Neural Stem Cells: What Happens When They Go Viral?
Abstract
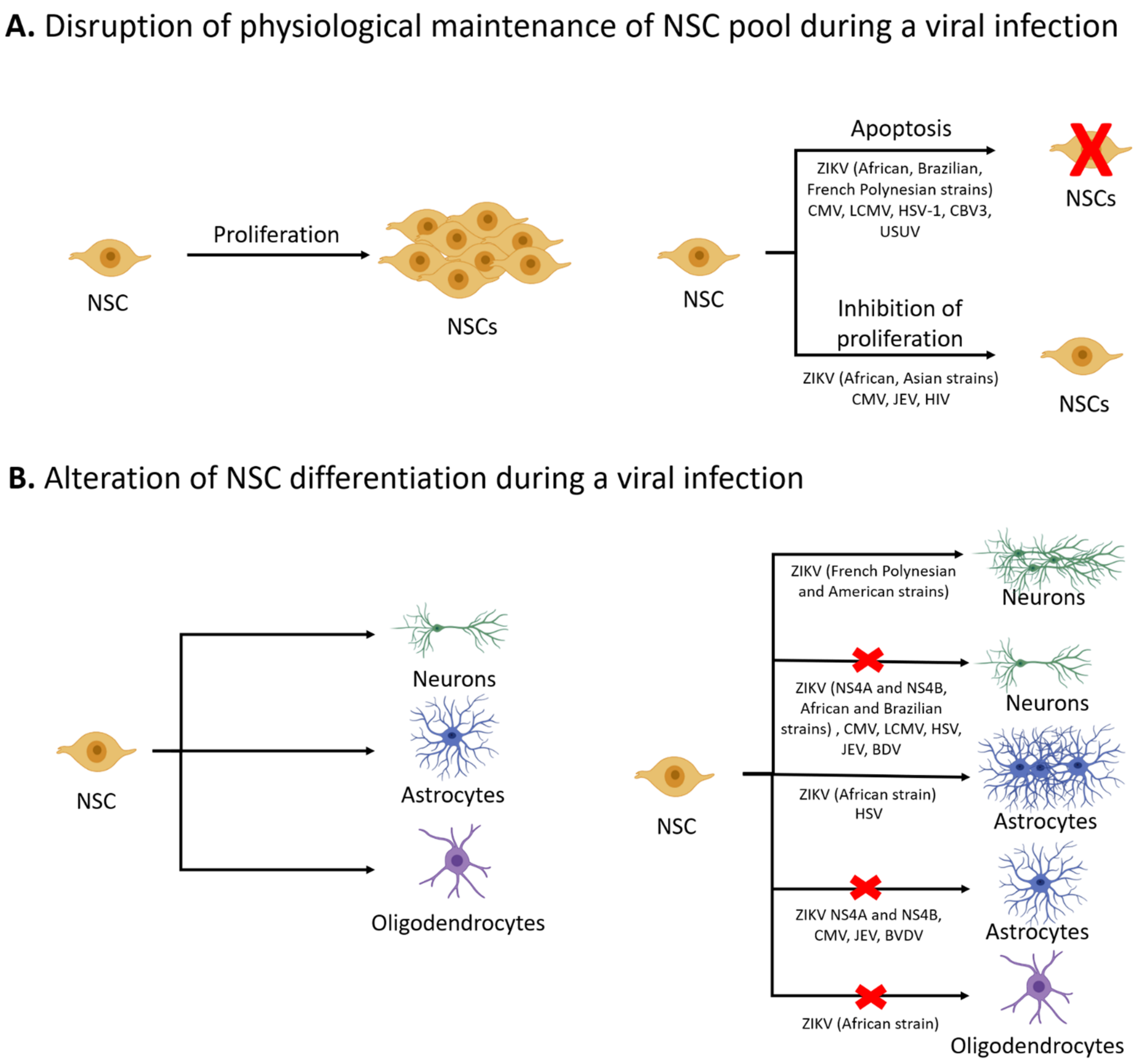
1. Introduction
2. NSC Survival and Proliferation
3. Differentiation of NSCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ariff, I.M.; Thounaojam, M.C.; Das, S.; Basu, A. Japanese Encephalitis Virus Infection Alters Both Neuronal and Astrocytic Differentiation of Neural Stem/Progenitor Cells. J. Neuroimmune Pharmacol. 2013, 8, 664–676. [Google Scholar] [CrossRef]
- Brnic, D.; Stevanovic, V.; Cochet, M.; Agier, C.; Richardson, J.; Montero-Menei, C.N.; Milhavet, O.; Eloit, M.; Coulpier, M. Borna Disease Virus Infects Human Neural Progenitor Cells and Impairs Neurogenesis. J. Virol. 2012, 86, 2512–2522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonthius, D.J. Lymphocytic Choriomeningitis Virus: An Underrecognized Cause of Neurologic Disease in the Fetus, Child, and Adult. Semin. Pediatric Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Basu, A. Japanese encephalitis virus infects neural progenitor cells and decreases their proliferation. J. Neurochem. 2008, 106, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Mutnal, M.B.; Cheeran, M.C.-J.; Hu, S.; Lokensgard, J.R. Murine Cytomegalovirus Infection of Neural Stem Cells Alters Neurogenesis in the Developing Brain. PLoS ONE 2011, 6, e16211. [Google Scholar] [CrossRef]
- Puccini, J.M.; Ruller, C.M.; Robinson, S.M.; Knopp, K.A.; Buchmeier, M.J.; Doran, K.S.; Feuer, R. Distinct neural stem cell tropism, early immune activation, and choroid plexus pathology following coxsackievirus infection in the neonatal central nervous system. Lab. Investig. 2014, 94, 161–181. [Google Scholar] [CrossRef]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Moore, D.L. Neural stem cells: Developmental mechanisms and disease modeling. Cell Tissue Res. 2018, 371, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.; Repic, M.; Knoblich, J.A. Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef]
- Liszewska, E.; Jaworski, J. Neural Stem Cell Dysfunction in Human Brain Disorders. Chem. Biol. Pteridines Folates 2018, 66, 283–305. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Gonzalez-Perez, O. Neural stem cells in the adult human brain. Biol. Biomed. Rep. 2012, 2, 59–69. [Google Scholar] [PubMed]
- Berdugo-Vega, G.; Arias-Gil, G.; López-Fernández, A.; Artegiani, B.; Wasielewska, J.M.; Lee, C.-C.; Lippert, M.T.; Kempermann, G.; Takagaki, K.; Calegari, F. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. The development of neural stem cells. Nat. Cell Biol. 2001, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Chandwani, M.N.; Creisher, P.S.; O’Donnell, L.A. Understanding the Role of Antiviral Cytokines and Chemokines on Neural Stem/Progenitor Cell Activity and Survival. Viral Immunol. 2019, 32, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S. Cytokine Control of Adult Neural Stem Cells. Ann. N.Y. Acad. Sci. 2009, 1153, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.A.; Palmer, T.D. Immune Influence on Adult Neural Stem Cell Regulation and Function. Neuron 2009, 64, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Latchney, S.E.; Eisch, A.J. Therapeutic application of neural stem cells and adult neurogenesis for neurodegenerative disorders: Regeneration and beyond. Eur. J. Neurodegener. Dis. 2012, 1, 335–351. [Google Scholar]
- Yau, S.-Y.; Li, A.; So, K.-F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef]
- Barton, L.L.; Peters, C.J.; Ksiazek, T.G.; Barton, L.L.; Peters, C.J.; Ksiazek, T.G. Lymphocytic Choriomeningitis Virus: An Unrecognized Teratogenic Pathogen. Emerg. Infect. Dis. 1995, 1, 152–153. [Google Scholar] [CrossRef]
- Dutta, K.; Rangarajan, P.N.; Vrati, S.; Basu, A. Japanese encephalitis: Pathogenesis, prophylactics and therapeutics. Curr. Sci. 2010, 98, 326–334. [Google Scholar]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Puma, D.D.L.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; O Melo, A.S.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Leal, M.D.C.; Muniz, L.F.; Neto, S.D.S.C.; Van Der Linden, V.; Ramos, R.C.F. Sensorineural hearing loss in a case of congenital Zika virus. Braz. J. Otorhinolaryngol. 2020, 86, 513–515. [Google Scholar] [CrossRef]
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat. Rev. Neurosci. 2019, 20, 34–48. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; De Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Altinisik, N.; Gopalakrishnan, J. Human Brain Organoids to Decode Mechanisms of Microcephaly. Front. Cell. Neurosci. 2020, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Hammack, C.; Ogden, S.C.; Madden, J.C.; Medina, A.; Xu, C.; Phillips, E.; Son, Y.; Cone, A.; Giovinazzi, S.; Didier, R.A.; et al. Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication. J. Virol. 2019, 93, e00638-19. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.; Wolmer, N.; Falci, S.; Westgren, M.; Seiger, A.; Söderberg-Nauclér, C. Human Cytomegalovirus Inhibits Neuronal Differentiation and Induces Apoptosis in Human Neural Precursor Cells. J. Virol. 2006, 80, 8929–8939. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, I.; Shinmura, Y.; Kawasaki, H.; Arai, Y.; Li, R.-Y.; Baba, S.; Tsutsui, Y. Cytomegalovirus Infection of the Central Nervous System Stem Cells from Mouse Embryo: A Model for Developmental Brain Disorders Induced by Cytomegalovirus. Lab. Investig. 2000, 80, 1373–1383. [Google Scholar] [CrossRef]
- Nakamura, H.; Liao, H.; Minami, K.; Toyoda, M.; Akutsu, H.; Miyagawa, Y.; Okita, H.; Kiyokawa, N.; Umezawa, A.; Imadome, K.-I.; et al. Human cytomegalovirus induces apoptosis in neural stem/progenitor cells derived from induced pluripotent stem cells by generating mitochondrial dysfunction and endoplasmic reticulum stress. Herpesviridae 2013, 4, 2. [Google Scholar] [CrossRef]
- Okamoto, S.-I.; Kang, Y.-J.; Brechtel, C.W.; Siviglia, E.; Russo, R.; Clemente, A.; Harrop, A.; McKercher, S.; Kaul, M.; Lipton, S.A. HIV/gp120 Decreases Adult Neural Progenitor Cell Proliferation via Checkpoint Kinase-Mediated Cell-Cycle Withdrawal and G1 Arrest. Cell Stem Cell 2007, 1, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Jiang, X.; Huang, S.-N.; Sun, J.-Y.; Zhao, F.; Zeng, W.-B.; Luo, M.-H. Human cytomegalovirus infection dysregulates neural progenitor cell fate by disrupting Hes1 rhythm and down-regulating its expression. Virol. Sin. 2017, 32, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Guillemot, F. HES1, two programs: Promoting the quiescence and proliferation of adult neural stem cells. Genes Dev. 2019, 33, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Kumari, R.; Schwamborn, J.C.; Mahadevan, A.; Shankar, S.K.; Raja, R.; Seth, P. Tripartite containing motif 32 modulates proliferation of human neural precursor cells in HIV-1 neurodegeneration. Cell Death Differ. 2016, 23, 776–786. [Google Scholar] [CrossRef]
- Sun, T.; Vasek, M.; Klein, R.S. Congenitally Acquired Persistent Lymphocytic Choriomeningitis Viral Infection Reduces Neuronal Progenitor Pools in the Adult Hippocampus and Subventricular Zone. PLoS ONE 2014, 9, e96442. [Google Scholar] [CrossRef]
- Huang, H.-I.; Lin, J.-Y.; Chen, H.-H.; Yeh, S.-B.; Kuo, R.-L.; Weng, K.-F.; Shih, S.-R. Enterovirus 71 infects brain-derived neural progenitor cells. Virology 2014, 468-470, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Feuer, R.; Pagarigan, R.R.; Harkins, S.; Liu, F.; Hunziker, I.P.; Whitton, J.L. Coxsackievirus Targets Proliferating Neuronal Progenitor Cells in the Neonatal CNS. J. Neurosci. 2005, 25, 2434–2444. [Google Scholar] [CrossRef]
- Ruller, C.M.; Tabor-Godwin, J.M.; Van Deren, D.A.; Robinson, S.M.; Maciejewski, S.; Gluhm, S.; Gilbert, P.E.; An, N.; Gude, N.A.; Sussman, M.A.; et al. Neural Stem Cell Depletion and CNS Developmental Defects After Enteroviral Infection. Am. J. Pathol. 2012, 180, 1107–1120. [Google Scholar] [CrossRef]
- Riccetti, S.; Sinigaglia, A.; Desole, G.; Nowotny, N.; Trevisan, M.; Barzon, L. Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells. Viruses 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Singh, N.; Sengupta, N.; Fatima, M.; Seth, P.; Mahadevan, A.; Shankar, S.K.; Bhattacharyya, A.; Basu, A. Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis. 2017, 8, e2556. [Google Scholar] [CrossRef]
- Choi, J.-A.; Song, C.-H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front. Immunol. 2019, 10, 3147. [Google Scholar] [CrossRef]
- Jheng, J.R.; Ho, J.Y.; Horng, J.T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014, 5, 388. [Google Scholar] [CrossRef]
- Zheng, W.; Klammer, A.M.; Naciri, J.N.; Yeung, J.; Demers, M.; Milosevich, J.; Kinchington, P.R.; Bloom, D.C.; Nimgaonkar, V.L.; D’Aiuto, L. Patterns of Herpes Simplex Virus 1 Infection in Neural Progenitor Cells. J. Virol. 2020, 94, e00994-20. [Google Scholar] [CrossRef]
- Qiao, H.; Guo, M.; Shang, J.; Zhao, W.; Wang, Z.; Liu, N.; Li, B.; Zhou, Y.; Wu, Y.; Chen, P. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLOS Pathog. 2020, 16, e1008899. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Nakayama, E.; Takahashi, K.; Taniguchi, S.; Shibasaki, K.-I.; Kato, F.; Maeki, T.; Suzuki, T.; Tajima, S.; Saijo, M.; et al. Increased growth ability and pathogenicity of American- and Pacific-subtype Zika virus (ZIKV) strains compared with a Southeast Asian-subtype ZIKV strain. PLoS Negl. Trop. Dis. 2019, 13, e0007387. [Google Scholar] [CrossRef]
- Ramos da Silva, S.; Ramos da Silva, S.; Cheng, F.; Huang, I.C.; Jung, J.U.; Gao, S.J. Efficiencies and kinetics of infection in different cell types/lines by African and Asian strains of Zika virus. J. Med. Virol. 2019, 91, 179–189. [Google Scholar] [CrossRef]
- Simonin, Y.; Van Riel, D.; Van de Perre, P.; Rockx, B.; Salinas, S. Differential virulence between Asian and African lineages of Zika virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.K.; Swiney, B.S.; Williams, S.L.; Huffman, J.N.; Lucas, K.; Wang, S.H.; Kapral, K.M.; Li, A.; Dikranian, K.T. Zika Virus Infection in the Developing Mouse Produces Dramatically Different Neuropathology Dependent on Viral Strain. J. Neurosci. 2020, 40, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Dang, J.W.; Lin, N.; Qin, Y.; Wang, S.; Rana, T.M. Zika virus depletes neural stem cells and evades selective autophagy by suppressing the Fanconi anemia protein FANCC. EMBO Rep. 2020, 21, e49183. [Google Scholar] [CrossRef]
- Lossia, O.V.; Conway, M.J.; Tree, M.O.; Williams, R.J.; Goldthorpe, S.C.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. Zika virus induces astrocyte differentiation in neural stem cells. J. NeuroVirology 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chavali, P.L.; Stojic, L.; Meredith, L.W.; Joseph, N.; Nahorski, M.S.; Sanford, T.J.; Sweeney, T.R.; Krishna, B.A.; Hosmillo, M.; Firth, A.E.; et al. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 2017, 357, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Kawahara, H.; Toriya, M.; Nakao, K.; Shibata, S.; Imai, T. Function of RNA-binding protein Musashi-1 in stem cells. Exp. Cell Res. 2005, 306, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Devhare, P.; Meyer, K.; Steele, R.; Ray, R.B.; Ray, R. Zika virus infection dysregulates human neural stem cell growth and inhibits differentiation into neuroprogenitor cells. Cell Death Dis. 2017, 8, e3106. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Gauthier, A.S. Timing Is Everything: Making Neurons versus Glia in the Developing Cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef]
- Hanners, N.W.; Eitson, J.L.; Usui, N.; Richardson, R.B.; Wexler, E.M.; Konopka, G.; Schoggins, J.W. Western Zika Virus in Human Fetal Neural Progenitors Persists Long Term with Partial Cytopathic and Limited Immunogenic Effects. Cell Rep. 2016, 15, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Tsueng, G.; Tabor-Godwin, J.M.; Gopal, A.; Ruller, C.M.; Deline, S.; An, N.; Frausto, R.F.; Milner, R.; Crocker, S.J.; Whitton, J.L.; et al. Coxsackievirus Preferentially Replicates and Induces Cytopathic Effects in Undifferentiated Neural Progenitor Cells. J. Virol. 2011, 85, 5718–5732. [Google Scholar] [CrossRef]
- Krathwohl, M.D.; Kaiser, J.L. HIV-1 Promotes Quiescence in Human Neural Progenitor Cells. J. Infect. Dis. 2004, 190, 216–226. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Leone, L.; Gironi, K.; Marcocci, M.E.; De Chiara, G.; Palamara, A.T.; Grassi, C. Herpes Simplex Virus Type-1 Infection Impairs Adult Hippocampal Neurogenesis via Amyloid-beta Protein Accumulation. Stem Cells 2019, 37, 1467–1480. [Google Scholar] [CrossRef]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.-L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef]
- Okano, H.; Temple, S. Cell types to order: Temporal specification of CNS stem cells. Curr. Opin. Neurobiol. 2009, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106-107, 1–16. [Google Scholar] [CrossRef]
- He, F.; Ge, W.; Martinowich, K.; Becker-Catania, S.; Coskun, V.; Zhu, W.; Wu, H.; Castro, D.; Guillemot, F.; Fan, G.; et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat. Neurosci. 2005, 8, 616–625. [Google Scholar] [CrossRef]
- Alonso, M.; Viollet, C.; Gabellec, M.-M.; Meas-Yedid, V.; Olivo-Marin, J.-C.; Lledo, P.-M. Olfactory Discrimination Learning Increases the Survival of Adult-Born Neurons in the Olfactory Bulb. J. Neurosci. 2006, 26, 10508–10513. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.; et al. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Dupret, D.; Revest, J.-M.; Koehl, M.; Ichas, F.; De Giorgi, F.; Costet, P.; Abrous, D.N.; Piazza, P.V. Spatial Relational Memory Requires Hippocampal Adult Neurogenesis. PLoS ONE 2008, 3, e1959. [Google Scholar] [CrossRef]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.-L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y. Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides. Hum. Cell 2019, 32, 88–94. [Google Scholar] [CrossRef]
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Barnes, C.C.; Pierce, K. Neuron Number and Size in Prefrontal Cortex of Children with Autism. JAMA 2011, 306, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.L.; Casanova, M.F. Genetics studies indicate that neural induction and early neuronal maturation are disturbed in autism. Front. Cell. Neurosci. 2014, 8, 397. [Google Scholar] [CrossRef]
- Pang, Y.; Fan, L.-W. Dysregulation of neurogenesis by neuroinflammation: Key differences in neurodevelopmental and neurological disorders. Neural Regen. Res. 2017, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Lie, D.C.; Rockenstein, E.; Aigner, R.; Aigner, L.; Masliah, E.; Kuhn, H.G.; Winkler, J. Human Wild-Type α-Synuclein Impairs Neurogenesis. J. Neuropathol. Exp. Neurol. 2004, 63, 1155–1166. [Google Scholar] [CrossRef]
- Desplats, P.; Spencer, B.; Crews, L.; Pathel, P.; Morvinski-Friedmann, D.; Kosberg, K.; Roberts, S.; Patrick, C.; Winner, B.; Winkler, J.; et al. α-Synuclein Induces Alterations in Adult Neurogenesis in Parkinson Disease Models via p53-mediated Repression of Notch1. J. Biol. Chem. 2012, 287, 31691–31702. [Google Scholar] [CrossRef]
- Iannitelli, A.; Quartini, A.; Tirassa, P.; Bersani, G. Schizophrenia and neurogenesis: A stem cell approach. Neurosci. Biobehav. Rev. 2017, 80, 414–442. [Google Scholar] [CrossRef]
- Dahm, T.; Rudolph, H.; Schwerk, C.; Schroten, H.; Tenenbaum, T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediat. Inflamm. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Das, S.; Basu, A. Viral infection and neural stem/progenitor cell’s fate: Implications in brain development and neurological disorders. Neurochem. Int. 2011, 59, 357–366. [Google Scholar] [CrossRef]
- Scordel, C.; Huttin, A.; Cochet-Bernoin, M.; Szelechowski, M.; Poulet, A.; Richardson, J.; Benchoua, A.; Gonzalez-Dunia, D.; Eloit, M.; Coulpier, M. Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis. PLoS Pathog. 2015, 11, e1004859. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kobayashi, S.; Kameyama, K.-I.; Sato, M.; Koiwa, M.; Sakoda, Y.; Taniyama, H. Non-cytopathic bovine viral diarrhea virus infection inhibits differentiation of bovine neural stem/progenitor cells into astrocytes in vitro. J. Vet. Med Sci. 2010, 72, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Li, X.; Sellier, Y.; Martin, H.; Perez-Berezo, T.; Rauwel, B.; Benchoua, A.; Bessières, B.; Aziza, J.; Cenac, N.; et al. PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells. PLoS Pathog. 2016, 12, e1005547. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Fernandes, L.; Cugola, F.R.; Russo, F.B.; Kawahara, R.; Freire, C.C.D.M.; Leite, P.E.C.; Stern, A.C.B.; Angeli, C.B.; De Oliveira, D.B.L.; Melo, S.R.; et al. Zika Virus Impairs Neurogenesis and Synaptogenesis Pathways in Human Neural Stem Cells and Neurons. Front. Cell. Neurosci. 2019, 13, 64. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-Buzhala, G.; Krut, O.; Peters, F.; Nikolić, M.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 2017, 20, 397–406.e5. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Nascimento, J.M.; De Vasconcelos, J.M.; Da Costa, R.M.; DelVecchio, R.; Trindade, P.; Loiola, E.C.; Higa, L.M.; Cassoli, J.S.; Vitória, G.; et al. Zika virus disrupts molecular fingerprinting of human neurospheres. Sci. Rep. 2017, 7, srep40780. [Google Scholar] [CrossRef]
- Ma, J.; Ketkar, H.; Geng, T.; Lo, E.; Wang, L.; Xi, J.; Sun, Q.; Zhu, Z.; Cui, Y.; Yang, L.; et al. Zika Virus Non-structural Protein 4A Blocks the RLR-MAVS Signaling. Front. Microbiol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Su, W.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y. Roles of mTOR Signaling in Brain Development. Exp. Neurobiol. 2015, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wahane, S.; Hellbach, N.; Prentzell, M.T.; Weise, S.C.; Vezzali, R.; Kreutz, C.; Timmer, J.; Krieglstein, K.; Thedieck, K.; Vogel, T. PI3K-p110-alpha-subtype signalling mediates survival, proliferation and neurogenesis of cortical progenitor cells via activation of mTORC2. J. Neurochem. 2014, 130, 255–267. [Google Scholar] [CrossRef]
- Stergiopoulos, A.; Politis, P.K. The role of nuclear receptors in controlling the fine balance between proliferation and differentiation of neural stem cells. Arch. Biochem. Biophys. 2013, 534, 27–37. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Sharma, A.; Valadi, N.; Miller, A.H.; Pearce, B.D. Neonatal Viral Infection Decreases Neuronal Progenitors and Impairs Adult Neurogenesis in the Hippocampus. Neurobiol. Dis. 2002, 11, 246–256. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Chavanas, S. Peroxisome proliferator-activated receptor γ (PPARγ) activation: A key determinant of neuropathogeny during congenital infection by cytomegalovirus. Neurogenesis 2016, 3, e1231654. [Google Scholar] [CrossRef]
- Ferraris, P.; Cochet, M.; Hamel, R.; Gladwyn-Ng, I.; Alfano, C.; Diop, F.; Garcia, D.; Talignani, L.; Montero-Menei, C.N.; Nougairède, A.; et al. Zika virus differentially infects human neural progenitor cells according to their state of differentiation and dysregulates neurogenesis through the Notch pathway. Emerg. Microbes Infect. 2019, 8, 1003–1016. [Google Scholar] [CrossRef]
- Garber, C.; Vasek, M.; Vollmer, L.; Sun, T.; Jiang, X.; Klein, R.S. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat. Immunol. 2018, 19, 151–161. [Google Scholar] [CrossRef]
- Green, H.F.; Treacy, E.; Keohane, A.; Sullivan, A.; O’Keeffe, G.W.; Nolan, Y.M. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol. Cell. Neurosci. 2012, 49, 311–321. [Google Scholar] [CrossRef]
- Wang, X.; Fu, S.; Wang, Y.; Yu, P.; Hu, J.; Gu, W.; Xu, X.-M.; Lu, P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell Neurosci. 2007, 36, 343–354. [Google Scholar] [CrossRef]
- Walter, J.; Honsek, S.D.; Illes, S.; Wellen, J.M.; Hartung, H.-P.; Rose, C.R.; Dihné, M. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol. Neurodegener. 2011, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Scully, T.J.; O’Donnell, L.A. The antiviral cytokine interferon-gamma restricts neural stem/progenitor cell proliferation through activation of STAT1 and modulation of retinoblastoma protein phosphorylation. J. Neurosci. Res. 2016, 95, 1582–1601. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rotschafer, J.H.; Lokensgard, J.R.; Cheeran, M.C.-J. Activated CD8+ T Lymphocytes Inhibit Neural Stem/Progenitor Cell Proliferation: Role of Interferon-Gamma. PLoS ONE 2014, 9, e105219. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, M.; Dell’Anno, M.T.; Pistello, M.; Conti, L.; Onorati, M. Human Neural Stem Cell Systems to Explore Pathogen-Related Neurodevelopmental and Neurodegenerative Disorders. Cells 2020, 9, 1893. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.A.; Mukherjee, S.; Mallick, S.; Akbar, I.; Basu, A. Atorvastatin ameliorates viral burden and neural stem/progenitor cell (NSPC) death in an experimental model of Japanese encephalitis. J. Biosci. 2020, 45, 1–17. [Google Scholar] [CrossRef]
- Choi, N.-Y.; Kim, J.Y.; Hwang, M.; Lee, E.-H.; Choi, H.; Lee, K.-Y.; Lee, Y.J.; Koh, S.-H. Atorvastatin Rejuvenates Neural Stem Cells Injured by Oxygen–Glucose Deprivation and Induces Neuronal Differentiation Through Activating the PI3K/Akt and ERK Pathways. Mol. Neurobiol. 2019, 56, 2964–2977. [Google Scholar] [CrossRef]
| Virus | Model System/Cell Type | Effect on NSCs | Notes |
|---|---|---|---|
| HSV-1 | Infection of adult mice or hippocampal NSC cultures from neonatal mice | Inhibits NSC proliferation [60] | Accumulation of amyloid β protein |
| Human iPSC-derived NSC monolayer culture | Cell death [45] | ||
| Human iPSC-derived cerebral organoids | Moderate cell death compared to monolayer hNSC cultures [46] | Activation of pro-apoptotic genes | |
| Human iPSC-derived NSCs and cerebral organoids | Decreased expression of neuronal markers and increased astroglial markers [46] | ||
| Murine neonatal hippocampal NSCs | Inhibits neuronal and promotes astroglial differentiation [60] | ||
| HCMV | Human fetal brain-derived NSCs | Inhibits NSC proliferation [34] | Disruption of Hes1 expression |
| Human fetal NSCs | Induction of apoptosis of infected cells [30] | Unfolded protein response activation | |
| Human iPSC-derived NSCs | Induction of apoptosis [32] | ||
| Human fetal NSCs | Inhibits neuronal differentiation when infection occurs in first 24 h after induction of differentiation [30] | ||
| NSCs derived from human embryonic stem cells (ESCs) | Inhibits neuronal differentiation [85] | Via elevated PPARγ levels | |
| MCMV | Murine embryonic cerebral stem cells | Reduction in NSC proliferation [31] | Inhibition of DNA replication |
| Murine neonatal brain cell after intracerebral MCMV infection | Decreased neuronal numbers and decreased expression of immature neuronal markers [5] | ||
| Murine embryonic NSCs | Inhibits neuronal and astroglial differentiation but neuronal differentiation is more severely affected [31] | ||
| BDV | Human fetal NSCs | Inhibits neuronal differentiation [2] | |
| Human fetal NSCs expressing BDV phosphoprotein P or X protein | Inhibits neuronal differentiation [83] | Driven by BDV phosphoprotein P | |
| BVDV | Bovine fetal NSCs | Inhibits astroglial differentiation [84] | |
| EV71 | Murine brain-derived NSCs | Induction of apoptosis [38] | |
| CVB3 | CVB3-infected neonatal mice | Induction of apoptosis and inhibition of proliferation of SVZ NSCs [39,40] | |
| JEV | JEV-infected neonatal mice | Loss of actively proliferating NSCs in the SVZ, impaired proliferation in vitro. [4] | |
| Human fetal NSC culture or NSCs isolated from brain of JEV-infected patients | Induction of apoptosis [42] | Increased expression of pro-apoptotic proteins and factors associated with ER stress. Increased cleavage of caspases-3, 7, 8, and 9. | |
| JEV-infected neonatal mice | Induction of apoptosis in the SVZ [108] | Reversed by atorvastatin treatment | |
| Murine neonatal SVZ NSCs | Inhibits neuronal and glial differentiation [1] | Downregulation of neuronal genes and decreased STAT3, JAK1, and increased STAT1 expression | |
| LCMV | LCMV-infected neonatal mice | Increased cell death in the SVZ during adulthood [37] | |
| Adult mouse brain harvested after neonatal LCMV infection | Decreased neuroblasts in the SVZ and SGZ [37] | Associated with chemokine expression | |
| Murine adult hippocampal and SVZ cells after congenital LCMV infection | Decrease in adult hippocampal neurogenesis [96] | ||
| WNV | Human fibroblast iPSC-derived NSCs | Induction of apoptosis [41] | |
| USUV | Human fibroblast iPSC-derived NSCs | Induction of mild apoptosis of NSCs compared to WNV [41] | |
| HIV | Human NSCs treated with HIV gp120 | Reduced proliferation and induction of quiescence. | Reduced Erk phosphorylation |
| Hippocampal tissue from HIV patients | Reduced NSC numbers [59] | ||
| Adult murine hippocampal NSCs or adult mice treated with HIV gp120 | Inhibition of NSC proliferation [33] | Cell cycle arrest in the G1 phase via MAPK pathway activation | |
| Fetal hNSCs treated with HIV tat | Increase in quiescent NSCs [36] | Increased nuclear localization of TRIM32 due to increased miR-155 | |
| Adult brain tissue from HIV seropositive patients | |||
| ZIKV African strain (MR766) | Human iPSC-derived forebrain NSCs | Reduced cell proliferation [29] | DNA damage, activation of the DNA damage response (DDR), and cell cycle arrest in the S-phase |
| Embryonic murine NSCs or human embryonic NSCs | Induction of apoptosis and autophagy [51] | Increases macroautophagy to promote viral replication and disrupts selective autophagy | |
| hNSC cell line | Induction of apoptosis [55] | Activation of DDR, increased phosphorylation of H2AX (cellular protein responsive to DDR), and increased PARP and cleaved caspase 3 | |
| African strain (IB H 30656) | Embryonic murine cortical NSCs | No effect on cell viability [52] | Cytopathic effect (alterations in NSC secretome) |
| Embryonic murine NSCs | Downregulation of neuronal and oligodendroglial genes and upregulation of astroglial genes [52] | ||
| American strain (PRVABC59) | Human iPSC-derived forebrain hNSCs infected with ZIKV | Reduction in proliferation [29] | DNA damage, activation of DDR, and cell cycle arrest in the S-phase |
| Embryonic murine cortical NSCs | No effect on cell viability [52] | Mild DDR induction in comparison to MR766 strain and activation of p53 | |
| hNSC cell line | Mild cytopathic effect (pyknotic nuclei) [55] | Poor innate immune activation in hNSCs | |
| Human fetal NSCs | Induction of apoptosis [57] | Persistent ZIKV infection | |
| Brazilian strain (Paraiba) | Embryonic murine NSCs or human embryonic NSCs | Induction of apoptosis and autophagy in NSCs [51] | Increases macroautophagy to promote viral replication and disrupts selective autophagy |
| Brazilian strain (ZIKV-BR) | NSCs from human iPSCs from exfoliated deciduous teeth | Aberrant neuronal differentiation with downregulation of mature neuron markers and upregulation of immature neuron markers [86] | |
| Brazilian strain (BR_ZIKV_AB_ES) | Human iPSC-derived NSCs | Inhibits neuronal differentiation [88] | |
| French Polynesia starin (H/PF/2013) | Human iPSC-derived NSCs | No changes in cell viability [41] | Cleaved caspase-3 |
| Human iPSC-derived NSCs | Premature differentiation to neurons [87] | ||
| American strain (FB-GWUH-2016) | Human iPSC-derived NSCs | Premature differentiation to neurons [87] | |
| ZIKV proteins (NS4A and NS4B) | Human fetal NSCs | Decreased neuronal and astrocytic numbers [91] | Inhibition of Akt-mTOR pathway |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamte, Y.S.; Chandwani, M.N.; Michaels, A.C.; O’Donnell, L.A. Neural Stem Cells: What Happens When They Go Viral? Viruses 2021, 13, 1468. https://doi.org/10.3390/v13081468
Kamte YS, Chandwani MN, Michaels AC, O’Donnell LA. Neural Stem Cells: What Happens When They Go Viral? Viruses. 2021; 13(8):1468. https://doi.org/10.3390/v13081468
Chicago/Turabian StyleKamte, Yashika S., Manisha N. Chandwani, Alexa C. Michaels, and Lauren A. O’Donnell. 2021. "Neural Stem Cells: What Happens When They Go Viral?" Viruses 13, no. 8: 1468. https://doi.org/10.3390/v13081468
APA StyleKamte, Y. S., Chandwani, M. N., Michaels, A. C., & O’Donnell, L. A. (2021). Neural Stem Cells: What Happens When They Go Viral? Viruses, 13(8), 1468. https://doi.org/10.3390/v13081468






