Modified-Live Feline Calicivirus Vaccination Reduces Viral RNA Loads, Duration of RNAemia, and the Severity of Clinical Signs after Heterologous Feline Calicivirus Challenge
Abstract
:1. Introduction
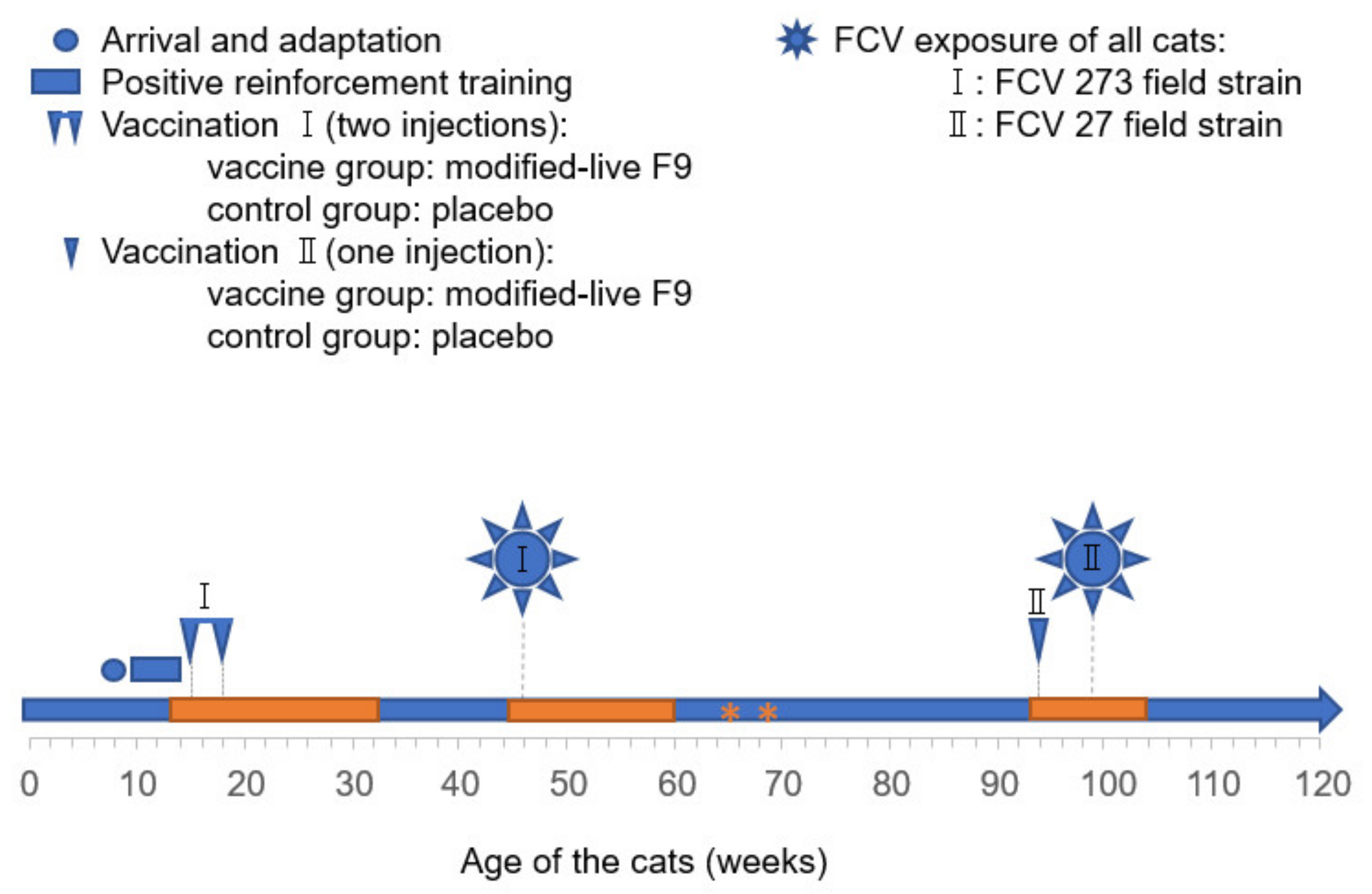
2. Materials and Methods
2.1. Animals
2.2. FCV Challenge Viruses
2.3. FCV Vaccinations and FCV Experimental Infections
2.4. Assessment of Clinical Signs
2.5. Oropharyngeal Cytobrush Sample Collection from Cats
2.6. Blood Collection, Processing, and Analyses
2.7. Nucleic Acid Extraction and PCR
2.8. Statistics
3. Results
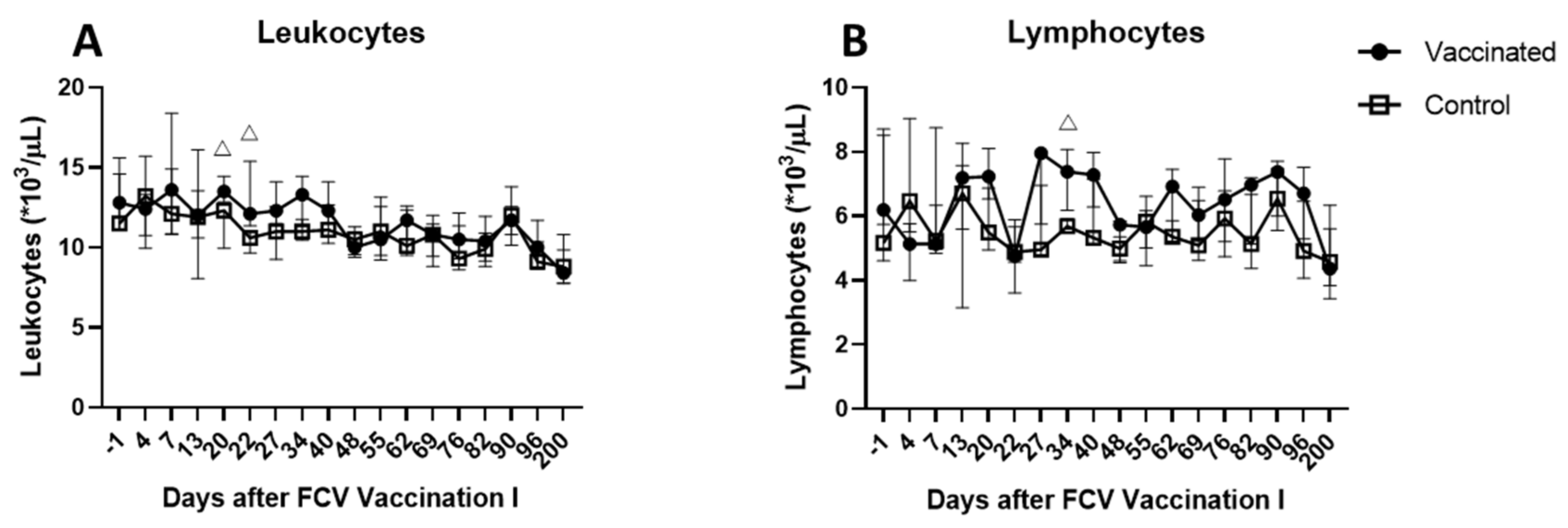
3.1. Outcome of the First FCV Vaccination (FCV Vaccination I)
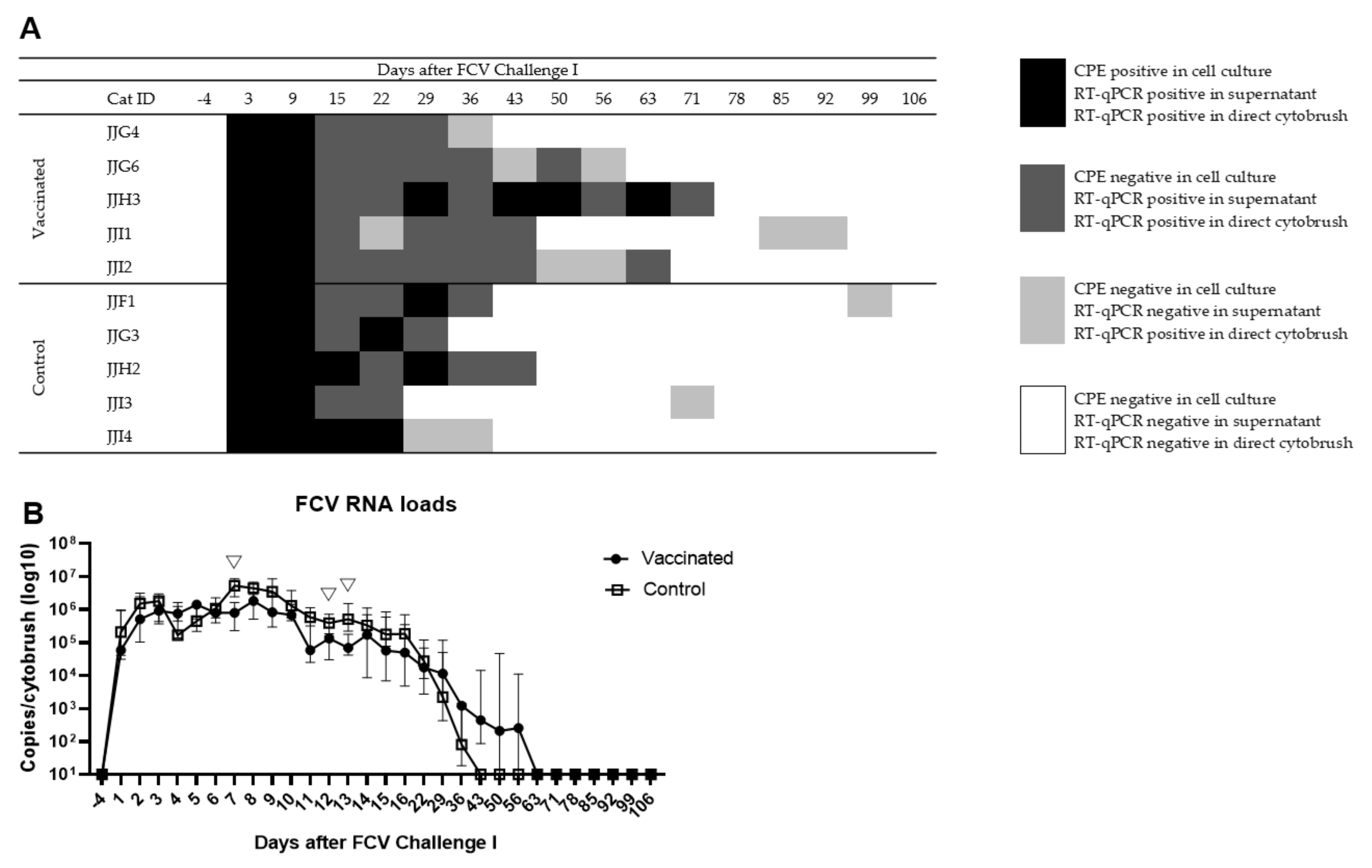
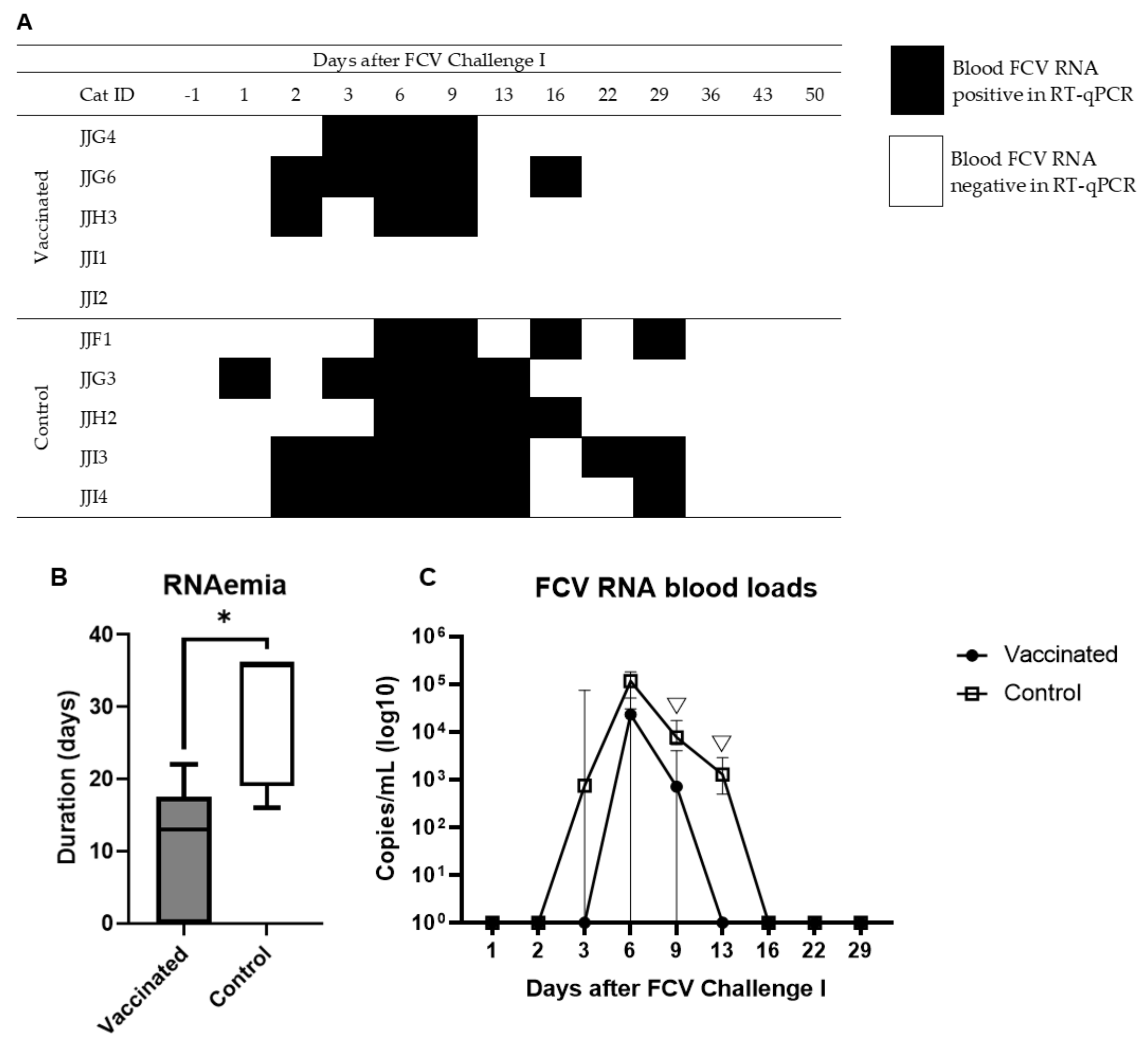
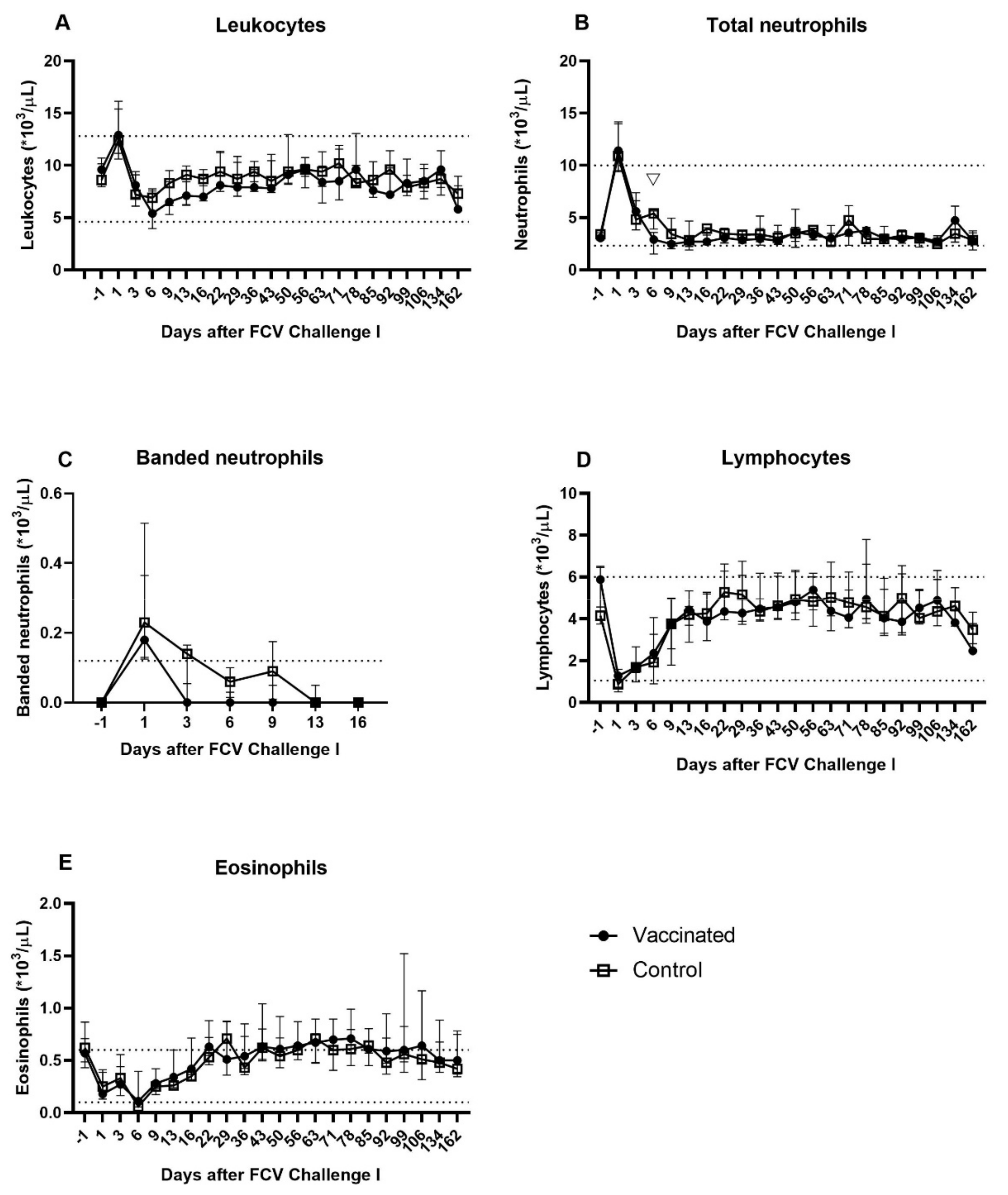
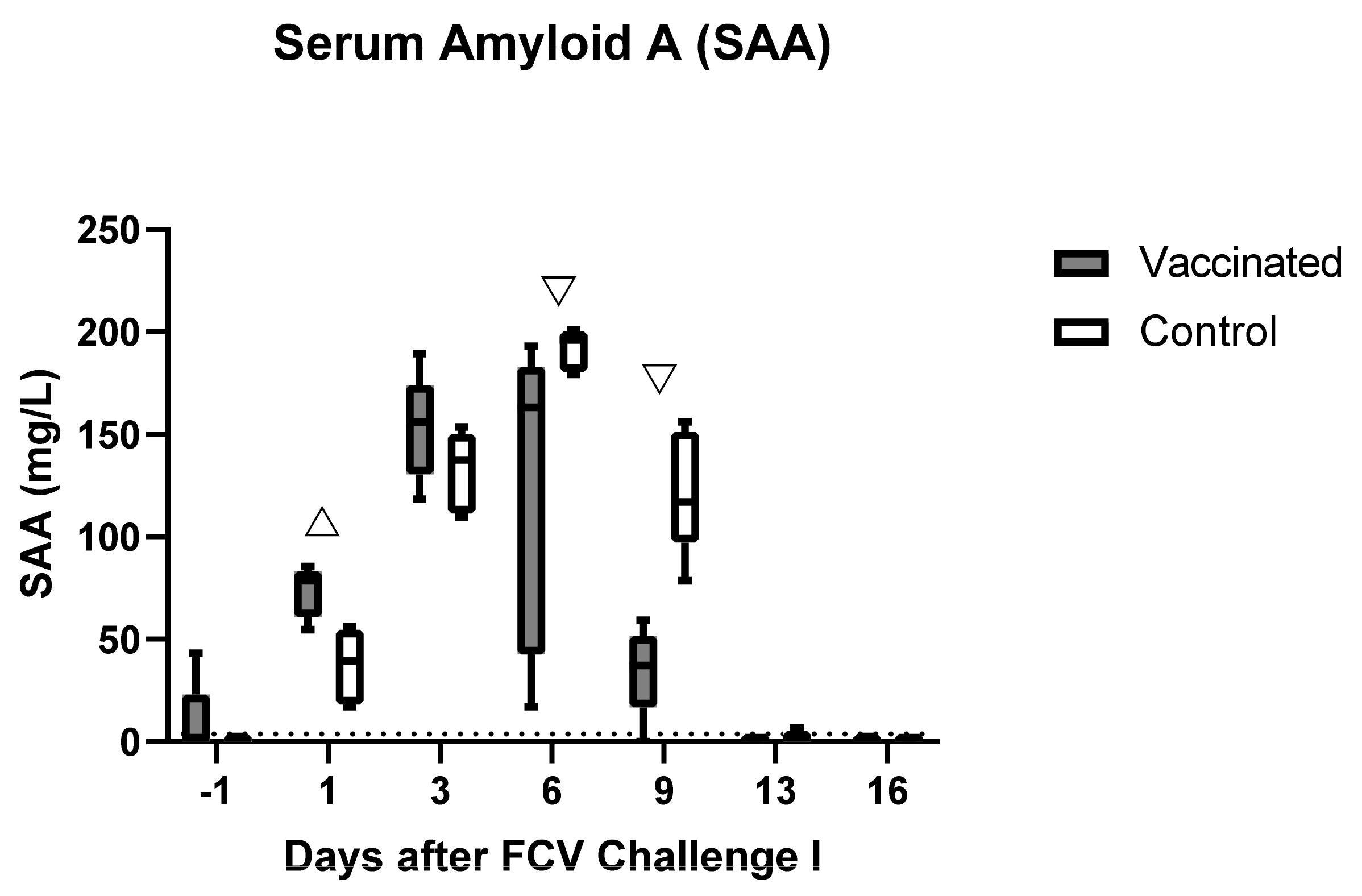
3.2. Outcome of the First Heterologous FCV Infection (FCV Challenge I)
3.3. Outcome of the Revaccination (FCV Vaccination II)
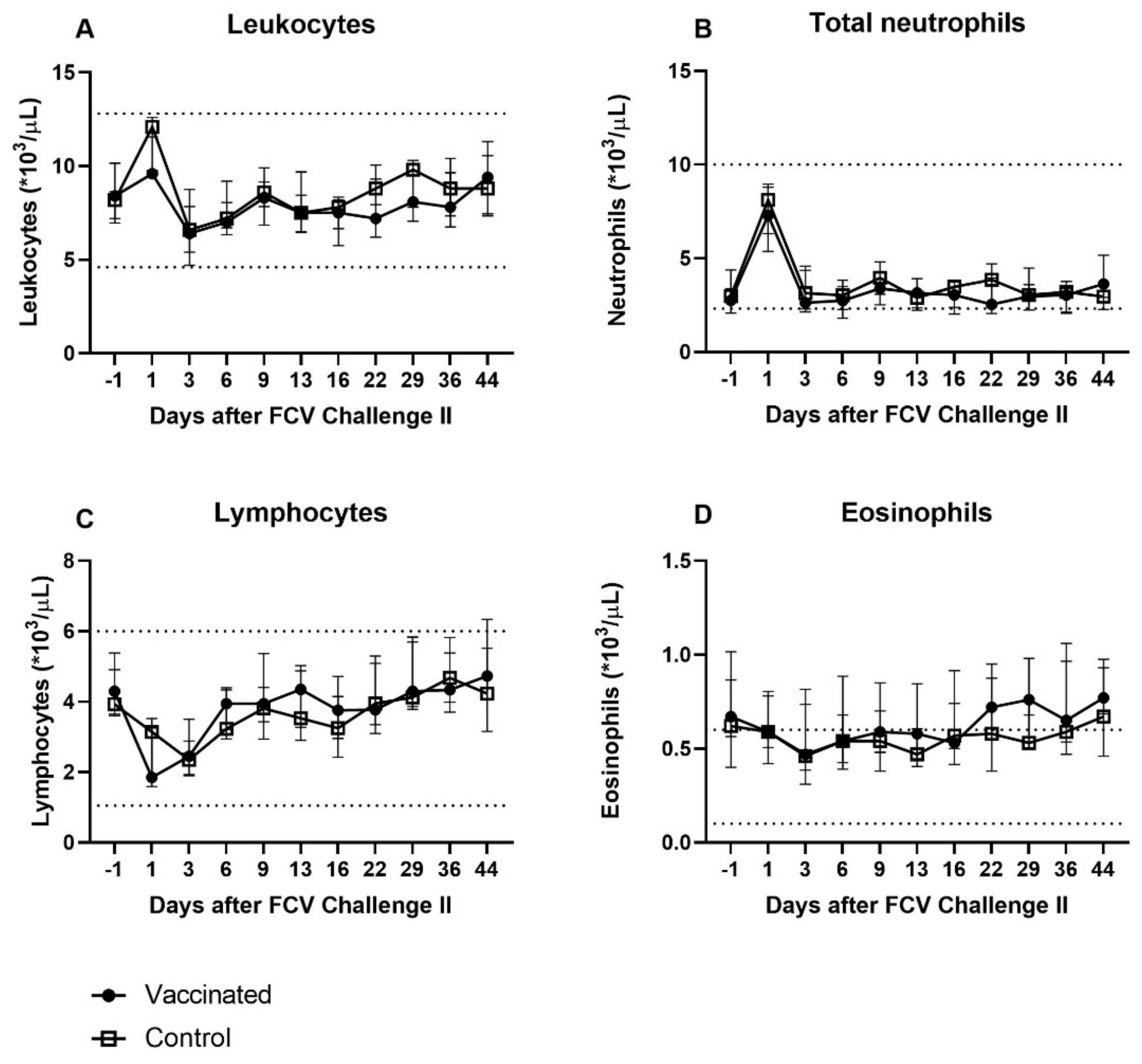
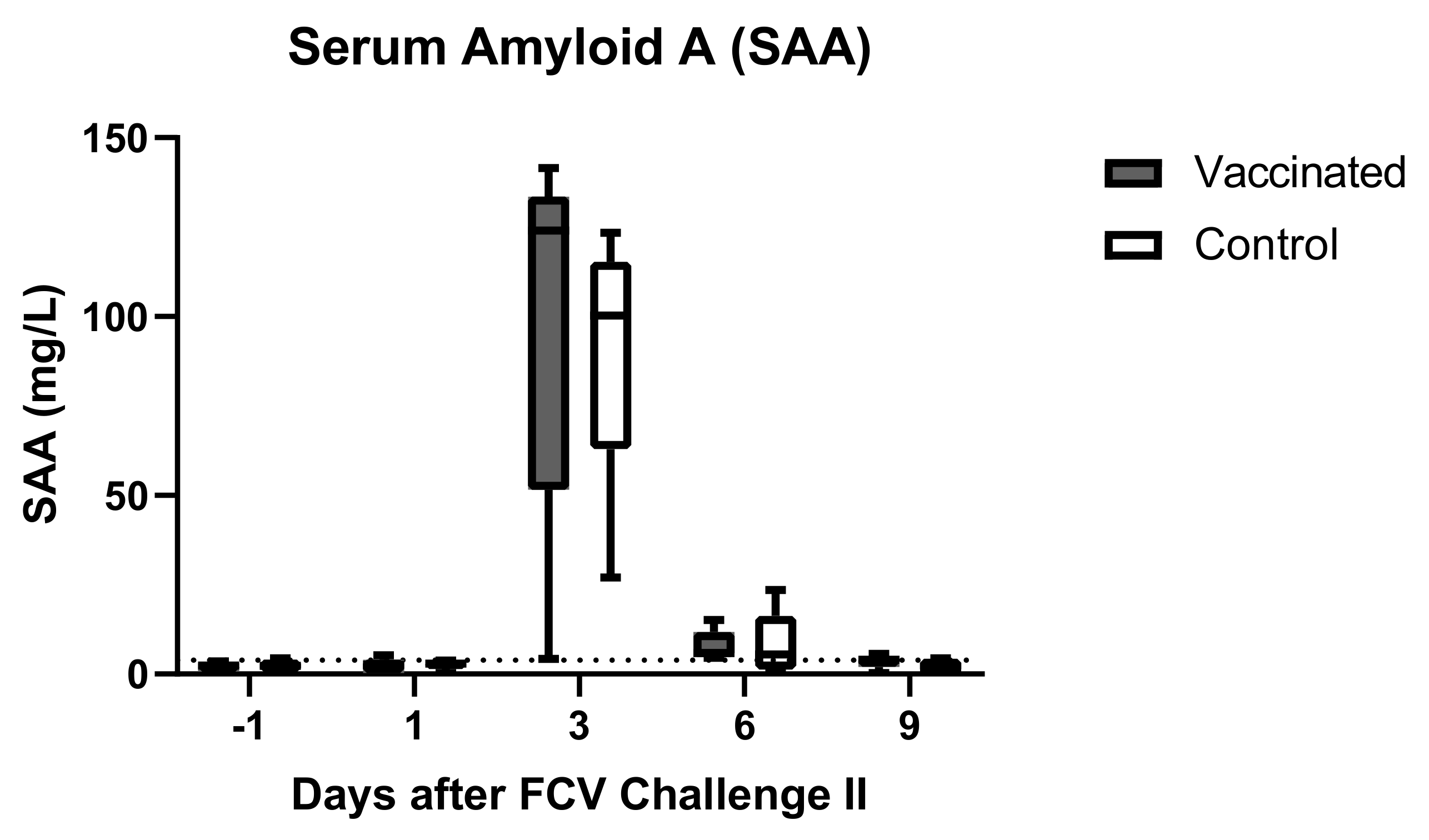
3.4. Outcome of the Second Heterologous FCV Infection (FCV Challenge II)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Change Over Time (Friedman and Dunn’s Post Tests) Vaccinated Group | Change Over Time (Friedman and Dunn’s Post Tests) Control Group | |
| Leukocytes | PF < 0.0001 Day 1 ↑/Day 6 (PD = 0.0011) Day 1 ↑/Dax 9 (PD = 0.0014) Day 1 ↑/Day 13 (PD = 0.008) Day 1 ↑/Day 162 (PD = 0.0038) Day 6 ↓/Day 78 (PD = 0.0122) Day 9 ↓/Day 78 (PD = 0.0151) Day 78 ↑/Day 162 (PD = 0.0371) | PF = 0.0012 Day 1 ↑/Day 6 (PD = 0.0042) Day 1 ↑/Day 162 (PD = 0.003) |
| Total (segmented and banded) neutrophils | PF = 0.0003 Day 1 ↑/Day 9 (PD = 0.001) Day 1 ↑/Day 13 (PD = 0.025) Day 3 ↑/Day 9 (PD = 0.0072) | PF = 0.0005 Day 1 ↑/Day 99 (PD = 0.0042) Day 1 ↑/Day 106 (PD = 0.0305) Day 1 ↑/Day 162 (PD = 0.008) |
| Banded neutrophils | PF = 0.0015 Significance lost in the post tests | PF = 0.0012 Day −1/Day 1 ↑ (PD = 0.016) Day 1 ↑/Day 16 (PD = 0.016) |
| Lymphocytes | PF < 0.0001 Day −1 ↑/Day 1 (PD = 0.0034) Day −1 ↑/Day 3 (PD = 0.0042) Day −1 ↑/Day 6 (PD = 0.0336) Day 1 ↓/Day 50 (PD = 0.011) Day 1 ↓/Day 78 (PD = 0.0065) Day 3 ↓/Day 50 (PD = 0.0136) Day 3 ↓/Day 78 (PD = 0.008) | PF < 0.0001 Day 1 ↓/Day 22 (PD = 0.0122) Day 1 ↓/Day 50 (PD = 0.0409) Day 1 ↓/Day 56 (PD = 0.0336) Day 3 ↓/Day 22 (PD = 0.0496) Day 6 ↓/Day 22 (PD = 0.0226) |
| Eosinophils | PF < 0.0001 Day 1 ↓/Day 78 (PD = 0.0034) Day 3 ↓/Day 78 (PD = 0.0058) Day 6 ↓/Day 78 (PD = 0.0014) Day 9 ↓/Day 78 (PD = 0.0052) Day 13 ↓/Day 78 (PD = 0.0305) | PF < 0.0001 Day 6 ↓/Day 43 (PD = 0.0047) Day 6 ↓/Day 63 (PD = 0.0305) Day 6 ↓/Day 78 (PD = 0.0336) Day 9 ↓/Day 43 (PD = 0.0450) |
| Body temperature | PF < 0.0001 Day 3 ↑/Day 13 (PD = 0.021) Day 4 ↑/Day 13 (PD = 0.0122) Day 5 ↑/Day 13 (PD = 0.0184) Day 3 ↑/Day 14 (PD = 0.0354) Day 4 ↑/Day 14 (PD = 0.021) Day 5 ↑/Day 14 (PD = 0.0311) | PF = 0.0004 Day 4 ↑/Day 13 (PD = 0.021) Day 4 ↑/Day 14 (PD = 0.021) Day 5 ↑/Day 13 (PD = 0.0354) Day 5 ↑/Day 14 (PD = 0.0354) |
| SAA | PF = 0.002 Day 3 ↑/Day 13 (PD = 0.016) Day 3 ↑/Day 16 (PD = 0.0269) Day 6 ↑/Day 13 (PD = 0.0269) Day 6 ↑/Day 16 (PD = 0.044) | PF < 0.001 Day 6 ↑/Day −1 (PD = 0.007) Day 6 ↑/Day 13 (PD = 0.0093) Day 6 ↑/Day 16 (PD = 0.0022) |
| Change Over Time (Friedman and Dunn’s Post Tests) Vaccinated Group | Change Over Time (Friedman and Dunn’s Post Tests) Control Group | |
| Leukocytes | PF = 0.0032 Day 3 ↓/Day 44 (PD = 0.0392) Day 16 ↓/Day 44 (PD = 0.0276) | PF = 0.0002 Day 1 ↑/Day 3 (PD = 0.0051) Day 1 ↑/Day 6 (PD = 0.0276) Day 1 ↑/Day 13 (PD = 0.016) |
| Total (segmented) neutrophils | Not significant | PF = 0.0098 Day 1 ↑/Day −1 (PD = 0.023) Day 1 ↑/Day 6 (PD = 0.0392) Day 1 ↑/Day 13 (PD = 0.0392) |
| Lymphocytes | PF < 0.0001 Day 1 ↓/Day 29 (PD = 0.0062) Day 1 ↓/Day 36 (PD = 0.0392) Day 3 ↓/Day 29 (PD = 0.0091) | PF < 0.0001 Day 1 ↓/Day 36 (PD = 0.016) Day 1 ↓/Day 44 (PD = 0.0075) Day 3 ↓/Day 29 (PD = 0.023) Day 3 ↓/Day 36 (PD = 0.0051) Day 3 ↓/Day 44 (PD = 0.0023) |
| Eosinophils | PF = 0.0034 Significance lost in the post test | Not significant |
| SAA | PF = 0.0118 Day −1 ↓/Day 3 (PD = 0.027) | PF = 0.0117 Day −1 ↓/Day 3 (PD = 0.0194) Day 3 ↑/Day 9 (PD = 0.0373) |
References
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Radford, A.D.; Sommerville, L.; Ryvar, R.; Cox, M.B.; Johnson, D.R.; Dawson, S.; Gaskell, R.M. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine 2001, 19, 4358–4362. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Ryvar, R.; Coyne, K.; Johnson, D.R.; Cox, M.B.; Acke, E.F.; Addie, D.D.; Gaskell, R.M. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes 2003, 27, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, M.J.; Foley, J.E. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J. Feline Med. Surg. 2005, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Helps, C.R.; Lait, P.; Damhuis, A.; Bjornehammar, U.; Bolta, D.; Brovida, C.; Chabanne, L.; Egberink, H.; Ferrand, G.; Fontbonne, A.; et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. Vet. Rec 2005, 156, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, K.P.; Dawson, S.; Radford, A.D.; Cripps, P.J.; Porter, C.J.; McCracken, C.M.; Gaskell, R.M. Long-term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Vet. Microbiol. 2006, 118, 12–25. [Google Scholar] [CrossRef]
- Berger, A.; Willi, B.; Meli, M.L.; Boretti, F.S.; Hartnack, S.; Dreyfus, A.; Lutz, H.; Hofmann-Lehmann, R. Feline calicivirus and other respiratory pathogens in cats with Feline calicivirus-related symptoms and in clinically healthy cats in Switzerland. BMC Vet. Res. 2015, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiri, A.M.; Meli, M.L.; Riond, B.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Environmental Contamination and Hygienic Measures after Feline Calicivirus Field Strain Infections of Cats in a Research Facility. Viruses 2019, 11, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, A.D.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 556–564. [Google Scholar] [CrossRef]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Samman, A.; Hoffmann, K.; Sydler, T.; Cattori, V.; Graf, F.; Diserens, K.A.; Padrutt, I.; et al. Molecular characterization and virus neutralization patterns of severe, non-epizootic forms of feline calicivirus infections resembling virulent systemic disease in cats in Switzerland and in Liechtenstein. Vet. Microbiol. 2016, 182, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, N.C.; Elliott, J.B.; Glasgow, A.; Poland, A.; Keel, K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 2000, 73, 281–300. [Google Scholar] [CrossRef]
- Hurley, K.E.; Pesavento, P.A.; Pedersen, N.C.; Poland, A.M.; Wilson, E.; Foley, J.E. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 2004, 224, 241–249. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Coyne, K.P.; Porter, C.J.; Gaskell, R.M. The challenge for the next generation of feline calicivirus vaccines. Vet. Microbiol. 2006, 117, 14–18. [Google Scholar] [CrossRef]
- Kahn, D.E.; Hoover, E.A.; Bittle, J.L. Induction of immunity to feline caliciviral disease. Infect. Immun. 1975, 11, 1003–1009. [Google Scholar] [CrossRef] [Green Version]
- Scott, F.W. Evaluation of a feline viral rhinotracheitis-feline calicivirus disease vaccine. Am. J. Vet. Res. 1977, 38, 229–234. [Google Scholar] [PubMed]
- Pedersen, N.C.; Hawkins, K.F. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet. Microbiol. 1995, 47, 141–156. [Google Scholar] [CrossRef]
- Poulet, H.; Brunet, S.; Leroy, V.; Chappuis, G. Immunisation with a combination of two complementary feline calicivirus strains induces a broad cross-protection against heterologous challenges. Vet. Microbiol. 2005, 106, 17–31. [Google Scholar] [CrossRef]
- Jas, D.; Aeberle, C.; Lacombe, V.; Guiot, A.L.; Poulet, H. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet. J. 2009, 182, 86–93. [Google Scholar] [CrossRef]
- Jas, D.; Frances-Duvert, V.; Vernes, D.; Guigal, P.M.; Poulet, H. Three-year duration of immunity for feline herpesvirus and calicivirus evaluated in a controlled vaccination-challenge laboratory trial. Vet. Microbiol. 2015, 177, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, A.; Jarrett, O.; Sabara, M. Serological analysis of feline calicivirus isolates from the United States and United Kingdom. Vet. Microbiol. 1997, 56, 55–63. [Google Scholar] [CrossRef]
- Addie, D.; Poulet, H.; Golder, M.C.; McDonald, M.; Brunet, S.; Thibault, J.C.; Hosie, M.J. Ability of antibodies to two new caliciviral vaccine strains to neutralise feline calicivirus isolates from the UK. Vet. Rec. 2008, 163, 355–357. [Google Scholar] [CrossRef]
- Porter, C.J.; Radford, A.D.; Gaskell, R.M.; Ryvar, R.; Coyne, K.P.; Pinchbeck, G.L.; Dawson, S. Comparison of the ability of feline calicivirus (FCV) vaccines to neutralise a panel of current UK FCV isolates. J. Feline Med. Surg. 2008, 10, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Afonso, M.M.; Pinchbeck, G.L.; Gaskell, R.M.; Dawson, S.; Radford, A.D. Temporally separated feline calicivirus isolates do not cluster phylogenetically and are similarly neutralised by high-titre vaccine strain FCV-F9 antisera in vitro. J. Feline Med. Surg. 2020, 22, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Spiri, A.M.; Theze, J.; Meli, M.L.; Cattori, V.; Berger, A.; Steinrigl, A.; Pybus, O.G.; Hofmann-Lehmann, R.; Willi, B. Genetic diversity and phenotypic associations of feline caliciviruses from cats in Switzerland. J. Gen. Virol. 2016, 97, 3253–3266. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Christley, R.M.; Pybus, O.G.; Dawson, S.; Gaskell, R.M.; Radford, A.D. Large-scale spatial and temporal genetic diversity of feline calicivirus. J. Virol. 2012, 86, 11356–11367. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.; Gaskell, R.M.; Mills, A.; Knowles, J.; Carter, S.; McArdle, F. Detection of feline calicivirus antigens in the joints of infected cats. Vet. Rec. 1989, 124, 329–332. [Google Scholar] [CrossRef]
- Russell, G.C.; Zadoks, R.N.; Willoughby, K.; Bachofen, C. Bovine viral diarrhoea virus loses quasispecies diversity rapidly in culture. Microb. Genom. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Geret, C.; Riond, B.; Cattori, V.; Meli, M.; Hofmann-Lehmann, R.; Lutz, H. Housing and care of laboratory cats: From requirements to practice. Schweiz. Arch. Tierheilkd 2011, 153, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Hofmann-Lehmann, R.; Holznagel, E.; Lutz, H. Female cats have lower rates of apoptosis in peripheral blood lymphocytes than male cats: Correlation with estradiol-17 beta, but not with progesterone blood levels. Vet. Immunol. Immunopathol. 1998, 65, 151–160. [Google Scholar] [CrossRef]
- Schweizerische Eidgenossenschaft. Schweizer Tierschutzgesetz (TSchG). Available online: https://www.admin.ch/opc/de/classified-compilation/20022103/index.html (accessed on 16 July 2019).
- Rong, S.; Lowery, D.; Floyd-Hawkins, K.; King, V. Characterization of an avirulent FCV strain with a broad serum cross-neutralization profile and protection against challenge of a highly virulent vs feline calicivirus. Virus Res. 2014, 188, 60–67. [Google Scholar] [CrossRef]
- Weissenbacher, S.; Riond, B.; Hofmann-Lehmann, R.; Lutz, H. Evaluation of a novel haematology analyser for use with feline blood. Vet. J. 2011, 187, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.; Jacobsen, S.; Ichiyanagi, T.; Kjelgaard-Hansen, M. Evaluation of an automated assay based on monoclonal anti-human serum amyloid A (SAA) antibodies for measurement of canine, feline, and equine SAA. Vet. J. 2012, 194, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.G.; Kahn, D.E.; Hoover, E.A.; Saxe, N.J.; Yohn, D.S. Differences in acute and convalescent-phase antibodies of cats infected with feline picornaviruses. Infect. Immun. 1974, 10, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Bittle, J.L.; Rubic, W.J. Immunization against feline calicivirus infection. Am. J. Vet. Res. 1976, 37, 275–278. [Google Scholar]
- Ruch-Gallie, R.A.; Veir, J.K.; Hawley, J.R.; Lappin, M.R. Results of molecular diagnostic assays targeting feline herpesvirus-1 and feline calicivirus in adult cats administered modified live vaccines. J. Feline Med. Surg. 2011, 13, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Poulet, H.; Jas, D.; Lemeter, C.; Coupier, C.; Brunet, S. Efficacy of a bivalent inactivated non-adjuvanted feline calicivirus vaccine: Relation between in vitro cross-neutralization and heterologous protection in vivo. Vaccine 2008, 26, 3647–3654. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A. WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, J.O.; McArdle, F.; Dawson, S.; Carter, S.D.; Gaskell, C.J.; Gaskell, R.M. Studies on the role of feline calicivirus in chronic stomatitis in cats. Vet. Microbiol. 1991, 27, 205–219. [Google Scholar] [CrossRef]
- Lesbros, C.; Martin, V.; Najbar, W.; Sanquer, A.; McGahie, D.; Eun, H.M.; Gueguen, S. Protective Efficacy of the Calicivirus Valency of the Leucofeligen Vaccine against a Virulent Heterologous Challenge in Kittens. Vet. Med. Int. 2013, 2013, 232397. [Google Scholar] [CrossRef]
- Tham, K.M.; Studdert, M.J. Antibody and cell-mediated immune responses to feline calicivirus following inactivated vaccine and challenge. J. Vet. Med. B 1987, 34, 640–654. [Google Scholar] [CrossRef]
- Lappin, M.R.; Andrews, J.; Simpson, D.; Jensen, W.A. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J. Am. Vet. Med. Assoc. 2002, 220, 38–42. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Chaintoutis, S.C.; Dovas, C.I.; Polizopoulou, Z.S.; Brellou, G.D.; Agianniotaki, E.I.; Tasioudi, K.E.; Chondrokouki, E.; Papadopoulos, O.; Karatzias, H.; et al. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transbound Emerg. Dis. 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Reinhardt, B.; Jaspert, R.; Niedrig, M.; Kostner, C.; L’Age-Stehr, J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: A model of human flavivirus infection. J. Med. Virol. 1998, 56, 159–167. [Google Scholar] [CrossRef]
- Decaro, N.; Crescenzo, G.; Desario, C.; Cavalli, A.; Losurdo, M.; Colaianni, M.L.; Ventrella, G.; Rizzi, S.; Aulicino, S.; Lucente, M.S.; et al. Long-term viremia and fecal shedding in pups after modified-live canine parvovirus vaccination. Vaccine 2014, 32, 3850–3853. [Google Scholar] [CrossRef]
- Reubel, G.H.; George, J.W.; Higgins, J.; Pedersen, N.C. Effect of chronic feline immunodeficiency virus infection on experimental feline calicivirus-induced disease. Vet. Microbiol. 1994, 39, 335–351. [Google Scholar] [CrossRef]
- Roberts, E.S.; VanLare, K.A.; Roycroft, L.M.; King, S. Effect of high-dose ciclosporin on the immune response to primary and booster vaccination in immunocompetent cats. J. Feline Med. Surg. 2015, 17, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.; Greene, C. Infectious Diseases of the Dog and Cat; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Almeras, T.; Schreiber, P.; Fournel, S.; Martin, V.; Nicolas, C.S.; Fontaine, C.; Lesbros, C.; Gueguen, S. Comparative efficacy of the Leucofeligen FeLV/RCP and Purevax RCP FeLV vaccines against infection with circulating feline Calicivirus. BMC Vet. Res. 2017, 13, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, S.; Smyth, N.R.; Bennett, M.; Gaskell, R.M.; McCracken, C.M.; Brown, A.; Gaskell, C.J. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. AIDS 1991, 5, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Povey, C.; Ingersoll, J. Cross-protection among feline caliciviruses. Infect. Immun. 1975, 11, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Sehata, G.; Okada, N.; Iwamoto, K.; Masubuchi, K.; Kainuma, R.; Noda, T.; Igarashi, T.; Sawada, T.; Noro, T.; et al. Intranasal immunization with inactivated feline calicivirus particles confers robust protection against homologous virus and suppression against heterologous virus in cats. J. Gen. Virol. 2017, 98, 1730–1738. [Google Scholar] [CrossRef]
- Liew, F.Y.; Russell, S.M.; Appleyard, G.; Brand, C.M.; Beale, J. Cross-Protection in Mice Infected with Influenza-a Virus by the Respiratory Route Is Correlated with Local Iga Antibody Rather Than Serum Antibody or Cyto-Toxic T-Cell Reactivity. Eur. J. Immunol. 1984, 14, 350–356. [Google Scholar] [CrossRef]
- Renegar, K.B.; Small, P.A. Passive Transfer of Local Immunity to Influenza-Virus Infection by Iga Antibody. J. Immunol. 1991, 146, 1972–1978. [Google Scholar]
- Pesavento, P.A.; Chang, K.O.; Parker, J.S. Molecular virology of feline calicivirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 775–786. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Stokol, T.; Liu, H.; van der List, D.A.; Gaffney, P.M.; Parker, J.S. Distribution of the feline calicivirus receptor junctional adhesion molecule a in feline tissues. Vet. Pathol. 2011, 48, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Kanellos, T.; Meli, M.L.; Sutton, D.J.; Gisler, R.; Gomes-Keller, M.A.; Hofmann-Lehmann, R.; Lutz, H. Antibody induction after combined application of an adjuvanted recombinant FeLV vaccine and a multivalent modified live virus vaccine with a chlamydial component. Vaccine 2006, 24, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Spiri, A.M.; Novacco, M.; Meli, M.L.; Stirn, M.; Riond, B.; Fogle, J.E.; Herbert, I.; Hosie, M.J.; Boretti, F.S.; Hofmann-Lehmann, R. Modified-live feline calicivirus vaccination elicits cellular immunity against a current feline calicivirus field strain in an experimental feline challenge study; unpublished; manuscript in preparation.
- Dalton, K.P.; Balseiro, A.; Juste, R.A.; Podadera, A.; Nicieza, I.; Del Llano, D.; Gonzalez, R.; Martin Alonso, J.M.; Prieto, J.M.; Parra, F.; et al. Clinical course and pathogenicity of variant rabbit haemorrhagic disease virus in experimentally infected adult and kit rabbits: Significance towards control and spread. Vet. Microbiol. 2018, 220, 24–32. [Google Scholar] [CrossRef]
- Newman, K.L.; Marsh, Z.; Kirby, A.E.; Moe, C.L.; Leon, J.S. Immunocompetent adults from human norovirus challenge studies do not exhibit norovirus viremia. J. Virol. 2015, 89, 6968–6969. [Google Scholar] [CrossRef] [Green Version]
- European Advisory Board on Cat Diseases (ABCD). Feline Calicivirus Infection. Available online: http://www.abcdcatsvets.org/feline-calicivirus-infection-2012-edition/ (accessed on 2 February 2021).
- Mencke, N.; Vobis, M.; Mehlhorn, H.D.H.; D’Haese, J.; Rehagen, M.; Mangold-Gehring, S.; Truyen, U. Transmission of feline calicivirus via the cat flea (Ctenocephalides felis). Parasitol. Res. 2009, 105, 185–189. [Google Scholar] [CrossRef]
- Boone, L.I. Disorders of White Blood Cells. In Handbook of Small Animal Practice; Morgan, R.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Von Dehn, B. Pediatric clinical pathology. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 205–219. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tohya, Y.; Horimoto, T.; Maeda, K.; Miyazawa, T.; Mikami, T. Carrier-state infection of feline T-lymphoblastoid cells with feline calicivirus. Vet. Microbiol. 1994, 40, 379–386. [Google Scholar] [CrossRef]
- Boonnak, K.; Vogel, L.; Feldmann, F.; Feldmann, H.; Legge, K.L.; Subbarao, K. Lymphopenia associated with highly virulent H5N1 virus infection due to plasmacytoid dendritic cell-mediated apoptosis of T cells. J. Immunol. 2014, 192, 5906–5912. [Google Scholar] [CrossRef] [PubMed]
- Yuki, M.; Aoyama, R.; Nakagawa, M.; Hirano, T.; Naitoh, E.; Kainuma, D. A Clinical Investigation on Serum Amyloid A Concentration in Client-Owned Healthy and Diseased Cats in a Primary Care Animal Hospital. Vet. Sci. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, K.; Ma, Z.; Khatlani, T.S.; Okuda, M.; Inokuma, H.; Onishi, T. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J. Vet. Med. Sci. 2003, 65, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Li, T.; Wang, C.; Liu, X.; Ouyang, H.; Ji, W.; Liu, J.; Liao, X.; Li, J.; Hu, C. A retrospective study of clinical and laboratory features and treatment on cats highly suspected of feline infectious peritonitis in Wuhan, China. Sci. Rep. 2021, 11, 5208. [Google Scholar] [CrossRef] [PubMed]
- Pineros, Y.S.S.; Bal, S.M.; Dijkhuis, A.; Majoor, C.J.; Dierdorp, B.S.; Dekker, T.; Hoefsmit, E.P.; Bonta, P.I.; Picavet, D.; van der Wel, N.N.; et al. Eosinophils capture viruses, a capacity that is defective in asthma. Allergy 2019, 74, 1898–1909. [Google Scholar] [CrossRef]


| Virus Neutralisation Against FCV F9 | ||
|---|---|---|
| Antisera | S1 1 | S2 1 |
| Neutralising antibody titre 2 | ||
| FCV 273 | <5 | 5 |
| FCV 27 | 15 | 15 |
| Clinical Sign | Timepoint of Occurrence (Day Post Challenge) | Score |
|---|---|---|
| Sneezing occasional | 1 (each day) | |
| Sneezing persistent | 2 (each day) | |
| Dyspnoea audible rales | 2 (each day) | |
| Dyspnoea coughing | 2 (each day) | |
| Open mouth breathing | 2 (each day) | |
| Oral ulcer (single, small < 4 mm diameter) | 1–5 | 2 |
| 6–9 | 3 | |
| ≥10 | 4 | |
| Oral ulcers (multiple, small < 4 mm diameter) | 1–4 | 3 |
| 5–8 | 5 | |
| ≥9 | 7 | |
| Oral ulcers (large, ≥ 4 mm diameter) | 1–4 | 5 |
| 5–8 | 7 | |
| ≥9 | 9 | |
| Dehydration | 1–2 | 3 |
| ≥3 | 4 | |
| External ulcers on lips or nares, nonbleeding | 4 (each day) | |
| External ulcers on lips or nares, bleeding | 6 (each day) | |
| Conjunctivitis—serous discharge | 1–3 | 1 |
| ≥4 | 2 | |
| Conjunctivitis—mucopurulent discharge | 1–2 | 2 |
| 3–5 | 4 | |
| ≥6 | 6 | |
| Rhinitis—serous discharge | 1–3 | 1 |
| ≥4 | 2 | |
| Rhinitis—mucopurulent discharge | 1–2 | 2 |
| 3–5 | 4 | |
| ≥6 | 6 | |
| Rectal temperature | <37.2 °C | 2 (each day) |
| 39.4–39.9 °C | 1 (each day) | |
| 40.0–40.5 °C | 2 (each day) | |
| >40.5 °C | 3 (each day) | |
| Anorexia | 1 (each day) | |
| Death | 15 |
| FCV Vaccination I * | FCV Challenge I | FCV Vaccination II | FCV Challenge II | |
|---|---|---|---|---|
| Clinical examination | −1, 1, 2, 3, 4, 5, 6, 7, 13, 20, 22, 25 and 27, then weekly until day 118 after injection I | −1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, then twice a week until day 108 after Challenge I, then weekly until FCV Vaccination II | −1, 1, 2, 3, 4, 5, 6, 7, 8 and 9, then weekly until FCV Challenge II | −1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16, then twice a week until day 64, then weekly until day 120 after FCV Challenge II |
| Clinical scoring | - | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15 | - | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15 |
| Oropharyngeal cytobrushes | −1, 1, 2, 3, 4, 5, 6, 7, 13, 20, 22, 25 and 27, then weekly until day 118 after injection I | −4, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 and 22, then weekly until day 106 after FCV Challenge I | −1, 1, 2, 3, 4, 5, 6, 7, 8 and 9, then weekly until FCV Challenge II | −4, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16, then weekly until day 42 after FCV Challenge II |
| EDTA blood collection | −1, 4, 7, 13, 20, 22 and 27, then weekly until day 96 and on day 200 after injection I | −1, 1, 2, 3, 6, 9, 13, 16, 22, 29, 36 and 43, then weekly until day 106 and day 134, 162 after FCV Challenge I | −1, 2, 3 and 8 | −1, 1, 3, 6, 9, 13 and 16, then weekly until day 44 after FCV Challenge II |
| Serum collection | - | −1, 1, 3, 6, 9, 13 and 16 | - | −1, 1, 3, 6 and 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiri, A.M.; Riond, B.; Stirn, M.; Novacco, M.; Meli, M.L.; Boretti, F.S.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Modified-Live Feline Calicivirus Vaccination Reduces Viral RNA Loads, Duration of RNAemia, and the Severity of Clinical Signs after Heterologous Feline Calicivirus Challenge. Viruses 2021, 13, 1505. https://doi.org/10.3390/v13081505
Spiri AM, Riond B, Stirn M, Novacco M, Meli ML, Boretti FS, Herbert I, Hosie MJ, Hofmann-Lehmann R. Modified-Live Feline Calicivirus Vaccination Reduces Viral RNA Loads, Duration of RNAemia, and the Severity of Clinical Signs after Heterologous Feline Calicivirus Challenge. Viruses. 2021; 13(8):1505. https://doi.org/10.3390/v13081505
Chicago/Turabian StyleSpiri, Andrea M., Barbara Riond, Martina Stirn, Marilisa Novacco, Marina L. Meli, Felicitas S. Boretti, Imogen Herbert, Margaret J. Hosie, and Regina Hofmann-Lehmann. 2021. "Modified-Live Feline Calicivirus Vaccination Reduces Viral RNA Loads, Duration of RNAemia, and the Severity of Clinical Signs after Heterologous Feline Calicivirus Challenge" Viruses 13, no. 8: 1505. https://doi.org/10.3390/v13081505
APA StyleSpiri, A. M., Riond, B., Stirn, M., Novacco, M., Meli, M. L., Boretti, F. S., Herbert, I., Hosie, M. J., & Hofmann-Lehmann, R. (2021). Modified-Live Feline Calicivirus Vaccination Reduces Viral RNA Loads, Duration of RNAemia, and the Severity of Clinical Signs after Heterologous Feline Calicivirus Challenge. Viruses, 13(8), 1505. https://doi.org/10.3390/v13081505









