Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up
Abstract
1. Introduction
2. Need for and Feasibility of an HCV Vaccine
3. Importance of T Cells to HCV Control
4. A Possible Role for Antibodies
5. HCV Subversion of Adaptive Immune Responses
6. HCV Genetic Diversity and Implications for Vaccine Design
7. Candidate HCV Prophylactic Vaccines
8. Novel Hepacivirus Models for HCV Study
9. Considerations for Using a Controlled Human Infection Model
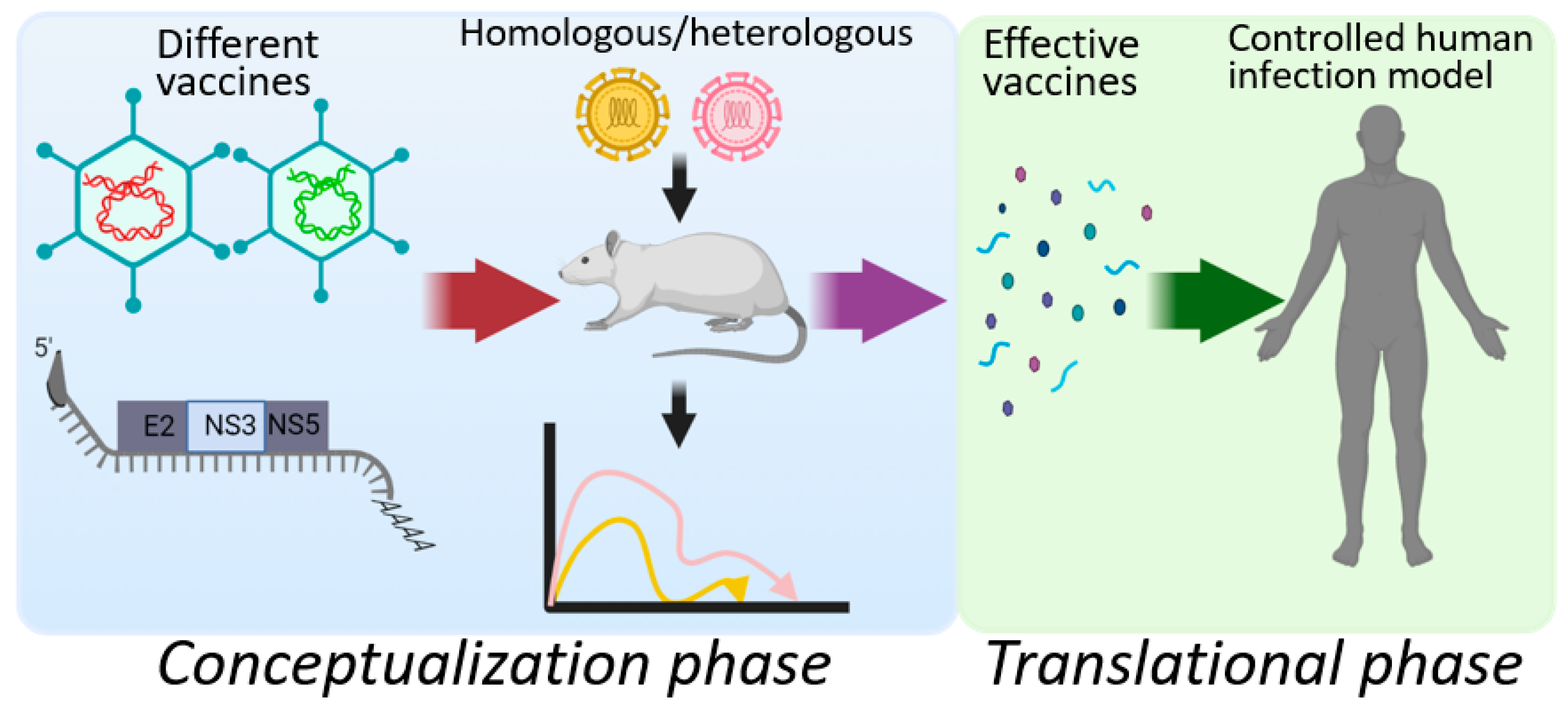
10. A Narrow Path forward for HCV Vaccine Development
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- Ly, K.N.; Hughes, E.M.; Jiles, R.B.; Holmberg, S.D. Rising Mortality Associated with Hepatitis C Virus in the United States, 2003–2013. Clin. Infect. Dis. 2016, 62, 1287–1288. [Google Scholar] [CrossRef] [PubMed]
- Zibbell, J.E.; Asher, A.K.; Patel, R.C.; Kupronis, B.; Iqbal, K.; Ward, J.W.; Holtzman, D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175–181. [Google Scholar] [CrossRef]
- Choo, Q.L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA clone derived from a bloodborne non-A, non-B viral hepatitis genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine. 2020. Available online: https://www.nobelprize.org/prizes/medicine/2020/summary/ (accessed on 18 May 2021).
- van der Meer, A.J.; Veldt, B.J.; Feld, J.J.; Wedemeyer, H.; Dufour, J.F.; Lammert, F.; Duarte-Rojo, A.; Heathcote, E.J.; Manns, M.P.; Kuske, L.; et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012, 308, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.Z.; Chong, M.; Kuo, M.; Woods, R.; Wong, J.; Yoshida, E.M.; Sherman, M.; Butt, Z.A.; Samji, H.; Cook, D.; et al. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J. Hepatol. 2017, 66, 504–513. [Google Scholar] [CrossRef]
- Walker, C.M. Designing an HCV vaccine: A unique convergence of prevention and therapy? Curr. Opin. Virol. 2017, 23, 113–119. [Google Scholar] [CrossRef]
- Bartenschlager, R.; Baumert, T.F.; Bukh, J.; Houghton, M.; Lemon, S.M.; Lindenbach, B.D.; Lohmann, V.; Moradpour, D.; Pietschmann, T.; Rice, C.M.; et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 2018, 248, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Konerman, M.A.; Lok, A.S. Hepatitis C Treatment and Barriers to Eradication. Clin. Transl. Gastroenterol. 2016, 7, e193. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Butt, Z.A.; Wong, S.; Buxton, J.; Islam, N.; Yu, A.; Darvishian, M.; Gilbert, M.; Wong, J.; Chapinal, N.; et al. Hepatitis C Virus Reinfection after Successful Treatment with Direct-Acting Antiviral Therapy in a Large Population-Based Cohort. Hepatology 2018, 68, 907a. [Google Scholar] [CrossRef]
- Callendret, B.; Eccleston, H.B.; Hall, S.; Satterfield, W.; Capone, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Walker, C.M. T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatology 2014, 60, 1531–1540. [Google Scholar] [CrossRef]
- Walker, C.M.; Grakoui, A. Hepatitis C virus: Why do we need a vaccine to prevent a curable persistent infection? Curr. Opin. Immunol. 2015, 35, 137–143. [Google Scholar] [CrossRef]
- Rehermann, B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J. Clin. Investig. 2009, 119, 1745–1754. [Google Scholar] [CrossRef]
- Holz, L.; Rehermann, B. T cell responses in hepatitis C virus infection: Historical overview and goals for future research. Antivir. Res. 2015, 114, 96–105. [Google Scholar] [CrossRef]
- Thimme, R.; Oldach, D.; Chang, K.M.; Steiger, C.; Ray, S.C.; Chisari, F.V. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 2001, 194, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.T.; Diepolder, H.M.; Jung, M.C.; Gruener, N.H.; Schraut, W.W.; Zachoval, R.; Hoffmann, R.; Schirren, C.A.; Santantonio, T.; Pape, G.R. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology 1999, 117, 933–941. [Google Scholar] [CrossRef]
- Schulze Zur Wiesch, J.; Ciuffreda, D.; Lewis-Ximenez, L.; Kasprowicz, V.; Nolan, B.E.; Streeck, H.; Aneja, J.; Reyor, L.L.; Allen, T.M.; Lohse, A.W.; et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 2012, 209, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Wolski, D.; Aneja, J.; Matsubara, L.; Robilotti, B.; Hauck, G.; de Sousa, P.S.F.; Subudhi, S.; Fernandes, C.A.; Hoogeveen, R.C.; et al. Hepatitis C virus-specific CD4+ T cell phenotype and function in different infection outcomes. J. Clin. Investig. 2020, 130, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Major, M.E.; Mihalik, K.; Puig, M.; Rehermann, B.; Nascimbeni, M.; Rice, C.M.; Feinstone, S.M. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 2002, 76, 6586–6595. [Google Scholar] [CrossRef]
- Nascimbeni, M.; Mizukoshi, E.; Bosmann, M.; Major, M.E.; Mihalik, K.; Rice, C.M.; Feinstone, S.M.; Rehermann, B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J. Virol. 2003, 77, 4781–4793. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.M.; Brotman, B.; Lee, D.H.; Pfahler, W.; Tricoche, N.; Andrus, L.; Shata, M.T. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J. Infect. Dis. 2005, 192, 1701–1709. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoukry, N.H.; Grakoui, A.; Houghton, M.; Chien, D.Y.; Ghrayeb, J.; Reimann, K.A.; Walker, C.M. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 2003, 197, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Lanford, R.E.; Guerra, B.; Chavez, D.; Bigger, C.; Brasky, K.M.; Wang, X.H.; Ray, S.C.; Thomas, D.L. Cross-genotype immunity to hepatitis C virus. J. Virol. 2004, 78, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Dahari, H.; Feinstone, S.M.; Major, M.E. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 2010, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Prins, M.; Hellard, M.; Cox, A.L.; Osburn, W.O.; Lauer, G.; Page, K.; Lloyd, A.R.; Dore, G.J.; International Collaboration of Incident, H.I.V.; et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: Towards a vaccine. Lancet. Infect. Dis. 2012, 12, 408–414. [Google Scholar] [CrossRef]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010, 138, 315–324. [Google Scholar] [CrossRef]
- Bowen, D.G.; Walker, C.M. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 2005, 436, 946–952. [Google Scholar] [CrossRef]
- Fitzmaurice, K.; Hurst, J.; Dring, M.; Rauch, A.; McLaren, P.J.; Gunthard, H.F.; Gardiner, C.; Klenerman, P.; Irish, H.C.V.R.C.; Swiss, H.I.V.C.S. Additive effects of HLA alleles and innate immune genes determine viral outcome in HCV infection. Gut 2015, 64, 813–819. [Google Scholar] [CrossRef]
- McKiernan, S.M.; Hagan, R.; Curry, M.; McDonald, G.S.; Kelly, A.; Nolan, N.; Walsh, A.; Hegarty, J.; Lawlor, E.; Kelleher, D. Distinct MHC class I and II alleles are associated with hepatitis C viral clearance, originating from a single source. Hepatology 2004, 40, 108–114. [Google Scholar] [CrossRef]
- Kuniholm, M.H.; Kovacs, A.; Gao, X.; Xue, X.; Marti, D.; Thio, C.L.; Peters, M.G.; Terrault, N.A.; Greenblatt, R.M.; Goedert, J.J.; et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology 2010, 51, 1514–1522. [Google Scholar] [CrossRef]
- Honegger, J.R.; Tedesco, D.; Kohout, J.A.; Prasad, M.R.; Price, A.A.; Lindquist, T.; Ohmer, S.; Moore-Clingenpeel, M.; Grakoui, A.; Walker, C.M. Influence of IFNL3 and HLA-DPB1 genotype on postpartum control of hepatitis C virus replication and T-cell recovery. Proc. Natl. Acad. Sci. USA 2016, 113, 10684–10689. [Google Scholar] [CrossRef] [PubMed]
- Grakoui, A.; Shoukry, N.H.; Woollard, D.J.; Han, J.H.; Hanson, H.L.; Ghrayeb, J.; Murthy, K.K.; Rice, C.M.; Walker, C.M. HCV persistence and immune evasion in the absence of memory T cell help. Science 2003, 302, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Current progress in development of hepatitis C virus vaccines. Nat. Med. 2013, 19, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Semmo, N.; Lucas, M.; Krashias, G.; Lauer, G.; Chapel, H.; Klenerman, P. Maintenance of HCV-specific T-cell responses in antibody-deficient patients a decade after early therapy. Blood 2006, 107, 4570–4571. [Google Scholar] [CrossRef][Green Version]
- Bassett, S.E.; Thomas, D.L.; Brasky, K.M.; Lanford, R.E. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. J. Virol. 1999, 73, 1118–1126. [Google Scholar] [CrossRef]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef]
- Pestka, J.M.; Zeisel, M.B.; Blaser, E.; Schurmann, P.; Bartosch, B.; Cosset, F.L.; Patel, A.H.; Meisel, H.; Baumert, J.; Viazov, S.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 2007, 104, 6025–6030. [Google Scholar] [CrossRef]
- Logvinoff, C.; Major, M.E.; Oldach, D.; Heyward, S.; Talal, A.; Balfe, P.; Feinstone, S.M.; Alter, H.; Rice, C.M.; McKeating, J.A. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 10149–10154. [Google Scholar] [CrossRef]
- Raghuraman, S.; Park, H.; Osburn, W.O.; Winkelstein, E.; Edlin, B.R.; Rehermann, B. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J. Infect. Dis. 2012, 205, 763–771. [Google Scholar] [CrossRef]
- Vanwolleghem, T.; Bukh, J.; Meuleman, P.; Desombere, I.; Meunier, J.C.; Alter, H.; Purcell, R.H.; Leroux-Roels, G. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology 2008, 47, 1846–1855. [Google Scholar] [CrossRef]
- Meuleman, P.; Bukh, J.; Verhoye, L.; Farhoudi, A.; Vanwolleghem, T.; Wang, R.Y.; Desombere, I.; Alter, H.; Purcell, R.H.; Leroux-Roels, G. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 2011, 53, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Bukh, J.; Engle, R.E.; Faulk, K.; Wang, R.Y.; Farci, P.; Alter, H.J.; Purcell, R.H. Immunoglobulin with High-Titer In Vitro Cross-Neutralizing Hepatitis C Virus Antibodies Passively Protects Chimpanzees from Homologous, but Not Heterologous, Challenge. J. Virol. 2015, 89, 9128–9132. [Google Scholar] [CrossRef]
- Krawczynski, K.; Alter, M.J.; Tankersley, D.L.; Beach, M.; Robertson, B.H.; Lambert, S.; Kuo, G.; Spelbring, J.E.; Meeks, E.; Sinha, S.; et al. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J. Infect. Dis. 1996, 173, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Morin, T.J.; Broering, T.J.; Leav, B.A.; Blair, B.M.; Rowley, K.J.; Boucher, E.N.; Wang, Y.; Cheslock, P.S.; Knauber, M.; Olsen, D.B.; et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012, 8, e1002895. [Google Scholar] [CrossRef]
- de Jong, Y.P.; Dorner, M.; Mommersteeg, M.C.; Xiao, J.W.; Balazs, A.B.; Robbins, J.B.; Winer, B.Y.; Gerges, S.; Vega, K.; Labitt, R.N.; et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci. Transl. Med. 2014, 6, 254ra129. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Wolski, D.; Foote, P.K.; Chen, D.Y.; Lewis-Ximenez, L.L.; Fauvelle, C.; Aneja, J.; Walker, A.; Tonnerre, P.; Torres-Cornejo, A.; Kvistad, D.; et al. Early Transcriptional Divergence Marks Virus-Specific Primary Human CD8(+) T Cells in Chronic versus Acute Infection. Immunity 2017, 47, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Bengsch, B.; Seigel, B.; Ruhl, M.; Timm, J.; Kuntz, M.; Blum, H.E.; Pircher, H.; Thimme, R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010, 6, e1000947. [Google Scholar] [CrossRef]
- Fuller, M.J.; Shoukry, N.H.; Gushima, T.; Bowen, D.G.; Callendret, B.; Campbell, K.J.; Hasselschwert, D.L.; Hughes, A.L.; Walker, C.M. Selection-driven immune escape is not a significant factor in the failure of CD4 T cell responses in persistent hepatitis C virus infection. Hepatology 2010, 51, 378–387. [Google Scholar] [CrossRef]
- Wang, H.; Eckels, D.D. Mutations in immunodominant T cell epitopes derived from the nonstructural 3 protein of hepatitis C virus have the potential for generating escape variants that may have important consequences for T cell recognition. J. Immunol. 1999, 162, 4177–4183. [Google Scholar] [PubMed]
- Fleming, V.M.; Harcourt, G.; Barnes, E.; Klenerman, P. Virological footprint of CD4+ T-cell responses during chronic hepatitis C virus infection. J. Gen. Virol. 2010, 91, 1396–1406. [Google Scholar] [CrossRef]
- Lucas, M.; Deshpande, P.; James, I.; Rauch, A.; Pfafferott, K.; Gaylard, E.; Merani, S.; Plauzolles, A.; Lucas, A.; McDonnell, W.; et al. Evidence of CD4+ T cell-mediated immune pressure on the Hepatitis C virus genome. Sci. Rep. 2018, 8, 7224. [Google Scholar] [CrossRef] [PubMed]
- Coss, S.L.; Torres-Cornejo, A.; Prasad, M.R.; Moore-Clingenpeel, M.; Grakoui, A.; Lauer, G.M.; Walker, C.M.; Honegger, J.R. CD4+ T cell restoration and control of hepatitis C virus replication after childbirth. J. Clin. Investig. 2020, 130, 748–753. [Google Scholar] [CrossRef]
- Smits, M.; Zoldan, K.; Ishaque, N.; Gu, Z.; Jechow, K.; Wieland, D.; Conrad, C.; Eils, R.; Fauvelle, C.; Baumert, T.F.; et al. Follicular T helper cells shape the HCV-specific CD4+ T cell repertoire after virus elimination. J. Clin. Investig. 2020, 130, 998–1009. [Google Scholar] [CrossRef]
- Timpe, J.M.; Stamataki, Z.; Jennings, A.; Hu, K.; Farquhar, M.J.; Harris, H.J.; Schwarz, A.; Desombere, I.; Roels, G.L.; Balfe, P.; et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 2008, 47, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Helle, F.; Goffard, A.; Morel, V.; Duverlie, G.; McKeating, J.; Keck, Z.Y.; Foung, S.; Penin, F.; Dubuisson, J.; Voisset, C. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J. Virol. 2007, 81, 8101–8111. [Google Scholar] [CrossRef]
- Dowd, K.A.; Netski, D.M.; Wang, X.H.; Cox, A.L.; Ray, S.C. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 2009, 136, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.J.; Geysen, H.M.; Christopherson, C.; Hall, J.E.; Mason, T.J.; Saracco, G.; Bonino, F.; Crawford, K.; Marion, C.D.; Crawford, K.A.; et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: Potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 1992, 89, 3468–3472. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, L.; Struble, E.B.; Watanabe, H.; Kachko, A.; Mihalik, K.; Virata-Theimer, M.L.; Alter, H.J.; Feinstone, S.; Major, M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc. Natl. Acad. Sci. USA 2009, 106, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef]
- Bailey, J.R.; Flyak, A.I.; Cohen, V.J.; Li, H.; Wasilewski, L.N.; Snider, A.E.; Wang, S.; Learn, G.H.; Kose, N.; Loerinc, L.; et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017, 2, e92872. [Google Scholar] [CrossRef]
- Hartlage, A.S.; Cullen, J.M.; Kapoor, A. The Strange, Expanding World of Animal Hepaciviruses. Annu. Rev. Virol. 2016, 3, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Swadling, L.; Brown, A.; Capone, S.; Folgori, A.; Salio, M.; Klenerman, P.; Barnes, E. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur. J. Immunol. 2015, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Folgori, A.; Capone, S.; Ruggeri, L.; Meola, A.; Sporeno, E.; Ercole, B.B.; Pezzanera, M.; Tafi, R.; Arcuri, M.; Fattori, E.; et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 2006, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef]
- Fytili, P.; Dalekos, G.N.; Schlaphoff, V.; Suneetha, P.V.; Sarrazin, C.; Zauner, W.; Zachou, K.; Berg, T.; Manns, M.P.; Klade, C.S.; et al. Cross-genotype-reactivity of the immunodominant HCV CD8 T-cell epitope NS3–1073. Vaccine 2008, 26, 3818–3826. [Google Scholar] [CrossRef]
- von Delft, A.; Donnison, T.A.; Lourenco, J.; Hutchings, C.; Mullarkey, C.E.; Brown, A.; Pybus, O.G.; Klenerman, P.; Chinnakannan, S.; Barnes, E. The generation of a simian adenoviral vectored HCV vaccine encoding genetically conserved gene segments to target multiple HCV genotypes. Vaccine 2018, 36, 313–321. [Google Scholar] [CrossRef]
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef]
- Kew, O.; Pallansch, M. Breaking the Last Chains of Poliovirus Transmission: Progress and Challenges in Global Polio Eradication. Annu. Rev. Virol. 2018, 5, 427–451. [Google Scholar] [CrossRef]
- Bailey, J.R.; Barnes, E.; Cox, A.L. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology 2019, 156, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.L.; Kuo, G.; Ralston, R.; Weiner, A.; Chien, D.; Van Nest, G.; Han, J.; Berger, K.; Thudium, K.; Kuo, C.; et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Coates S, K.G.; Crawford, K.; Dong, C.; Wininger, M.; Houghton, M. Protection of chimpanzees against heterologous 1a viral challenge using a gpE1/gpE2 heterodimer vaccine. In Proceedings of the 11th International Symposium on Viral Hepatitis and Liver Disease; Wiley: Hoboken, NJ, USA, 2003; pp. 118–123. [Google Scholar]
- Leroux-Roels, G.; Depla, E.; Hulstaert, F.; Tobback, L.; Dincq, S.; Desmet, J.; Desombere, I.; Maertens, G. A candidate vaccine based on the hepatitis C E1 protein: Tolerability and immunogenicity in healthy volunteers. Vaccine 2004, 22, 3080–3086. [Google Scholar] [CrossRef]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef] [PubMed]
- Law, J.L.; Chen, C.; Wong, J.; Hockman, D.; Santer, D.M.; Frey, S.E.; Belshe, R.B.; Wakita, T.; Bukh, J.; Jones, C.T.; et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS ONE 2013, 8, e59776. [Google Scholar] [CrossRef]
- Drane, D.; Maraskovsky, E.; Gibson, R.; Mitchell, S.; Barnden, M.; Moskwa, A.; Shaw, D.; Gervase, B.; Coates, S.; Houghton, M.; et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: A phase I study in healthy volunteers. Hum. Vaccin. 2009, 5, 151–157. [Google Scholar] [CrossRef]
- Verstrepen, B.E.; Depla, E.; Rollier, C.S.; Mares, G.; Drexhage, J.A.; Priem, S.; Verschoor, E.J.; Koopman, G.; Granier, C.; Dreux, M.; et al. Clearance of genotype 1b hepatitis C virus in chimpanzees in the presence of vaccine-induced E1-neutralizing antibodies. J. Infect. Dis. 2011, 204, 837–844. [Google Scholar] [CrossRef]
- Puig, M.; Major, M.E.; Mihalik, K.; Feinstone, S.M. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine 2004, 22, 991–1000. [Google Scholar] [CrossRef]
- Esumi, M.; Rikihisa, T.; Nishimura, S.; Goto, J.; Mizuno, K.; Zhou, Y.H.; Shikata, T. Experimental vaccine activities of recombinant E1 and E2 glycoproteins and hypervariable region 1 peptides of hepatitis C virus in chimpanzees. Arch. Virol. 1999, 144, 973–980. [Google Scholar] [CrossRef]
- Forns, X.; Payette, P.J.; Ma, X.; Satterfield, W.; Eder, G.; Mushahwar, I.K.; Govindarajan, S.; Davis, H.L.; Emerson, S.U.; Purcell, R.H.; et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology 2000, 32, 618–625. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra111. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Cicconi, P.; D’Alise, A.M.; Brown, A.; Esposito, M.; Swadling, L.; Holst, P.J.; Bassi, M.R.; Stornaiuolo, M.; Mori, F.; et al. MHC class II invariant chain-adjuvanted viral vectored vaccines enhances T cell responses in humans. Sci. Transl. Med. 2020, 12, eaaz7715. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.W.; Hu, Y.W.; Tricoche, N.; Pfahler, W.; Shata, M.T.; Dreux, M.; Cosset, F.L.; Folgori, A.; Lee, D.H.; Brotman, B.; et al. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J. Virol. 2008, 82, 10896–10905. [Google Scholar] [CrossRef]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef]
- Rollier, C.S.; Paranhos-Baccala, G.; Verschoor, E.J.; Verstrepen, B.E.; Drexhage, J.A.; Fagrouch, Z.; Berland, J.L.; Komurian-Pradel, F.; Duverger, B.; Himoudi, N.; et al. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology 2007, 45, 602–613. [Google Scholar] [CrossRef]
- Puig, M.; Mihalik, K.; Tilton, J.C.; Williams, O.; Merchlinsky, M.; Connors, M.; Feinstone, S.M.; Major, M.E. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology 2006, 44, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.W.; Park, S.H.; Lavillette, D.; Cosset, F.L.; Yang, S.H.; Lee, C.G.; Jin, H.T.; Kim, C.M.; Shata, M.T.; Lee, D.H.; et al. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology 2005, 42, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Zubkova, I.; Choi, Y.H.; Chang, E.; Pirollo, K.; Uren, T.; Watanabe, H.; Wells, F.; Kachko, A.; Krawczynski, K.; Major, M.E. T-cell vaccines that elicit effective immune responses against HCV in chimpanzees may create greater immune pressure for viral mutation. Vaccine 2009, 27, 2594–2602. [Google Scholar] [CrossRef]
- Rollier, C.; Depla, E.; Drexhage, J.A.; Verschoor, E.J.; Verstrepen, B.E.; Fatmi, A.; Brinster, C.; Fournillier, A.; Whelan, J.A.; Whelan, M.; et al. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J. Virol. 2004, 78, 187–196. [Google Scholar] [CrossRef]
- Elmowalid, G.A.; Qiao, M.; Jeong, S.H.; Borg, B.B.; Baumert, T.F.; Sapp, R.K.; Hu, Z.; Murthy, K.; Liang, T.J. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc. Natl. Acad. Sci. USA 2007, 104, 8427–8432. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol. Rev. 2011, 239, 99–108. [Google Scholar] [CrossRef]
- Meunier, J.C.; Gottwein, J.M.; Houghton, M.; Russell, R.S.; Emerson, S.U.; Bukh, J.; Purcell, R.H. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis. 2011, 204, 1186–1190. [Google Scholar] [CrossRef]
- Logan, M.; Law, J.; Wong, J.A.J.; Hockman, D.; Landi, A.; Chen, C.; Crawford, K.; Kundu, J.; Baldwin, L.; Johnson, J.; et al. Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG. J. Virol. 2016, 91, e01552-16. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Shin, E.C.; Capone, S.; Caggiari, L.; De Re, V.; Nicosia, A.; Folgori, A.; Rehermann, B. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology 2012, 143, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Burm, R.; Collignon, L.; Mesalam, A.A.; Meuleman, P. Animal Models to Study Hepatitis C Virus Infection. Front. Immunol. 2018, 9, 1032. [Google Scholar] [CrossRef]
- Bukh, J. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 2012, 142, 1279–1287. [Google Scholar] [CrossRef]
- Scheel, T.K.; Kapoor, A.; Nishiuchi, E.; Brock, K.V.; Yu, Y.; Andrus, L.; Gu, M.; Renshaw, R.W.; Dubovi, E.J.; McDonough, S.P.; et al. Characterization of nonprimate Hepacivirus and construction of a functional molecular clone. Proc. Natl. Acad. Sci. USA 2015, 112, 2192–2197. [Google Scholar] [CrossRef]
- Pfaender, S.; Cavalleri, J.M.; Walter, S.; Doerrbecker, J.; Campana, B.; Brown, R.J.; Burbelo, P.D.; Postel, A.; Hahn, K.; Kusuma, A.; et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like non-primate Hepaciviruses. Hepatology 2015, 61, 447–459. [Google Scholar] [CrossRef]
- Pfaender, S.; Walter, S.; Grabski, E.; Todt, D.; Bruening, J.; Romero-Brey, I.; Gather, T.; Brown, R.J.; Hahn, K.; Puff, C.; et al. Immune protection against reinfection with nonprimate Hepacivirus. Proc. Natl. Acad. Sci. USA 2017, 114, E2430–E2439. [Google Scholar] [CrossRef]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Wolfisberg, R.; Fahnoe, U.; Xiao, J.W.; Quirk, C.; Luna, J.M.; Cullen, J.M.; Hartlage, A.S.; Chiriboga, L.; Ghoshal, K.; et al. Mouse models of acute and chronic Hepacivirus infection. Science 2017, 357, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Murthy, S.; Sharma, H.; Hartlage, A.S.; Kumar, A.; Gadi, S.; Simmonds, P.; Chauhan, L.V.; Scheel, T.K.H.; Billerbeck, E.; et al. Viral persistence, liver disease and host response in Hepatitis C-like virus rat model. Hepatology 2018, 68, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, A.S.; Walker, C.M.; Kapoor, A. Priming of Antiviral CD8 T Cells without Effector Function by a Persistently Replicating Hepatitis C-Like Virus. J. Virol. 2020, 94, e00035-20. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, A.S.; Murthy, S.; Kumar, A.; Trivedi, S.; Dravid, P.; Sharma, H.; Walker, C.M.; Kapoor, A. Vaccination to prevent T cell subversion can protect against persistent Hepacivirus infection. Nat. Commun. 2019, 10, 1113. [Google Scholar] [CrossRef]
- Atcheson, E.; Li, W.; Bliss, C.M.; Chinnakannan, S.; Heim, K.; Sharpe, H.; Hutchings, C.; Dietrich, I.; Nguyen, D.; Kapoor, A.; et al. Use of an Outbred Rat Hepacivirus Challenge Model for Design and Evaluation of Efficacy of Different Immunization Strategies for Hepatitis C Virus. Hepatology 2020, 71, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Wolfisberg, R.; Holmbeck, K.; Nielsen, L.; Kapoor, A.; Rice, C.M.; Bukh, J.; Scheel, T.K.H. Replicons of a Rodent Hepatitis C Model Virus Permit Selection of Highly Permissive Cells. J. Virol. 2019, 93, e00733-19. [Google Scholar] [CrossRef]
| Vaccine Type | Antigen | Delivery/Adjuvant | Species | Immunogenicity | Challenge | Clinical Trial | Outcome | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Recombinant protein | GT1a E1E2 | MF59 or MF75 | Chimpanzees (n = 21) | Anti-E1E2 antibodies | Homologous | - | 5 aviremic, 14 resolved, 2 chronic | Protected against acute and chronic infection. | [75,76] |
| GT1b E1 | Alum | Humans (n = 20) | Anti-E1 antibodies, CD4 T cells | - | Phase I | - | - | [77] | |
| GT1a E1E2 | MF59 | Humans (n = 60) | Neutralizing antibodies, CD4 T cells | - | Phase I | - | - | [78,79] | |
| GT1a core | ISCOMATRIX | Humans (n = 30) | CD4 and CD8 T cells | - | Phase I | - | - | [80] | |
| GT1b E1 or E2△HVR-1 | Alum | Chimpanzees (n = 4) | CD4 T cells; neutralizing antibodies in E1 recipients | Heterologous GT1b | - | 2 resolved, 2 chronic | E1 but not E2 vaccine recipients protected against chronic infection. | [81] | |
| GT1a E1E2 | RIBIs | Chimpanzees (n = 1) | Anti-E1E2 antibodies, T cells | Homologous | - | 1 chronic | Delayed infection by 3 weeks but did not prevent persistence | [82] | |
| Recombinant protein and peptide | GT2 E1E2 and HVR-1 peptides | Complete and incomplete Freund’s | Chimpanzees (n = 1) | Anti-E1E2 and HVR-1 antibodies | Homologous | 1 resolved | Protected against chronic infection. | [83] | |
| DNA | GT1a E2 | None | Chimpanzees (n = 2) | Anti-E2 antibodies, T cells | Homologous | - | 2 resolved | Protected against chronic infection. | [84] |
| Viral vector | GT1b NS3-NS5B | Ad6/ChAd3 | Humans (n = 41) | Heterotypic CD4 and CD8 T cells | - | Phase I | - | - | [85] |
| GT1b NS3-NS5B | Chad3/MVA | Humans (n = 55) | Heterotypic CD4 and CD8 T cells | - | Phase I | - | - | [69] | |
| GT1b NS3-NS5B fused to MHC class II-associated invariant chain | Chad3/MVA | Humans (n = 17) | Heterotypic CD4 and CD8 T cells | Phase I | - | - | [86] | ||
| GT1b core, E1, E2, p7, NS2, NS3 | Vaccinia | Chimpanzees (n = 4) | T cells | Homologous | - | 4 resolved | Protected against chronic infection. | [87] | |
| Gt1b NS3-NS5B | Chad3/MVA | Humans (n = 548) | T cells | - | Phase I/II | - | Reduced peak viremia but did not protect against chronic infection upon natural exposure | [88] | |
| DNA and viral vector | GT1b Core-E1E2, NS3 | DNA/MVA | Chimpanzees (n = 4) | CD4 and CD8 T cells | Homologous | - | 1 resolved, 3 chronic | Reduced peak viremia but did not protect against chronic infection. | [89] |
| GT1b NS3-NS5B | Ad6/Ad24 + electroporated DNA | Chimpanzees (n = 5) | CD4 and CD8 T cells | Heterologous GT1a | - | 4 resolved, 1 chronic | Protected against chronic infection. | [68] | |
| GT1a NS3, NS5A, NS5B | DNA/vaccinia-expressing B7.1, ICAM-1, LFA-3; CpGs | Chimpanzees (n = 1) | CD4 and CD8 T cells | Homologous | - | 1 chronic | Early suppression of viremia but late resurgence and eventual persistence | [90] | |
| GT1b Core-E1E2, NS3-NS5B | DNA/Ad5; hIL-12 expressing plasmid | Chimpanzees (n = 6) | Neutralizing antibodies, T cells | Homologous | 1 aviremic, 1 resolved, 4 chronic | Sterilizing immunity associated with high anti-E2 neutralizing antibody response | [91] | ||
| GT1a NS3-NS5B | DNA/Ad5; hIL-12 expressing plasmid | Chimpanzees (n = 2) | T cells | Homologous | - | 1 resolved, 1 chronic | Early suppression of viremia | [92] | |
| DNA and recombinant protein | GT1a Core, NS3; GT1b Core, E1E2, NS3 | Alum | Chimpanzees (n = 2) | Anti-E1E2 antibodies, T cells | Homologous | - | 1 resolved, 1 chronic | Reduced peak viremia. | [93] |
| Virus-like particles | GT1b Core-E1E2 | AS01B (n = 2) | Chimpanzees (n = 4) | CD4 and CD8 T cells | Homologous | - | 4 resolved | Protected against chronic infection. | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartlage, A.S.; Kapoor, A. Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses 2021, 13, 1596. https://doi.org/10.3390/v13081596
Hartlage AS, Kapoor A. Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses. 2021; 13(8):1596. https://doi.org/10.3390/v13081596
Chicago/Turabian StyleHartlage, Alex S., and Amit Kapoor. 2021. "Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up" Viruses 13, no. 8: 1596. https://doi.org/10.3390/v13081596
APA StyleHartlage, A. S., & Kapoor, A. (2021). Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses, 13(8), 1596. https://doi.org/10.3390/v13081596






