut Epithelium in the Insect Vector. J. Virol. 2014, 88, 10488–10500. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zheng, L.; Chen, H.; Jia, D.; Li, F.; Wei, T. Nonstructural protein NS4 of rice stripe virus plays a critical role in viral spread in the body of vector insects. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Zhang, Q.; Zeng, T.; Zheng, Y.; Chen, H.; Zhang, X.F.; Wei, T. Rice yellow stunt nucleorhabdovirus matrix protein mediates viral axonal transport in the central nervous system of its insect vector. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Kikuchi, A.; Moriyasu, Y.; Tomaru, M.; Jin, Y.; Suga, H.; Hagiwara, K.; Akita, F.; Shimizu, T.; Netsu, O.; et al. Rice Dwarf Viruses with Dysfunctional Genomes Generated in Plants Are Filtered Out in Vector Insects: Implications for the Origin of the Virus. J. Virol. 2011, 85, 2975–2979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, Q.; Liao, Z.; Li, J.; Liu, Y.; Wu, W.; Chen, H.; Chen, Q.; Jia, D.; Wei, T. Filamentous Structures Induced by a Phytoreovirus Mediate Viral Release from Salivary Glands in Its Insect Vector. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Liu, W.; Zhang, F.; Chen, X.; Li, L.; Liu, Q.; Zhou, Y.; Wei, T.; Fang, R.; Wang, X. Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef]
- Światek, P. Oogenesis in the leech Glossiphonia heteroclita (Annelida, Hirudinea, Glossiphoniidae). II. Vitellogenesis, follicle cell structure and egg shell formation. Tissue Cell 2006, 38, 263–270. [Google Scholar] [CrossRef]
- Liao, Z.; Mao, Q.; Li, J.; Lu, C.; Wu, W.; Chen, H.; Chen, Q.; Jia, D.; Wei, T. Virus-induced tubules: A vehicle for spread of virions into ovary oocyte cells of an insect vector. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 88–104. [Google Scholar] [CrossRef]
- Wu, P.; Yu, X.; Wang, P.; Cheng, G. Arbovirus lifecycle in mosquito: Acquisition, propagation and transmission. Expert Rev. Mol. Med. 2019, 21. [Google Scholar] [CrossRef]
- Lequime, S.; Lambrechts, L. Vertical transmission of arboviruses in mosquitoes: A historical perspective. Infect. Genet. Evol. 2014, 28, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Mosso, C.; Galván-Mendoza, I.J.; Ludert, J.E.; del Angel, R.M. Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT. Virology 2008, 378, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.H.; Leong, P.W.H.; Ng, M.L. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology 2006, 349, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, T.M.; Cox, J.; Nguyen, A.; Feitosa, F.; Krishnan, M.N.; Fikrig, E. Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector. Virology 2011, 417, 179–187. [Google Scholar] [CrossRef]
- Paingankar, M.S.; Gokhale, M.D.; Deobagkar, D.N. Dengue-2-virus-interacting polypeptides involved in mosquito cell infection. Arch. Virol. 2010, 155, 1453–1461. [Google Scholar] [CrossRef]
- Mairiang, D.; Zhang, H.; Sodja, A.; Murali, T.; Suriyaphol, P.; Malasit, P.; Limjindaporn, T.; Finley, R.L. Identification of New Protein Interactions between Dengue Fever Virus and Its Hosts, Human and Mosquito. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Sigle, L.T.; McGraw, E.A. Expanding the canon: Non-classical mosquito genes at the interface of arboviral infection. Insect Biochem. Mol. Biol. 2019, 109, 72–80. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Dunn, W.A.; Campbell, C.L.; Olson, K.E.; Marinotti, O.; James, A.A. Complex Modulation of the Aedes aegypti Transcriptome in Response to Dengue Virus Infection. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, P.; Mooney, B.P.; Franz, A.W.E. Quantitative Proteomic Analysis of Chikungunya Virus-Infected Aedes aegypti Reveals Proteome Modulations Indicative of Persistent Infection. J. Proteome Res. 2020, 19, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ protein regulating signaling pathways and cellular functions. Int. J. Mol. Sci. 2019, 20, 4171. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Te Riet, J.; Eich, C.; Harkes, R.; Smisdom, N.; Wenger, J.B.; Ameloot, M.; Holt, M.; Kanger, J.S.; Figdor, C.G.; et al. Syntenin-1 and ezrin proteins link activated leukocyte cell adhesion molecule to the actin cytoskeleton. J. Biol. Chem. 2014, 289, 13445–13460. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khorramnejad, A.; Perdomo, H.D.; Palatini, U.; Bonizzoni, M.; Gasmi, L. Cross Talk between Viruses and Insect Cells Cytoskeleton. Viruses 2021, 13, 1658. https://doi.org/10.3390/v13081658
Khorramnejad A, Perdomo HD, Palatini U, Bonizzoni M, Gasmi L. Cross Talk between Viruses and Insect Cells Cytoskeleton. Viruses. 2021; 13(8):1658. https://doi.org/10.3390/v13081658
Chicago/Turabian StyleKhorramnejad, Ayda, Hugo D. Perdomo, Umberto Palatini, Mariangela Bonizzoni, and Laila Gasmi. 2021. "Cross Talk between Viruses and Insect Cells Cytoskeleton" Viruses 13, no. 8: 1658. https://doi.org/10.3390/v13081658
APA StyleKhorramnejad, A., Perdomo, H. D., Palatini, U., Bonizzoni, M., & Gasmi, L. (2021). Cross Talk between Viruses and Insect Cells Cytoskeleton. Viruses, 13(8), 1658. https://doi.org/10.3390/v13081658






