Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases
Abstract
1. Introduction
1.1. Advantages and Utility of Ad-Based Vectors
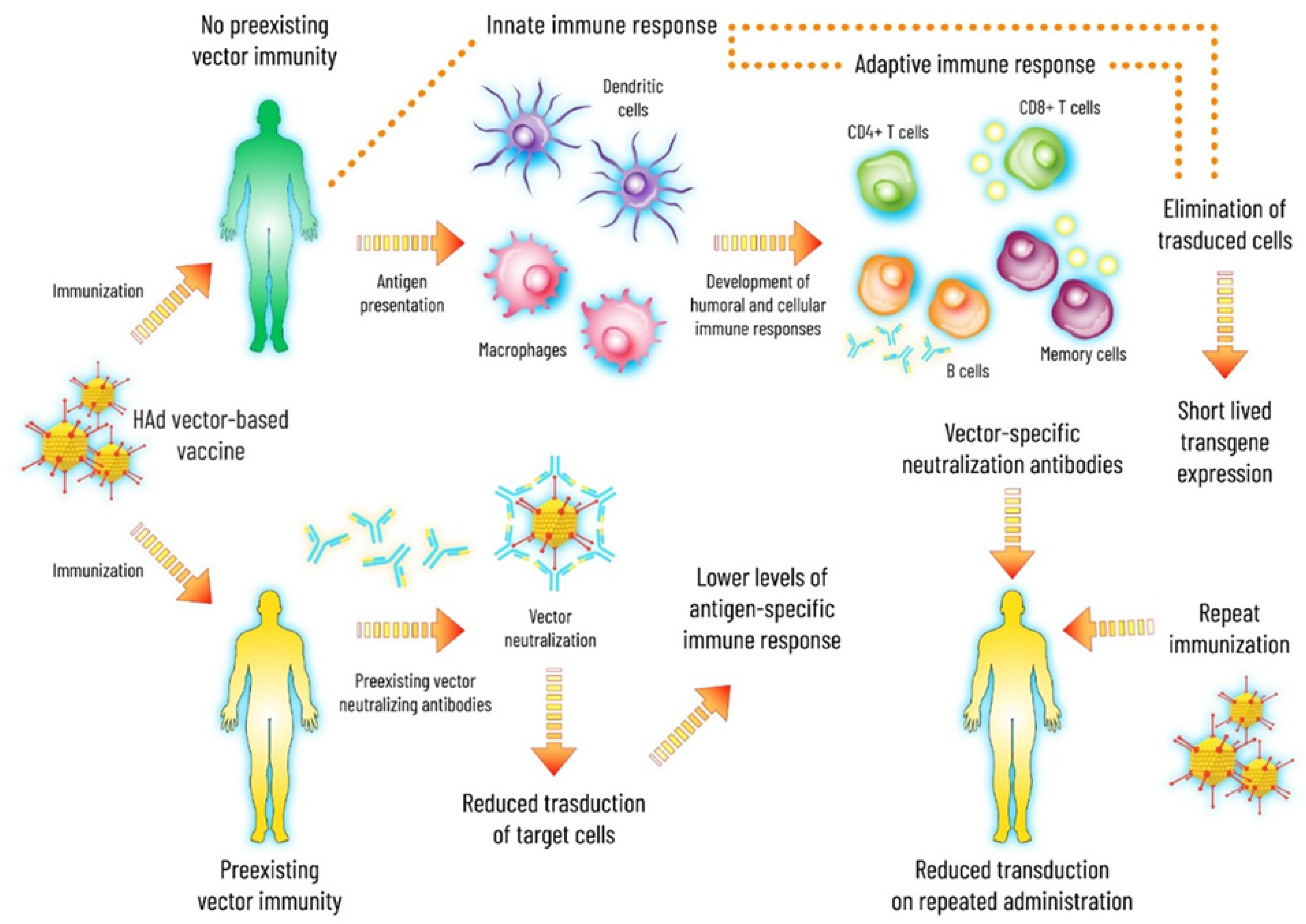
1.2. Ad-Induced Innate Immunity
1.3. Basic Features of Ad Vector-Based Vaccines
1.4. Preexisting Ad Vector Immunity
2. Chimpanzee Ad Vectors
2.1. ChAd63
2.2. ChAd3
2.3. ChAdOx1
2.4. ChAdOx2
2.5. Simian Ad36
2.6. ChAd155
2.7. PanAd3
2.8. ChAd7
3. Bovine Ad Vectors
4. Canine Ad Vectors
5. Porcine Ad Vectors
6. Ovine Ad Vectors
7. Avian Ad Vectors
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGrory, W.J.; Bautista, D.S.; Graham, F.L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology 1988, 163, 614–617. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Shayakhmetov, D.M. Adenovirus sensing by the immune system. Curr. Opin. Virol. 2016, 21, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Doronin, K.; Flatt, J.W.; Di Paolo, N.C.; Khare, R.; Kalyuzhniy, O.; Acchione, M.; Sumida, J.P.; Ohto, U.; Shimizu, T.; Akashi-Takamura, S.; et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science 2012, 338, 795–798. [Google Scholar] [CrossRef]
- McEwan, W.A.; Tam, J.C.; Watkinson, R.E.; Bidgood, S.R.; Mallery, D.L.; James, L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 2013, 14, 327–336. [Google Scholar] [CrossRef]
- Tam, J.C.; Bidgood, S.R.; McEwan, W.A.; James, L.C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 2014, 345, 1256070. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Z.; Smith, J.S.; Hofherr, S.E.; Barry, M.A.; Byrnes, A.P. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: Indirect complement activation by virions in vivo. J. Virol. 2009, 83, 5648–5658. [Google Scholar] [CrossRef]
- Nemerow, G.R.; Stewart, P.L.; Reddy, V.S. Structure of human adenovirus. Curr. Opin. Virol. 2012, 2, 115–121. [Google Scholar] [CrossRef]
- Koizumi, N.; Kawabata, K.; Sakurai, F.; Watanabe, Y.; Hayakawa, T.; Mizuguchi, H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alphav integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum. Gene Ther. 2006, 17, 264–279. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Baldwin, L.K.; Irons, E.E.; Papayannopoulou, T.; Tomlinson, S.; Shayakhmetov, D.M. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014, 10, e1004035. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Lyons, M.; Onion, D.; Green, N.K.; Aslan, K.; Rajaratnam, R.; Bazan-Peregrino, M.; Phipps, S.; Hale, S.; Mautner, V.; Seymour, L.W.; et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006, 14, 118–128. [Google Scholar] [CrossRef]
- Anghelina, D.; Lam, E.; Falck-Pedersen, E. Diminished innate antiviral response to adenovirus vectors in cGAS/STING-deficient mice minimally impacts adaptive immunity. J. Virol. 2016, 90, 5915–5927. [Google Scholar] [CrossRef]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef]
- Lee, H.C.; Chathuranga, K.; Lee, J.S. Intracellular sensing of viral genomes and viral evasion. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Shah, G.A.; O’Shea, C.C. Viral and cellular genomes activate distinct DNA damage responses. Cell 2015, 162, 987–1002. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef] [PubMed]
- Appledorn, D.; Patial, S.; McBride, A.; Godbehere, S.; Van Rooijen, N.; Parameswaran, N.; Amalfitano, A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008, 181, 2134–2144. [Google Scholar] [CrossRef]
- Hehir, K.M.; Armentano, D.; Cardoza, L.M.; Choquette, T.L.; Berthelette, P.B.; White, G.A.; Couture, L.A.; Everton, M.B.; Keegan, J.; Martin, J.M.; et al. Molecular characterization of replication-competent variants of adenovirus vectors and genome modifications to prevent their occurrence. J. Virol. 1996, 70, 8459–8467. [Google Scholar] [CrossRef]
- Shiver, J.W.; Fu, T.M.; Chen, L.; Casimiro, D.R.; Davies, M.E.; Evans, R.K.; Zhang, Z.Q.; Simon, A.J.; Trigona, W.L.; Dubey, S.A.; et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002, 415, 331–335. [Google Scholar] [CrossRef]
- Crosby, C.M.; Barry, M.A. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 2014, 462–463, 158–165. [Google Scholar] [CrossRef]
- Crosby, C.M.; Nehete, P.; Sastry, K.J.; Barry, M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J. Virol. 2015, 89, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating single-cycle adenovirus vectors generate amplified influenza vaccine responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Toth, K.; Lee, S.R.; Ying, B.; Spencer, J.F.; Tollefson, A.E.; Sagartz, J.E.; Kong, I.K.; Wang, Z.; Wold, W.S. STAT2 knockout Syrian hamsters support enhanced replication and pathogenicity of human adenovirus, revealing an important role of type I interferon response in viral control. PLoS Pathog. 2015, 11, e1005084. [Google Scholar] [CrossRef]
- Guo, J.; Mondal, M.; Zhou, D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018, 14, 1679–1685. [Google Scholar] [CrossRef]
- Colloca, S.; Barnes, E.; Folgori, A.; Ammendola, V.; Capone, S.; Cirillo, A.; Siani, L.; Naddeo, M.; Grazioli, F.; Esposito, M.L.; et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 2012, 4, 115ra112. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.M.; Da Costa, A.; Yamamoto, A.; Berry, D.; Lindsay, R.W.; Darrah, P.A.; Wang, L.; Cheng, C.; Kong, W.P.; Gall, J.G.; et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013, 190, 2720–2735. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee adenovirus vector Ebola vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Gao, G.P.; Reyes-Sandoval, A.; Pavlakis, G.N.; Xiang, Z.Q.; Wlazlo, A.P.; Giles-Davis, W.; Wilson, J.M.; Ertl, H.C. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 2003, 170, 1416–1422. [Google Scholar] [CrossRef]
- Chen, E.C.; Yagi, S.; Kelly, K.R.; Mendoza, S.P.; Tarara, R.P.; Canfield, D.R.; Maninger, N.; Rosenthal, A.; Spinner, A.; Bales, K.L.; et al. Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony. PLoS Pathog. 2011, 7, e1002155. [Google Scholar] [CrossRef]
- Roy, S.; Vandenberghe, L.H.; Kryazhimskiy, S.; Grant, R.; Calcedo, R.; Yuan, X.; Keough, M.; Sandhu, A.; Wang, Q.; Medina-Jaszek, C.A.; et al. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog. 2009, 5, e1000503. [Google Scholar] [CrossRef]
- Roy, S.; Gao, G.; Lu, Y.; Zhou, X.; Lock, M.; Calcedo, R.; Wilson, J.M. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum. Gene Ther. 2004, 15, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef]
- Machitani, M.; Sakurai, F.; Wakabayashi, K.; Tachibana, M.; Fujiwara, T.; Mizuguchi, H. Enhanced oncolytic activities of the telomerase-specific replication-competent adenovirus expressing short-hairpin RNA against dicer. Mol. Cancer Ther. 2017, 16, 251–259. [Google Scholar] [CrossRef]
- Dudareva, M.; Andrews, L.; Gilbert, S.C.; Bejon, P.; Marsh, K.; Mwacharo, J.; Kai, O.; Nicosia, A.; Hill, A.V. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine 2009, 27, 3501–3504. [Google Scholar] [CrossRef]
- Capone, S.; Reyes-Sandoval, A.; Naddeo, M.; Siani, L.; Ammendola, V.; Rollier, C.S.; Nicosia, A.; Colloca, S.; Cortese, R.; Folgori, A.; et al. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine 2010, 29, 256–265. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, G.A.; Duncan, C.J.; Ewer, K.J.; Collins, K.A.; Elias, S.C.; Halstead, F.D.; Goodman, A.L.; Edwards, N.J.; Reyes-Sandoval, A.; Bird, P.; et al. Clinical assessment of a recombinant simian adenovirus ChAd63: A potent new vaccine vector. J. Infect. Dis. 2012, 205, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Rampling, T.; Ewer, K.J.; Bowyer, G.; Bliss, C.M.; Edwards, N.J.; Wright, D.; Payne, R.O.; Venkatraman, N.; de Barra, E.; Snudden, C.M.; et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS, S/AS01B with chimpanzee adenovirus 63 and modified vaccinia Ankara vectored vaccines expressing ME-TRAP. J. Infect. Dis. 2016, 214, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, S.H.; Duncan, C.J.; Elias, S.C.; Biswas, S.; Collins, K.A.; O’Hara, G.A.; Halstead, F.D.; Ewer, K.J.; Mahungu, T.; Spencer, A.J.; et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS ONE 2012, 7, e31208. [Google Scholar] [CrossRef]
- Sheehy, S.H.; Duncan, C.J.; Elias, S.C.; Collins, K.A.; Ewer, K.J.; Spencer, A.J.; Williams, A.R.; Halstead, F.D.; Moretz, S.E.; Miura, K.; et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol. Ther. 2011, 19, 2269–2276. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Lyke, K.E.; Haidara, F.C.; Diallo, F.; Doumbia, M.; Traore, A.; Coulibaly, F.; Kodio, M.; Onwuchekwa, U.; et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: A phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2016, 16, 31–42. [Google Scholar] [CrossRef]
- Stanley, D.A.; Honko, A.N.; Asiedu, C.; Trefry, J.C.; Lau-Kilby, A.W.; Johnson, J.C.; Hensley, L.; Ammendola, V.; Abbate, A.; Grazioli, F.; et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014, 20, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.D.; Sow, S.O.; Mbaye, K.D.; Thiongane, A.; Ndiaye, B.P.; Ndour, C.T.; Mboup, S.; Keshinro, B.; Kinge, T.N.; Vernet, G.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Ewer, K.; Rampling, T.; Venkatraman, N.; Bowyer, G.; Wright, D.; Lambe, T.; Imoukhuede, E.B.; Payne, R.; Fehling, S.K.; Strecker, T.; et al. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N. Engl. J. Med. 2016, 374, 1635–1646. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra111. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Mele, A.; Berton, A.; Ruggeri, L.; Ferrigno, L.; Garbuglia, A.R.; Perrone, M.P.; Girelli, G.; Del Porto, P.; Piccolella, E.; et al. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 2004, 53, 1673–1681. [Google Scholar] [CrossRef]
- Lechner, F.; Wong, D.K.; Dunbar, P.R.; Chapman, R.; Chung, R.T.; Dohrenwend, P.; Robbins, G.; Phillips, R.; Klenerman, P.; Walker, B.D. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 2000, 191, 1499–1512. [Google Scholar] [CrossRef]
- Folgori, A.; Capone, S.; Ruggeri, L.; Meola, A.; Sporeno, E.; Ercole, B.B.; Pezzanera, M.; Tafi, R.; Arcuri, M.; Fattori, E.; et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 2006, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef]
- Fischer, R.J.; Purushotham, J.N.; van Doremalen, N.; Sebastian, S.; Meade-White, K.; Cordova, K.; Letko, M.; Jeremiah Matson, M.; Feldmann, F.; Haddock, E.; et al. ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects guinea pigs against lethal Lassa virus challenge. NPJ Vaccines 2021, 6, 32. [Google Scholar] [CrossRef]
- Antrobus, R.D.; Coughlan, L.; Berthoud, T.K.; Dicks, M.D.; Hill, A.V.; Lambe, T.; Gilbert, S.C. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. 2014, 22, 668–674. [Google Scholar] [CrossRef]
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine 2018, 29, 146–154. [Google Scholar] [CrossRef]
- Von Delft, A.; Donnison, T.A.; Lourenço, J.; Hutchings, C.; Mullarkey, C.E.; Brown, A.; Pybus, O.G.; Klenerman, P.; Chinnakannan, S.; Barnes, E. The generation of a simian adenoviral vectored HCV vaccine encoding genetically conserved gene segments to target multiple HCV genotypes. Vaccine 2018, 36, 313–321. [Google Scholar] [CrossRef]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime–MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Griffiths, K.L.; Poyntz, H.C.; Harrington-Kandt, R.; Dicks, M.D.; Stockdale, L.; Betts, G.; McShane, H. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine 2015, 33, 6800–6808. [Google Scholar] [CrossRef]
- van Doremalen, N.; Haddock, E.; Feldmann, F.; Meade-White, K.; Bushmaker, T.; Fischer, R.J.; Okumura, A.; Hanley, P.W.; Saturday, G.; Edwards, N.J.; et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci. Adv. 2020, 6, eaba8399. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Swanson, P.A.; Padilla, M.; Hoyland, W.; McGlinchey, K.; Fields, P.A.; Bibi, S.; Faust, S.N.; McDermott, A.B.; Lambe, T.; Pollard, A.J.; et al. T-cell mediated immunity after AZD1222 vaccination: A polyfunctional spike-specific Th1 response with a diverse TCR repertoire. medRxiv 2021. [Google Scholar] [CrossRef]
- Morris, S.J.; Sebastian, S.; Spencer, A.J.; Gilbert, S.C. Simian adenoviruses as vaccine vectors. Future Virol. 2016, 11, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Bellamy, D.; Roberts, R.; Powlson, J.; Edwards, N.J.; Mair, C.F.; Bowyer, G.; Poulton, I.; Mitton, C.H.; Green, N.; et al. Safety and immunogenicity of a novel recombinant simian adenovirus ChAdOx2 as a vectored vaccine. Vaccines 2019, 7, 40. [Google Scholar] [CrossRef]
- Wang, C.; Dulal, P.; Zhou, X.; Xiang, Z.; Goharriz, H.; Banyard, A.; Green, N.; Brunner, L.; Ventura, R.; Collin, N.; et al. A simian-adenovirus-vectored rabies vaccine suitable for thermostabilisation and clinical development for low-cost single-dose pre-exposure prophylaxis. PLoS Negl. Trop. Dis. 2018, 12, e0006870. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Cicconi, P.; Jones, C.; Sarkar, E.; Silva-Reyes, L.; Klenerman, P.; de Lara, C.; Hutchings, C.; Moris, P.; Janssens, M.; Fissette, L.A.; et al. First-in-human randomized study to assess the safety and immunogenicity of an investigational respiratory syncytial virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector-expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clin. Infect. Dis. 2020, 70, 2073–2081. [Google Scholar] [CrossRef]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Napolitano, F.; Merone, R.; Abbate, A.; Ammendola, V.; Horncastle, E.; Lanzaro, F.; Esposito, M.; Contino, A.M.; Sbrocchi, R.; Sommella, A.; et al. A next generation vaccine against human rabies based on a single dose of a chimpanzee adenovirus vector serotype C. PLoS Negl. Trop. Dis. 2020, 14, e0008459. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Scarselli, E.; Sande, C.J.; Thompson, A.J.; de Lara, C.M.; Taylor, K.S.; Haworth, K.; Del Sorbo, M.; Angus, B.; Siani, L.; et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci. Transl. Med. 2015, 7, 300ra126. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Sande, C.J.; Scarselli, E.; Capone, S.; Vitelli, A.; Nicosia, A.; Silva-Reyes, L.; Thompson, A.J.; de Lara, C.M.; Taylor, K.S.; et al. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J. Infect. 2019, 78, 382–392. [Google Scholar] [CrossRef]
- Abbink, P.; Maxfield, L.F.; Ng’ang’a, D.; Borducchi, E.N.; Iampietro, M.J.; Bricault, C.A.; Teigler, J.E.; Blackmore, S.; Parenteau, L.; Wagh, K.; et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J. Virol. 2015, 89, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, M.; Zhang, C.; Yang, Y.; Chi, Y.; Tang, X.; Zhang, H.; Xiong, S.; Yu, L.; Zhou, D. Neutralizing antibody responses to enterovirus and adenovirus in healthy adults in China. Emerg. Microbes Infect. 2014, 3, e30. [Google Scholar] [CrossRef]
- Zhi, Y.; Figueredo, J.; Kobinger, G.P.; Hagan, H.; Calcedo, R.; Miller, J.R.; Gao, G.; Wilson, J.M. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 2006, 17, 500–506. [Google Scholar] [CrossRef]
- Xu, K.; Song, Y.; Dai, L.; Zhang, Y.; Lu, X.; Xie, Y.; Zhang, H.; Cheng, T.; Wang, Q.; Huang, Q.; et al. Recombinant chimpanzee adenovirus vaccine AdC7-M/E protects against Zika virus infection and testis damage. J. Virol. 2018, 92, e01722-17. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, X.; Song, Y.; Tang, X.; Zhang, C.; Zhang, H.; Jin, X.; Zhou, D. Chimpanzee adenovirus vector-based avian influenza vaccine completely protects mice against lethal challenge of H5N1. Vaccine 2016, 34, 4875–4883. [Google Scholar] [CrossRef]
- Chen, T.; Li, D.; Song, Y.; Yang, X.; Liu, Q.; Jin, X.; Zhou, D.; Huang, Z. A heterologous prime-boost Ebola virus vaccine regimen induces durable neutralizing antibody response and prevents Ebola virus-like particle entry in mice. Antiviral. Res. 2017, 145, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kobinger, G.P.; Figueredo, J.M.; Rowe, T.; Zhi, Y.; Gao, G.; Sanmiguel, J.C.; Bell, P.; Wivel, N.A.; Zitzow, L.A.; Flieder, D.B.; et al. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine 2007, 25, 5220–5231. [Google Scholar] [CrossRef]
- Sharma, A.; Wendland, R.; Sung, B.; Wu, W.; Grunwald, T.; Worgall, S. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine 2014, 32, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W. CHAPTER 42–Viral Diseases of the Bovine Respiratory Tract. In Food Animal Practice, 5th ed.; Anderson, D.E., Rings, D.M., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2009; pp. 171–191. [Google Scholar] [CrossRef]
- Harrach, B.; Tarján, Z.L.; Benkő, M. Adenoviruses across the animal kingdom: A walk in the zoo. FEBS Lett. 2019, 593, 3660–3673. [Google Scholar] [CrossRef]
- Mittal, S.K.; Prevec, L.; Graham, F.L.; Babiuk, L.A. Development of a bovine adenovirus type 3-based expression vector. J. Gen. Virol. 1995, 76, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zakhartchouk, A.N.; Reddy, P.S.; Baxi, M.; Baca-Estrada, M.E.; Mehtali, M.; Babiuk, L.A.; Tikoo, S.K. Construction and characterization of E3-deleted bovine adenovirus type 3 expressing full-length and truncated form of bovine herpesvirus type 1 glycoprotein gD. Virology 1998, 250, 220–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, P.S.; Idamakanti, N.; Chen, Y.; Whale, T.; Babiuk, L.A.; Mehtali, M.; Tikoo, S.K. Replication-defective bovine adenovirus type 3 as an expression vector. J. Virol. 1999, 73, 9137–9144. [Google Scholar] [CrossRef]
- Reddy, P.S.; Idamakanti, N.; Zakhartchouk, L.N.; Babiuk, L.A.; Mehtali, M.; Tikoo, S.K. Optimization of bovine coronavirus hemagglutinin-estrase glycoprotein expression in E3 deleted bovine adenovirus-3. Virus Res. 2000, 70, 65–73. [Google Scholar] [CrossRef]
- Baxi, M.K.; Deregt, D.; Robertson, J.; Babiuk, L.A.; Schlapp, T.; Tikoo, S.K. Recombinant bovine adenovirus type 3 expressing bovine viral diarrhea virus glycoprotein E2 induces an immune response in cotton rats. Virology 2000, 278, 234–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittal, S.K.; Middleton, D.M.; Tikoo, S.K.; Babiuk, L.A. Pathogenesis and immunogenicity of bovine adenovirus type 3 in cotton rats (Sigmodon hispidus). Virology 1995, 213, 131–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittal, S.K.; Tikoo, S.K.; Van Donkersgoed, J.; Beskorwayne, T.; Godson, D.L.; Babiuk, L.A. Experimental inoculation of heifers with bovine adenovirus type 3. Can. J. Vet. Res. 1999, 63, 153–156. [Google Scholar] [PubMed]
- Sayedahmed, E.E.; Hassan, A.O.; Kumari, R.; Cao, W.; Gangappa, S.; York, I.; Sambhara, S.; Mittal, S.K. A bovine adenoviral vector-based H5N1 influenza -vaccine provides enhanced immunogenicity and protection at a significantly low dose. Mol. Ther. Methods Clin. Dev. 2018, 10, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.; Kumar, P.; Babiuk, L.A.; Tikoo, S.K. Recombinant bovine adenovirus-3 co-expressing bovine respiratory syncytial virus glycoprotein G and truncated glycoprotein gD of bovine herpesvirus-1 induce immune responses in cotton rats. Mol. Biotechnol. 2015, 57, 58–64. [Google Scholar] [CrossRef]
- Bangari, D.S.; Sharma, A.; Mittal, S.K. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem. Biophys. Res. Commun. 2005, 331, 1478–1484. [Google Scholar] [CrossRef]
- Li, X.; Bangari, D.S.; Sharma, A.; Mittal, S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 2009, 392, 162–168. [Google Scholar] [CrossRef]
- Tandon, M.; Sharma, A.; Vemula, S.V.; Bangari, D.S.; Mittal, S.K. Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 2012, 163, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tandon, M.; Ahi, Y.S.; Bangari, D.S.; Vemulapalli, R.; Mittal, S.K. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010, 17, 634–642. [Google Scholar] [CrossRef]
- Sharma, A.; Bangari, D.S.; Tandon, M.; Hogenesch, H.; Mittal, S.K. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 2010, 153, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bangari, D.S.; Tandon, M.; Pandey, A.; HogenEsch, H.; Mittal, S.K. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology 2009, 386, 44–54. [Google Scholar] [CrossRef]
- Sharma, A.; Bangari, D.S.; Vemula, S.V.; Mittal, S.K. Persistence and the state of bovine and porcine adenoviral vector genomes in human and nonhuman cell lines. Virus Res. 2011, 161, 181–187. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, A.; Jayashankar, L.; Mittal, S.K. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 2008, 16, 965–971. [Google Scholar] [CrossRef]
- Khan, A.; Sayedahmed, E.E.; Singh, V.; Mishra, A.; Dorta-Estremera, S.; Nookala, S.; Canaday, D.; Chen, M.; Wang, J.; Sastry, J.; et al. A recombinant bovine adenoviral mucosal vaccine expressing mycobacterial antigen-85B generates robust protection against tuberculosis in mice. Cell Rep. Med. 2021, in press. [Google Scholar]
- Fernandes, P.; Peixoto, C.; Santiago, V.M.; Kremer, E.J.; Coroadinha, A.S.; Alves, P.M. Bioprocess development for canine adenovirus type 2 vectors. Gene Ther. 2013, 20, 353–360. [Google Scholar] [CrossRef]
- Klonjkowski, B.; Gilardi-Hebenstreit, P.; Hadchouel, J.; Randrianarison, V.; Boutin, S.; Yeh, P.; Perricaudet, M.; Kremer, E.J. A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum. Gene Ther. 1997, 8, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Faber, M.; Papaneri, A.; Faber, M.L.; McGettigan, J.P.; Schnell, M.J.; Dietzschold, B. A single immunization with a recombinant canine adenovirus expressing the rabies virus G protein confers protective immunity against rabies in mice. Virology 2006, 356, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, S.; Fooks, A.R.; Yuan, H.; Liu, Y.; Li, H.; Tu, C.; Xia, X.; Xiao, Y. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes Infect. 2006, 8, 1090–1097. [Google Scholar] [CrossRef]
- Bouet-Cararo, C.; Contreras, V.; Fournier, A.; Jallet, C.; Guibert, J.M.; Dubois, E.; Thiery, R.; Bréard, E.; Tordo, N.; Richardson, J.; et al. Canine adenoviruses elicit both humoral and cell-mediated immune responses against rabies following immunisation of sheep. Vaccine 2011, 29, 1304–1310. [Google Scholar] [CrossRef]
- Hu, R.L.; Liu, Y.; Zhang, S.F.; Zhang, F.; Fooks, A.R. Experimental immunization of cats with a recombinant rabies-canine adenovirus vaccine elicits a long-lasting neutralizing antibody response against rabies. Vaccine 2007, 25, 5301–5307. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Ma, G.; Zhang, F.; Hu, R. Efficacy and safety of a live canine adenovirus-vectored rabies virus vaccine in swine. Vaccine 2008, 26, 5368–5372. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschauwer, A.R.; Zhou, X.; Lefebvre, D.J.; Garnier, A.; Watier, F.; Pignon, C.; Lacour, S.A.; Zientara, S.; Bakkali-Kassimi, L.; De Clercq, K.; et al. A canine adenovirus type 2 vaccine vector confers protection against foot-and-mouth disease in guinea pigs. Vaccine 2018, 36, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, Z.; Liu, J.S.; Kong, D.S.; Guo, D.C.; Quan, C.S.; Li, B.T.; Hu, X.L.; Qu, L. Recombinant canine adenovirus type 2 expressing rabbit hemorrhagic disease virus VP60 protein provided protection against RHD in rabbits. Vet. Microbiol. 2018, 213, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Wang, X.H.; Xia, L.J.; Weng, Y.B.; Hernandez, J.A.; Tu, L.Q.; Li, L.T.; Li, S.J.; Yuan, Z.G. Protective efficacy of recombinant canine adenovirus type-2 expressing TgROP18 (CAV-2-ROP18) against acute and chronic Toxoplasma gondii infection in mice. BMC Infect. Dis. 2015, 15, 114. [Google Scholar] [CrossRef]
- Bangari, D.S.; Shukla, S.; Mittal, S.K. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem. Biophys. Res. Commun. 2005, 327, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Idamakanti, N.; Hyun, B.H.; Tikoo, S.K.; Babiuk, L.A. Development of porcine adenovirus-3 as an expression vector. J. Gen. Virol. 1999, 80, 563–570. [Google Scholar] [CrossRef]
- Zhou, Y.; Tikoo, S.K. Analysis of early region 1 of porcine adenovirus type 3. Virology 2001, 291, 68–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleiboeker, S.B. Sequence analysis of putative E3, pVIII, and fiber genomic regions of a porcine adenovirus. Virus Res. 1994, 31, 17–25. [Google Scholar] [CrossRef]
- Hammond, J.M.; McCoy, R.J.; Jansen, E.S.; Morrissy, C.J.; Hodgson, A.L.; Johnson, M.A. Vaccination with a single dose of a recombinant porcine adenovirus expressing the classical swine fever virus gp55 (E2) gene protects pigs against classical swine fever. Vaccine 2000, 18, 1040–1050. [Google Scholar] [CrossRef]
- Hammond, J.M.; Jansen, E.S.; Morrissy, C.J.; van der Heide, B.; Goff, W.V.; Williamson, M.M.; Hooper, P.T.; Babiuk, L.A.; Tikoo, S.K.; Johnson, M.A. Vaccination of pigs with a recombinant porcine adenovirus expressing the gD gene from pseudorabies virus. Vaccine 2001, 19, 3752–3758. [Google Scholar] [CrossRef]
- Tuboly, T.; Nagy, É. Construction and characterization of recombinant porcine adenovirus serotype 5 expressing the transmissible gastroenteritis virus spike gene. J. Gen. Virol. 2001, 82, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Tuboly, T. Porcine adenoviruses: An update on genome analysis and vector development. Acta Vet. Hung. 2000, 48, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.M.; Johnson, M.A. Porcine adenovirus as a delivery system for swine vaccines and immunotherapeutics. Vet. J. 2005, 169, 17–27. [Google Scholar] [CrossRef]
- Bangari, D.S.; Mittal, S.K. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004, 105, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bangari, D.S.; Mittal, S.K. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 2006, 24, 849–862. [Google Scholar] [CrossRef]
- Zakhartchouk, A.; Zhou, Y.; Tikoo, S.K. A recombinant E1-deleted porcine adenovirus-3 as an expression vector. Virology 2003, 313, 377–386. [Google Scholar] [CrossRef][Green Version]
- Patel, A.; Tikoo, S.; Kobinger, G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PLoS ONE 2010, 5, e15301. [Google Scholar] [CrossRef] [PubMed]
- Vidovszky, M.Z.; Szeredi, L.; Doszpoly, A.; Harrach, B.; Hornyák, Á. Isolation and complete genome sequence analysis of a novel ovine adenovirus type representing a possible new mastadenovirus species. Arch. Virol. 2019, 164, 2205–2207. [Google Scholar] [CrossRef]
- Both, G.W. Ovine atadenovirus: A review of its biology, biosafety profile and application as a gene delivery vector. Immunol. Cell Biol. 2004, 82, 189–195. [Google Scholar] [CrossRef]
- Pantelic, R.S.; Lockett, L.J.; Rothnagel, R.; Hankamer, B.; Both, G.W. Cryoelectron microscopy map of Atadenovirus reveals cross-genus structural differences from human adenovirus. J. Virol. 2008, 82, 7346–7356. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Both, G.W. Altered tropism of an ovine adenovirus carrying the fiber protein cell binding domain of human adenovirus type 5. Virology 1998, 248, 156–163. [Google Scholar] [CrossRef][Green Version]
- Bridgeman, A.; Roshorm, Y.; Lockett, L.J.; Xu, Z.Z.; Hopkins, R.; Shaw, J.; Both, G.W.; Hanke, T. Ovine atadenovirus, a novel and highly immunogenic vector in prime-boost studies of a candidate HIV-1 vaccine. Vaccine 2009, 28, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.K.; Diener, K.R.; Lousberg, E.L.; Both, G.W.; Ward, L.; Brown, M.P.; Hayball, J.D. Induction of both cellular and humoral immunity following a rational prime-boost immunization regimen that incorporates recombinant ovine atadenovirus and fowlpox virus. Clin. Vaccine Immunol. 2010, 17, 1679–1686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wüest, T.; Both, G.W.; Prince, A.M.; Hofmann, C.; Löser, P. Recombinant ovine atadenovirus induces a strong and sustained T cell response against the hepatitis C virus NS3 antigen in mice. Vaccine 2004, 22, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Li, K.; Wilson, M.; Both, G.W.; Taylor, J.A.; Young, S.L. Potent antietumor immunity in mice induced by vaccination with an ovine atadenovirus vector. J. Immunother. 2012, 35, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ojkic, D.; Nagy, E. The complete nucleotide sequence of fowl adenovirus type 8. J. Gen. Virol. 2000, 81, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Ojkić, D.; Krell, P.J.; Tuboly, T.; Nagy, E. Characterization of fowl adenoviruses isolated in Ontario and Quebec, Canada. Can. J. Vet. Res. 2008, 72, 236–241. [Google Scholar] [PubMed]
- Corredor, J.C.; Garceac, A.; Krell, P.J.; Nagy, E. Sequence comparison of the right end of fowl adenovirus genomes. Virus Genes 2008, 36, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, M.; Werner, W.; Tsatas, E.; McCoy, R.; Prowse, S.; Johnson, M. Fowl adenovirus recombinant expressing VP2 of infectious bursal disease virus induces protective immunity against bursal disease. Arch. Virol. 1998, 143, 915–930. [Google Scholar] [CrossRef]
- Johnson, M.A.; Pooley, C.; Ignjatovic, J.; Tyack, S.G. A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine 2003, 21, 2730–2736. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- David, P.; Dotan, A.; Mahroum, N.; Shoenfeld, Y. Immune thrombocytopenic purpura (ITP) triggered by COVID-19 infection and vaccination. Isr. Med. Assoc. J. 2021, 23, 378–380. [Google Scholar]
- Sayedahmed, E.E.; Kumari, R.; Shukla, S.; Hassan, A.O.; Mohammed, S.I.; York, I.A.; Gangappa, S.; Sambhara, S.; Mittal, S.K. Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy. Vaccine 2018, 36, 6744–6751. [Google Scholar] [CrossRef] [PubMed]
| Prototype Strain of Non-Human Adenovirus | Max Transgene Insertion Capacity | Deletion/Insertion Sites | Primary Receptor Utilized | Cell Line Used for Propagation |
|---|---|---|---|---|
| Simian adenovirus type 25 | ~8 kb | E1 and E3 regions | CAR | HEK293 cells or any HAd5 E1 transformed cell lines |
| Bovine adenovirus type 3 | ~5.5 kb | E1 and E3 regions | α(2,3)-linked and α(2,6)-linked SA | Bovine-human hybrid cells (BHH3 and BHH8); BAd3 E1 complementing cell lines; or HAd5 E1 transformed fetal bovine retinal cells (VIDO R2 and FBRT HE1) |
| Canine adenovirus type 2 | ~4.0 kb | E1 and E3 regions | CAR | CAd2 E1 transformed DK cells |
| Ovine adenovirus type 7 | ~6.3 kb | Site I: pVIII and fiber intergenic region; Site II: unique SalI site within ORF RH2; & Site III: region between the putative E4 transcription units and the right end | INT | Ovine Fetal skin fibroblast producer cell line (HVO156) or Sheep fetal lung cells (CSL503) |
| Porcine adenovirus type 3 | ~4.7 kb | E1 and E3 regions | HAd5 E1 transformed porcine fetal retinal cell lines (VIDO R1 and FPRT HE1-5) | |
| Avian adenovirus (Fowl adenovirus type 1) | ~4.0 kb | Region between E4 promotor and right ITR; Region between 938 and 2900 (requires trans-complementation); and Three ORFs adjacent to right end of the genome | CAR | Leghorn male hepatoma (LMH) cell line |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhashimi, M.; Elkashif, A.; Sayedahmed, E.E.; Mittal, S.K. Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses 2021, 13, 1493. https://doi.org/10.3390/v13081493
Alhashimi M, Elkashif A, Sayedahmed EE, Mittal SK. Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses. 2021; 13(8):1493. https://doi.org/10.3390/v13081493
Chicago/Turabian StyleAlhashimi, Marwa, Ahmed Elkashif, Ekramy E. Sayedahmed, and Suresh K. Mittal. 2021. "Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases" Viruses 13, no. 8: 1493. https://doi.org/10.3390/v13081493
APA StyleAlhashimi, M., Elkashif, A., Sayedahmed, E. E., & Mittal, S. K. (2021). Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses, 13(8), 1493. https://doi.org/10.3390/v13081493








