Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cases and Controls
2.3. HPV Testing
2.4. IgG-Specific Anti-HPV16 L1 VLP-Based Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Serum Samples
2.6. Statistical Analysis
3. Results
3.1. Comparison between Cases and Controls
3.2. Subgroup Analysis: A Longitudinal Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2010, 128, 927–935. [Google Scholar] [CrossRef]
- Serrano, B.; de Sanjosé, S.; Tous, S.; Quiros, B.; Muñoz, N.; Bosch, X.; Alemany, L. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer 2015, 51, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Chen, Z.; Rodríguez, A.C.; Hildesheim, A.; Porras, C.; Herrero, R.; Wacholder, S.; Panagiotou, O.; Befano, B.; Burk, R.D.; et al. Cross-protection of the Bivalent Human Papillomavirus (HPV) Vaccine Against Variants of Genetically Related High-Risk HPV Infections. J. Infect. Dis. 2016, 213, 939–947. [Google Scholar] [CrossRef]
- Kuhs, K.A.L.; Gonzalez, P.; Rodriguez, A.C.; Van Doorn, L.-J.; Schiffman, M.; Struijk, L.; Chen, S.; Quint, W.; Lowy, D.R.; Porras, C.; et al. Reduced Prevalence of Vulvar HPV16/18 Infection among Women Who Received the HPV16/18 Bivalent Vaccine: A Nested Analysis Within the Costa Rica Vaccine Trial. J. Infect. Dis. 2014, 210, 1890–1899. [Google Scholar] [CrossRef]
- Apter, D.; Wheeler, C.M.; Paavonen, J.; Castellsagué, X.; Garland, S.M.; Skinner, S.R.; Naud, P.; Salmerón, J.; Chow, S.-N.; Kitchener, H.C.; et al. Efficacy of Human Papillomavirus 16 and 18 (HPV-16/18) AS04-Adjuvanted Vaccine against Cervical Infection and Precancer in Young Women: Final Event-Driven Analysis of the Randomized, Double-Blind PATRICIA Trial. Clin. Vaccine Immunol. 2015, 22, 361–373. [Google Scholar] [CrossRef]
- Pils, S.; Joura, E. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef]
- Cameron, R.L.; Kavanagh, K.; Watt, D.C.; Robertson, C.; Cuschieri, K.; Ahmed, S.; Pollock, K.G.J. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: Reducing the gap. J. Epidemiol. Community Health 2017, 71, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Machalek, D.A.; Garland, S.M.; Brotherton, J.M.L.; Bateson, D.; McNamee, K.; Stewart, M.; Skinner, R.; Liu, B.; Cornall, A.M.; Kaldor, J.M.; et al. Very Low Prevalence of Vaccine Human Papillomavirus Types Among 18- to 35-Year Old Australian Women 9 Years Following Implementation of Vaccination. J. Infect. Dis. 2018, 217, 1590–1600. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Lehtinen, M.; Lagheden, C.; Luostarinen, T.; Eriksson, T.; Apter, D.; Harjula, K.; Kuortti, M.; Natunen, K.; Palmroth, J.; Petäjä, T.; et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point—Registry-based follow-up of three cohorts from randomized trials. BMJ Open 2017, 7, e015867. [Google Scholar] [CrossRef] [PubMed]
- Ault, K.A. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: A combined analysis of four randomised clinical trials. Lancet 2007, 369, 1861–1868. [Google Scholar] [CrossRef]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent Vaccine against Human Papillomavirus to Prevent Anogenital Diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef]
- Muñoz, N.; Kjaer, S.; Sigurdsson, K.; Iversen, O.-E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; et al. Impact of Human Papillomavirus (HPV)-6/11/16/18 Vaccine on All HPV-Associated Genital Diseases in Young Women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.; Penny, M.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of Quadrivalent HPV Vaccine against HPV Infection and Disease in Males. N. Engl. J. Med. 2011, 364, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev. Vaccines 2018, 17, 1085–1091. [Google Scholar] [CrossRef]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; De Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- Levin, M.J.; Moscicki, A.-B.; Song, L.-Y.; Fenton, T.; Meyer, W.A.; Read, J.S.; Handelsman, E.L.; Nowak, B.; Sattler, C.A.; Saah, A.; et al. Safety and Immunogenicity of a Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Vaccine in HIV-Infected Children 7 to 12 Years Old. JAIDS J. Acquir. Immune Defic. Syndr. 2010, 55, 197–204. [Google Scholar] [CrossRef]
- Kahn, J.A.; Xu, J.; Kapogiannis, B.G.; Rudy, B.; Gonin, R.; Liu, N.; Wilson, C.M.; Worrell, C.; Squires, K.E. Immunogenicity and Safety of the Human Papillomavirus 6, 11, 16, 18 Vaccine in HIV-Infected Young Women. Clin. Infect. Dis. 2013, 57, 735–744. [Google Scholar] [CrossRef][Green Version]
- Kojic, E.M.; Kang, M.; Cespedes, M.S.; Umbleja, T.; Godfrey, C.; Allen, R.T.; Firnhaber, C.; Grinsztejn, B.; Palefsky, J.M.; Webster-Cyriaque, J.Y.; et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1–Infected Women. Clin. Infect. Dis. 2014, 59, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Huang, S.; Moscicki, A.-B.; Song, L.-Y.; Read, J.S.; Meyer, W.A.; Saah, A.J.; Richardson, K.; Weinberg, A. Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: Effect of a fourth dose of vaccine. Vaccine 2017, 35, 1712–1720. [Google Scholar] [CrossRef]
- Mugo, N.R.; Eckert, L.; Magaret, A.S.; Cheng, A.; Mwaniki, L.; Ngure, K.; Celum, C.; Baeten, J.M.; Galloway, D.A.; Wamalwa, D.; et al. Quadrivalent HPV vaccine in HIV-1-infected early adolescent girls and boys in Kenya: Month 7 and 12 post vaccine immunogenicity and correlation with immune status. Vaccine 2018, 36, 7025–7032. [Google Scholar] [CrossRef] [PubMed]
- Mugo, N.; Eckert, L.O.; Odero, L.; Gakuo, S.; Ngure, K.; Celum, C.; Baeten, J.M.; Barnabas, R.V.; Wald, A. Antibody responses to prophylactic quadrivalent human papillomavirus vaccine at 48 months among HIV-infected girls and boys ages 9–14 in Kenya, Africa. Vaccine 2021, 39, 4751–4758. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Hubbert, N.L.; Wheeler, C.M.; Becker, T.M.; Lowy, D.R.; Schiller, J.T. A Virus-Like Particle Enzyme-Linked Immunosorbent Assay Detects Serum Antibodies in a Majority of Women Infected with Human Papillomavirus Type 16. J. Natl. Cancer Inst. 1994, 86, 494–499. [Google Scholar] [CrossRef]
- Dillner, J.; Wiklund, F.; Lenner, P.; Eklund, C.; Fredriksson-Shanazarian, V.; Schiller, J.T.; Hibma, M.; Hallmans, G.; Stendahl, U. Antibodies against linear and conformational epitopes of human papillomavirus type 16 that independently associate with incident cervical cancer. Int. J. Cancer 2006, 60, 377–382. [Google Scholar] [CrossRef]
- Harro, C.D.; Pang, Y.-Y.S.; Roden, R.B.S.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and Immunogenicity Trial in Adult Volunteers of a Human Papillomavirus 16 L1 Virus-Like Particle Vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef]
- Frazer, I.H. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 2004, 4, 46–55. [Google Scholar] [CrossRef]
- Carter, J.J.; Koutsky, L.A.; Wipf, G.C.; Christensen, N.D.; Lee, S.-K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. The Natural History of Human Papillomavirus Type 16 Capsid Antibodies among a Cohort of University Women. J. Infect. Dis. 1996, 174, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Dillner, J.; Lehtinen, M.; Björge, T.; Luostarinen, T.; Youngman, L.; Jellum, E.; Koskela, P.; Gislefoss, R.E.; Hallmans, G.; Paavonen, J.; et al. Prospective seroepidemiologic study of human papillomavirus infection as a risk factor for invasive cervical cancer. J. Natl. Cancer Inst. 1997, 89, 1293–1299. [Google Scholar] [CrossRef]
- Ho, G.Y.F.; Studentsov, Y.; Hall, C.; Bierman, R.; Beardsley, L.; Lempa, M.; Burk, R.D. Risk Factors for Subsequent Cervicovaginal Human Papillomavirus (HPV) Infection and the Protective Role of Antibodies to HPV-16 Virus-Like Particles. J. Infect. Dis. 2002, 186, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.F.; Studentsov, Y.Y.; Bierman, R.; Burk, R.D. Natural History of Human Papillomavirus Type 16 Virus-Like Particle Antibodies in Young Women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Joura, E.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of neutralizing antibody immune responses to non-vaccine targeted high-risk HPV types induced by the bivalent and the quadrivalent vaccines. Vaccine 2021, 39, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, N.F.; Diaz, A.; Nucci-Sack, A.; Shyhalla, K.; Shankar, V.; Guillot, M.; Holloman, D.; Strickler, H.D.; Burk, R.D. Incidence and Types of Human Papillomavirus Infections in Adolescent Females Immunized with the Quadrivalent Human Papillomavirus Vaccine. JAMA Netw. Open 2021, 4, e2121893. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Burk, R.D.; Nucci-Sack, A.; Shankar, V.; Peake, K.; Lorde-Rollins, E.; Porter, R.; Linares, L.O.; Rojas, M.; Strickler, H.D.; et al. Cervical, Anal and Oral HPV in an Adolescent Inner-City Health Clinic Providing Free Vaccinations. PLoS ONE 2012, 7, e37419. [Google Scholar] [CrossRef] [PubMed]
- Braun-Courville, D.K.; Schlecht, N.F.; Burk, R.D.; Strickler, H.D.; Rojas, M.; Lorde-Rollins, E.; Nucci-Sack, A.; Hollman, D.; Linares, L.O.; Diaz, A. Strategies for Conducting Adolescent Health Research in the Clinical Setting: The Mount Sinai Adolescent Health Center HPV Experience. J. Pediatr. Adolesc. Gynecol. 2014, 27, e103–e108. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Diaz, A.; Shankar, V.; Szporn, A.H.; Wu, M.; Nucci-Sack, A.; Peake, K.; Strickler, H.D.; Burk, R.D. Risk of Delayed Human Papillomavirus Vaccination in Inner-City Adolescent Women. J. Infect. Dis. 2016, 214, 1952–1960. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Taub, J.; Greenstone, H.; Roden, R.; Dürst, M.; Gissmann, L.; Lowy, D.R.; Schiller, J.T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993, 67, 6929–6936. [Google Scholar] [CrossRef]
- Studentsov, Y.Y.; Schiffman, M.; Strickler, H.D.; Ho, G.Y.F.; Pang, Y.-Y.S.; Schiller, J.; Herrero, R.; Burk, R.D. Enhanced Enzyme-Linked Immunosorbent Assay for Detection of Antibodies to Virus-Like Particles of Human Papillomavirus. J. Clin. Microbiol. 2002, 40, 1755–1760. [Google Scholar] [CrossRef]
- Walker, J.M. The Bicinchoninic Acid (BCA) Assay for Protein Quantitation. Basic Protein Peptide Protoc. 2003, 32, 5–8. [Google Scholar] [CrossRef]
- Shields, T.S.; Brinton, L.A.; Burk, R.D.; Wang, S.S.; Weinstein, S.J.; Ziegler, R.G.; Studentsov, Y.Y.; McAdams, M.; Schiffman, M. A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1574–1582. [Google Scholar]
- Studentsov, Y.Y.; Ho, G.Y.F.; Marks, M.A.; Bierman, R.; Burk, R.D. Polymer-Based Enzyme-Linked Immunosorbent Assay Using Human Papillomavirus Type 16 (HPV16) Virus-Like Particles Detects HPV16 Clade-Specific Serologic Responses. J. Clin. Microbiol. 2003, 41, 2827–2834. [Google Scholar] [CrossRef]
- Sasagawa, T.; Yamazaki, H.; Dong, Y.Z.; Satake, S.; Tateno, M.; Inoue, M. Immunoglobulin-A and -G responses against virus-like particles (VLP) of human papillomavirus type 16 in women with cervical cancer and cervical intra-epithelial lesions. Int. J. Cancer 1998, 75, 529–535. [Google Scholar] [CrossRef]
- Bogaards, J.A.; Van Der Weele, P.; Woestenberg, P.J.; Van Benthem, B.H.B.; King, A.J. Bivalent Human Papillomavirus (HPV) Vaccine Effectiveness Correlates with Phylogenetic Distance From HPV Vaccine Types 16 and 18. J. Infect. Dis. 2019, 220, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Fappani, C.; Bianchi, S.; Panatto, D.; Petrelli, F.; Colzani, D.; Scuri, S.; Gori, M.; Amendola, A.; Grappasonni, I.; Tanzi, E.; et al. HPV Type-Specific Prevalence a Decade after the Implementation of the Vaccination Program: Results from a Pilot Study. Vaccines 2021, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.L.; Perez, G.; Kjaer, S.K.; Paavonen, J.; Lehtinen, M.; Munoz, N.; Sigurdsson, K.; Hernandez-Avila, M.; Skjeldestad, F.E.; Thoresen, S.; et al. Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.; Kitchener, H.C.; Castellsagué, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Lehtinen, M.; Dillner, J. Clinical trials of human papillomavirus vaccines and beyond. Nat. Rev. Clin. Oncol. 2013, 10, 400–410. [Google Scholar] [CrossRef]
- Mesher, D.; Panwar, K.; Thomas, S.L.; Edmundson, C.; Choi, Y.H.; Beddows, S.; Soldan, K. The Impact of the National HPV Vaccination Program in England Using the Bivalent HPV Vaccine: Surveillance of Type-Specific HPV in Young Females, 2010–2016. J. Infect. Dis. 2018, 218, 911–921. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Boily, M.-C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.L.; Cummings, T.; et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef]
- Muñoz, N.; Manalastas, R.; Pitisuttithum, P.; Tresukosol, D.; Monsonego, J.; Ault, K.; Clavel, C.; Luna, J.; Myers, E.; Hood, S.; et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: A randomised, double-blind trial. Lancet 2009, 373, 1949–1957. [Google Scholar] [CrossRef]
| Variables | Cases (n = 43) | Random Controls (n = 43) | High-Risk Controls (n = 43) | p-Value * |
|---|---|---|---|---|
| Age at Baseline Mean (years ± SD) | 18.13 ± 1.33 | 17.85 ± 1.23 | 17.93 ± 1.19 | 0.5702 |
| Age at Coitarche Mean (years ± SD) | 14.84 ± 1.15 | 14.74 ± 1.38 | 14.49 ± 1.52 | 0.4701 |
| Age at First 4vHPV Dose Mean (years ± SD) | 15.09 ± 2.49 | 15.19 ± 2.12 | 15.19 ± 2.25 | 0.9717 |
| Racen(%) | ||||
| Hispanic | 26 (60.47) | 23 (53.49) | 24 (55.81) | 0.1156 & |
| African American | 17 (39.53) | 15 (34.88) | 18 (41.86) | |
| Other | 0 (0) | 5 (11.63) | 1 (2.33) | |
| Lifetime Number of Partners n(%) | ||||
| 1 | 2 (4.65) | 7 (16.28) | 0 (0) | 0.0461 & |
| 2 | 3 (6.98) | 3 (6.98) | 1 (2.33) | |
| 3 | 11 (25.58) | 12 (27.91) | 9 (20.93) | |
| 4+ | 27 (62.79) | 21 (48.84) | 33 (76.74) | |
| Number of Past Partners (6 months) n(%) | ||||
| 0 | 2 (4.65) | 3 (6.98) | 0 (0) | 0.0405 & |
| 1 | 21 (48.84) | 29 (67.44) | 19 (44.19) | |
| 2+ | 20 (46.51) | 11 (25.58) | 24 (55.81) | |
| Chlamydia Trachomatis n(%) | ||||
| Yes | 22 (51.16) | 15 (34.88) | 35 (81.40) | <0.0001 & |
| Any Pregnancyn(%) | ||||
| Yes | 18 (41.86) | 15 (34.88) | 21 (48.84) | 0.4260 & |
| Emergency Contraception Ever (n(%) | ||||
| Yes | 32 (74.42) | 25 (58.14) | 38 (88.37) | 0.0065 & |
| Condom Usen(%) | ||||
| Never | 17 (39.53) | 15 (34.88) | 30 (69.77) | 0.0022 & |
| Anal Sex Evern(%) | ||||
| Yes | 17 (39.53) | 12 (27.91) | 41 (95.35) | <0.0001 & |
| Lifetime Number of Anal Sex Partners n(%) | ||||
| 0 | 26 (60.47) | 31 (72.09) | 2 (4.65) | <0.0001 & |
| 1 | 8 (18.60) | 4 (9.30) | 20 (46.51) | |
| 2+ | 9 (20.93) | 8 (18.60) | 21 (48.84) | |
| Risk Score Mean ± SD | 7.95 ± 2.53 | 6.65 ± 2.64 | 10.58 ± 0.59 | <0.0001 |
| Vaccine Before Coitarche n(%) | ||||
| Yes | 19 (44.19) | 17 (39.53) | 13 (30.23) | 0.4008 & |
| Follow-up Time (years) Median (Min, Max) | 2.17 (0.50, 7.46) | 5.88 (0.54, 8.90) | 6.15 (0.84, 10.00) | <0.0001 # |
| Serum Titer Median (Min, Max) | 3872.86 (100.00–68,527.80) | 4893.76 (248.90–102,450.03) | 7859.28 (588.18–51,658.15) | 0.0557 # |
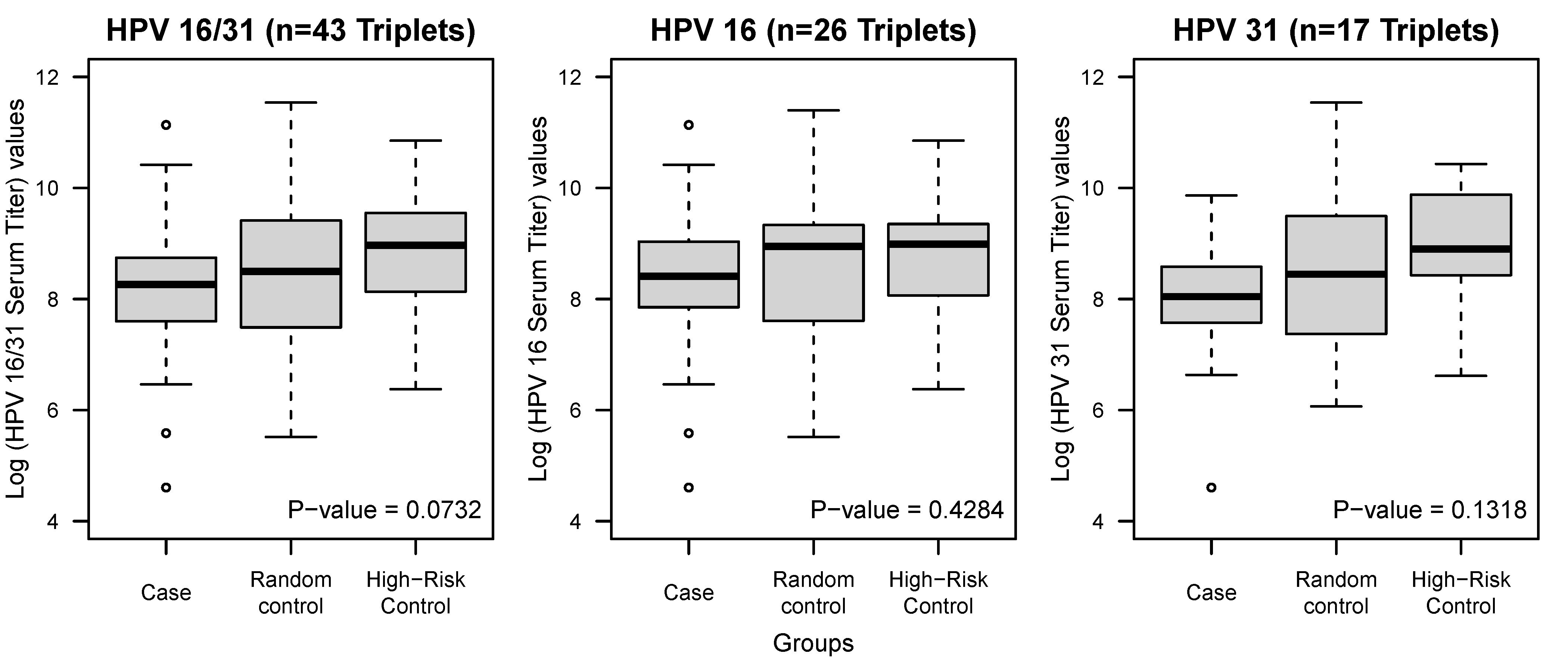
| Log Serum Titer Mean ± SD | 8.16 ± 1.31 | 8.54 ± 1.41 | 8.79 ± 1.13 | 0.0732 |
| Log Serum Titer Case vs. Random Control | 0.1914 ^ | |||
| Log Serum Titer Case vs. High-Risk Control | 0.0179 ^ |
| HPV16/31 (n = 43) | HPV16 Only (n = 26) | HPV31 ONLY (n = 17) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) § | p-Value | OR (95% CI) § | p-Value | OR (95% CI) § | p-Value | |
| Cases vs. Controls | 1.41 (1.01–1.96) | 0.0415 | 1.26 (0.82–1.92) | 0.2884 | 1.67 (0.96–2.92) | 0.0704 |
| Cases vs. Random Controls | 1.25 (0.88–1.79) | 0.2143 | 1.11 (0.69–1.77) | 0.6746 | 1.47 (0.81–2.66) | 0.2065 |
| Cases vs. HR Controls | 1.73 (1.03–2.88) | 0.0373 | 1.59 (0.80–3.14) | 0.1832 | 1.97 (0.84–4.60) | 0.1177 |
| 4vHPV Completed before Coitarche (Case/Control = 19/30) | |
|---|---|
| OR (95% CI) † | p-Value |
| 1.47 (0.95–2.28) | 0.0824 |
| Variables | Pre-Infection (n = 43) Mean ± SD | Peri-Infection (n = 10) Mean ± SD | Post-Infection (n = 32) Mean ± SD |
|---|---|---|---|
| Log Serum Titers | 8.16 ± 1.31 | 8.36 ± 1.30 | 8.20 ± 0.77 |
| Duration of Time from Last Dose (years) | 2.99 ± 2.94 | 4.40 ± 2.92 | 6.52 ± 3.04 |
| Serum Collection Time Relative to Infection (years) | −1.41 ± 0.70 | 0 ± 0 | 1.51 ± 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradissimo, A.; Shankar, V.; Wiek, F.; St. Peter, L.; Studentsov, Y.; Nucci-Sack, A.; Diaz, A.; Pickering, S.; Schlecht, N.F.; Burk, R.D. Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection. Viruses 2021, 13, 1548. https://doi.org/10.3390/v13081548
Gradissimo A, Shankar V, Wiek F, St. Peter L, Studentsov Y, Nucci-Sack A, Diaz A, Pickering S, Schlecht NF, Burk RD. Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection. Viruses. 2021; 13(8):1548. https://doi.org/10.3390/v13081548
Chicago/Turabian StyleGradissimo, Ana, Viswanathan Shankar, Fanua Wiek, Lauren St. Peter, Yevgeniy Studentsov, Anne Nucci-Sack, Angela Diaz, Sarah Pickering, Nicolas F. Schlecht, and Robert D. Burk. 2021. "Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection" Viruses 13, no. 8: 1548. https://doi.org/10.3390/v13081548
APA StyleGradissimo, A., Shankar, V., Wiek, F., St. Peter, L., Studentsov, Y., Nucci-Sack, A., Diaz, A., Pickering, S., Schlecht, N. F., & Burk, R. D. (2021). Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection. Viruses, 13(8), 1548. https://doi.org/10.3390/v13081548







