Identification, Genetic Characterization and Validation of Highly Diverse HIV-1 Viruses for Reference Panel Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Cell Culture
2.2. Specimens Characterization
2.2.1. Viral Load (VL), p24, and AlphaLISA Assays
2.2.2. RNA Extraction, RT-PCR, and Sequencing
2.2.3. Bioinformatics Data Analysis
3. Results
3.1. Virus Culture and HIV-1 Storage
3.2. HIV-1 Viruses Represent a High Viral Load and p24 Antigen
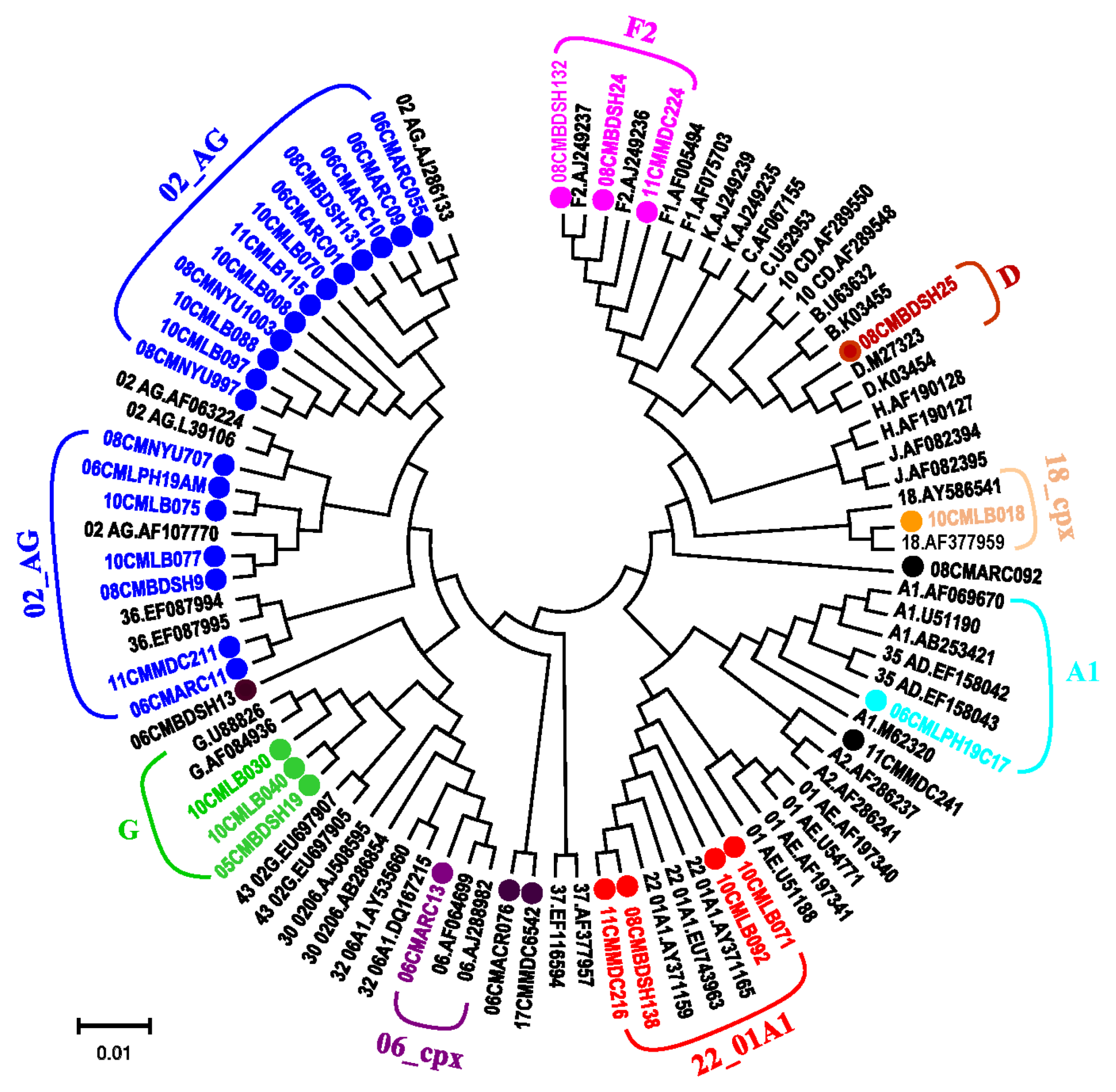
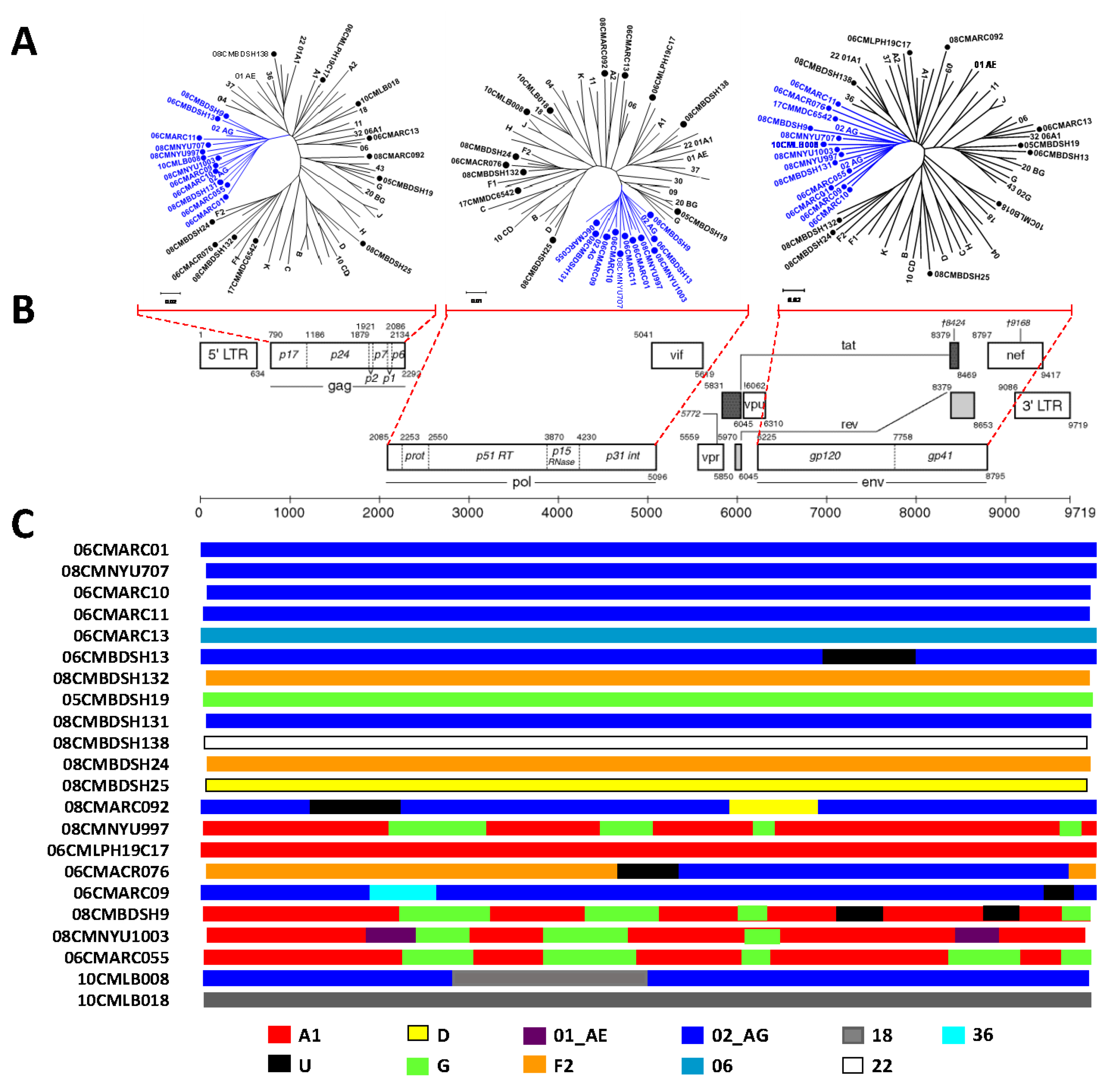
3.3. Phylogenetic Analysis Determines Diverse HIV-1 Viruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Hora, B.; Keating, S.; Chen, Y.; Sanchez, A.M.; Sabino, E.; Hunt, G.; Ledwaba, J.; Hackett, J.; Swanson, P.; Hewlett, I.; et al. Genetic Characterization of a Panel of Diverse HIV-1 Isolates at Seven International Sites. PLoS ONE 2016, 11, e0157340. [Google Scholar] [CrossRef]
- Sanchez, A.M.; DeMarco, C.T.; Hora, B.; Keinonen, S.; Chen, Y.; Brinkley, C.; Stone, M.; Tobler, L.; Keating, S.; Schito, M.; et al. Development of a contemporary globally diverse HIV viral panel by the EQAPOL program. J. Immunol. Methods 2014, 409, 117–130. [Google Scholar] [CrossRef]
- Manak, M.; Sina, S.; Anekella, B.; Hewlett, I.; Sanders-Buell, E.; Ragupathy, V.; Kim, J.; Vermeulen, M.; Stramer, S.L.; Sabino, E.; et al. Pilot Studies for Development of an HIV Subtype Panel for Surveillance of Global Diversity. Aids Res. Hum. Retrovir. 2012, 28, 594–606. [Google Scholar] [CrossRef]
- Price, M.A.; Rida, W.; Kilembe, W.; Karita, E.; Inambao, M.; Ruzagira, E.; Kamali, A.; Sanders, E.J.; Anzala, O.; Hunter, E.; et al. HIV-1 viral control varies by viral subtype in a large cohort of African adults with incident HIV-1 infection. J. Infect. Dis. 2019, 220, 432–441. [Google Scholar] [CrossRef]
- Banin, A.N.; Tuen, M.; Bimela, J.S.; Tongo, M.; Zappile, P.; Khodadadi-Jamayran, A.; Nanfack, A.J.; Okonko, I.O.; Meli, J.; Wang, X.; et al. Near full genome characterization of HIV-1 unique recombinant forms in Cameroon reveals dominant CRF02_AG and F2 recombination patterns. J. Int. AIDS Soc. 2019, 22, e25362. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.K.; Torimiro, J.N.; Wolfe, N.D.; Eitel, M.N.; Kim, B.; Sanders-Buell, E.; Jagodzinski, L.L.; Gotte, D.; Burke, D.S.; Birx, D.L.; et al. The AG recombinant IbNG and novel strains of group M HIV-1 are common in Cameroon. Virology 2001, 286, 168–181. [Google Scholar] [CrossRef][Green Version]
- Gao, F.; Bailes, E.; Robertson, D.L.; Chen, Y.; Rodenburg, C.M.; Michael, S.F.; Cummins, L.B.; Arthur, L.O.; Peeters, M.; Shaw, G.M.; et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nat. Cell Biol. 1999, 397, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H.; Shaw, G.M.; De Cock, K.M.; Sharp, P.M. AIDS as a zoonosis: Scientific and public health implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef]
- Keele, B.F.; Van Heuverswyn, F.; Li, Y.; Bailes, E.; Takehisa, J.; Santiago, M.L.; Bibollet-Ruche, F.; Chen, Y.; Wain, L.; Liegeois, F.; et al. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1. Science 2006, 313, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Plantier, J.-C.; Leoz, M.; Dickerson, J.E.; De Oliveira, F.; Cordonnier, F.; Lemée, V.; Damond, F.; Robertson, D.L.; Simon, F. A new human immunodeficiency virus derived from gorillas. Nat. Med. 2009, 15, 871–872. [Google Scholar] [CrossRef]
- Powell, R.L.; Zhao, J.; Konings, F.A.; Tang, S.; Ewane, L.; Burda, S.; Urbanski, M.M.; Saa, D.; Hewlett, I.; Nyambi, P.N. Circulating Recombinant Form (CRF) 37_cpx: An Old Strain in Cameroon Composed of Diverse, Genetically Distant Lineages of Subtypes A and G. AIDS Res. Hum. Retrovir. 2007, 23, 923–933. [Google Scholar] [CrossRef]
- Powell, R.L.; Zhao, J.; Konings, F.A.; Tang, S.; Nanfack, A.; Burda, S.; Urbanski, M.M.; Saa, D.; Hewlett, I.; Nyambi, P.N. Identification of a Novel Circulating Recombinant Form (CRF) 36_cpx in Cameroon That Combines Two CRFs (01_AE and 02_AG) with Ancestral Lineages of Subtypes A and G. AIDS Res. Hum. Retrovir. 2007, 23, 1008–1019. [Google Scholar] [CrossRef]
- Vergne, L.; Bourgeois, A.; Mpoudi-Ngole, E.; Mougnutou, R.; Mbuagbaw, J.; Liegeois, F.; Laurent, C.; Butel, C.; Zekeng, L.; Delaporte, E.; et al. Biological and genetic characteristics of HIV infections in Cameroon reveals dual group M and O infections and a correlation between SI-inducing phenotype of the predominant CRF02_AG variant and disease stage. Virology 2003, 310, 254–266. [Google Scholar] [CrossRef]
- Wilbe, K.; Casper, C.; Albert, J.; Leitner, T. Identification of Two CRF11-cpx Genomes and Two Preliminary Representatives of a New Circulating Recombinant Form (CRF13-cpx) of HIV Type 1 in Cameroon. AIDS Res. Hum. Retrovir. 2002, 18, 849–856. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, S.; Ragupathy, V.; Carr, J.K.; Wolfe, N.D.; Awazi, B.; Hewlett, I. Identification and Genetic Characterization of a Novel CRF22_01A1 Recombinant Form of HIV Type 1 in Cameroon. AIDS Res. Hum. Retrovir. 2010, 26, 1033–1045. [Google Scholar] [CrossRef]
- Zhong, P.; Burda, S.; Urbanski, M.; Kenfack, H.; Tongo, M.; Heyndrickx, L.; Nanfack, A.; Shang, J.; Agyingi, L.; Zolla-Pazner, S.; et al. HIV type 1 group M clades infecting subjects from rural villages in equatorial rain forests of Cameroon. J. Acquir. Immune Defic. Syndr. 2002, 31, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Bodelle, P.; Vallari, A.S.; Coffey, R.; McArthur, C.P.; Schochetman, G.; Devare, S.G.; Brennan, C.A. HIV infections in northwestern Cameroon: Identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res. Hum. Retrovir. 2004, 20, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Badreddine, S.; Swanson, P.; Bodelle, P.; Devare, S.G.; Brennan, C.A. Identification of New CRF43_02G and CRF25_cpx in Saudi Arabia Based on Full Genome Sequence Analysis of Six HIV Type 1 Isolates. AIDS Res. Hum. Retrovir. 2008, 24, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Yebra, G.; Rivas, P.; Herrero, M.D.; López, M.; De Mulder, M.; Puente, S.; Ramírez-Olivencia, G.; Soriano, V.; Holguín, A. Clinical Differences and Viral Diversity between Newly HIV Type 1-Diagnosed African and Non-African Patients in Spain (2005–2007). AIDS Res. Hum. Retrovir. 2009, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.T.; Hackett, J.; Holzmayer, V.; Hillyard, D.R. Large-Scale Analysis of the Prevalence and Geographic Distribution of HIV-1 Non-B Variants in the United States. J. Clin. Microbiol. 2013, 51, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.; de Mendoza, C.; Joshi, Y.; Golden, A.; Hodinka, R.L.; Soriano, V.; Devare, S.G.; Hackett, J., Jr. Impact of Human Immunodeficiency Virus Type 1 (HIV-1) Genetic Diversity on Performance of Four Commercial Viral Load Assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol 2005, 43, 3860–3868. [Google Scholar] [CrossRef][Green Version]
- Church, D.; Gregson, D.; Lloyd, T.; Klein, M.; Beckthold, B.; Laupland, K.; Gill, M.J. Comparison of the RealTime HIV-1, COBAS TaqMan 48 v1.0, Easy Q v1.2, and Versant v3.0 assays for Determination of HIV-1 Viral Loads in a Cohort of Canadian Patients with Diverse HIV Subtype Infections. J. Clin. Microbiol. 2010, 49, 118–124. [Google Scholar] [CrossRef]
- Gueudin, M.; Plantier, J.-C.; Lemée, V.; Schmitt, M.P.; Chartier, L.; Bourlet, T.; Ruffault, A.; Damond, F.; Vray, M.; Simon, F. Evaluation of the Roche Cobas TaqMan and Abbott Real Time Extraction-Quantification Systems for HIV-1 Subtypes. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 44, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Sobieszczyk, M.E.; McCutchan, F.E.; Hammer, S.M. The Challenge of HIV-1 Subtype Diversity. N. Engl. J. Med. 2008, 358, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, M.A.; Brenner, B.G. The Impact of HIV Genetic Polymorphisms and Subtype Differences on the Occurrence of Resistance to Antiretroviral Drugs. Mol. Biol. Int. 2012, 2012, 256982. [Google Scholar] [CrossRef] [PubMed]
- Machuca, A.; Tang, S.; Hu, J.; Lee, S.; Wood, O.; Vockley, C.; Vutukuri, S.G.; Deshmukh, R.; Awazi, B.; Hewlett, I. Increased Genetic Diversity and Intersubtype Recombinants of HIV-1 in Blood Donors From Urban Cameroon. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 45, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tang, S.; Ragupathy, V.; Gaddam, D.; Wang, X.; Zhang, P.; Nyambi, P.N.; Hewlett, I. CRF22_01A1 is Involved in the Emergence of New HIV-1 Recombinants in Cameroon. J. Acquir. Immune Defic. Syndr. 2012, 60, 344–350. [Google Scholar] [CrossRef]
- UNAIDS. Propagation of Primary HIV-1 Isolates. NIH AIDS Research & Reference Reagent Program. Available online: https://www.aidsreagent.org/support_docs/virus.pdf (accessed on 20 November 2020).
- Zhang, M.; Huang, Q.; Huang, Y.; Wood, O.; Yuan, W.; Chancey, C.; Daniel, S.; Rios, M.; Hewlett, I.; Clouse, K.A.; et al. beta-estradiol attenuates the anti-HIV-1 efficacy of Stavudine (D4T) in primary PBL. Retrovirology 2008, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Ragupathy, V.; Zhao, J.; Wood, O.; Tang, S.; Lee, S.; Nyambi, P.; Hewlett, I. Identification of new, emerging HIV-1 unique recombinant forms and drug resistant viruses circulating in Cameroon. Virol. J. 2011, 8, 185. [Google Scholar] [CrossRef]
- APHL. Best Practice Guidance: Specimen and Specimen-Product Storage and Retention. APHL Issues In Brief. Infect. Dis. 2016, 1, 1–6. [Google Scholar]
- Scott, L.E.; Crump, J.A.; Msuya, E.; Morrissey, A.B.; Venter, W.F.; Stevens, W.S. Abbott RealTime HIV-1 m2000rt viral load testing: Manual extraction versus the automated m2000sp extraction. J. Virol. Methods 2011, 172, 78–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pulido-Olmo, H.; Rodríguez-Sánchez, E.; García, J.A.N.; Barderas, M.G.; Alvarez-Llamas, G.; Segura, J.; Fernandez-Alfonso, M.S.; Ruilope, L.M.; Ruiz-Hurtado, G. Rapid, Automated, and Specific Immunoassay to Directly Measure Matrix Metalloproteinase-9–Tissue Inhibitor of Metalloproteinase-1 Interactions in Human Plasma Using AlphaLISA Technology: A New Alternative to Classical ELISA. Front. Immunol. 2017, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- CRFs. HIV Sequence Database. Los Alamos National Laboratory, the Circulating Recombinant Forms (CRFs). Available online: https://www.hiv.lanl.gov/content/sequence/HIV/CRFs/CRFs.html (accessed on 29 April 2021).
- Kuiken, T.; Fouchier, R.; Rimmelzwaan, G.; Osterhaus, A. Emerging viral infections in a rapidly changing world. Curr. Opin. Biotechnol. 2003, 14, 641–646. [Google Scholar] [CrossRef] [PubMed]
- NCBI. National Center for Biotechnology Information (NCBI) Genotyping. Available online: https://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi (accessed on 29 April 2021).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Davis, C.; Berry, N.; Heath, A.; Holmes, H. An international collaborative study to establish a replacement World Health Organization International Standard for human immunodeficiency virus 1 RNA nucleic acid assays. Vox Sang. 2008, 95, 218–225. [Google Scholar] [CrossRef]
- Holmes, H.; Davis, C.; Heath, A. Development of the 1st International Reference Panel for HIV-1 RNA genotypes for use in nucleic acid-based techniques. J. Virol. Methods 2008, 154, 86–91. [Google Scholar] [CrossRef]
- WHO. World Health Organization HIV Reference Panels. 2001. Available online: https://www.who.int/bloodproducts/cs/031961.pdf (accessed on 29 April 2021).
| Patient ID | Year | VL (107 Copies/mL) | p24 (ng/mL) | AlphaLISA (ng/mL) | Assay’s Agreement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W1 | W2 | W3 | W4 | W1 | W2 | W3 | W4 | |||
| 06CMARC01 | 2006 | 1.4 | 92.4 | 322.7 | - | 0.6 | 49.4 | 112.3 | - | 3.9 | 272.3 | 0.0 ** | - | yes |
| 06CMARC07 | 2006 | 0 | 6.1 | 5.6 | - | 2 | 3.2 | 1.9 | - | 23.5 | 34.7 | 21.8 | - | yes |
| 06CMARC09 | 2006 | 147.2 | 80.8 | 64.8 | - | 56.5 | 91.4 | 27 | - | 290.4 | 330.1 | 60.4 | - | yes * |
| 06CMARC10 | 2006 | 120.8 | 329.6 | 183.4 | - | 58.5 | 110.1 | 93.6 | - | 130.3 | 0.0 ** | 0.0 ** | - | yes |
| 06CMARC11 | 2006 | 12.6 | 27.4 | 14.7 | - | 5.9 | 14.2 | 6.5 | - | 0.9 | 4 | 1.8 | - | yes |
| 06CMARC13 | 2006 | 0.4 | 1.8 | 1.2 | - | 0.2 | 0.9 | 0.8 | - | 0.3 | 1 | 0.7 | - | yes |
| 08CMBDSH24 | 2008 | 17.5 | 121.6 | 22.5 | - | 8.2 | 10.5 | 13.1 | - | 29.7 | 150.8 | 40.8 | - | yes |
| 06CMLPH19C17 | 2006 | 34.6 | 56.7 | 67.7 | - | 13 | 21.4 | 18.5 | - | 101.6 | 59.8 | 136.2 | - | yes * |
| 06CMLPH19AM | 2006 | N.D. | N.D. | N.D. | - | 1.7 | 63.2 | 107 | - | 13.3 | 128.1 | 253.6 | - | yes |
| 06CMARC071 | 2006 | 25 | - | 65.3 | - | 8 | - | 28.2 | - | 58.3 | - | 8.4 | - | yes * |
| 06CMARC076 | 2006 | 25.2 | - | 111.7 | - | 9.5 | - | 41.2 | - | 57.6 | - | 1134 | - | yes |
| 08CMBDSH131 | 2008 | - | 91.7 | 93.6 | - | - | 24.1 | 26.3 | - | - | 32.8 | 28.7 | - | yes * |
| 05CMBDSH19 | 2005 | - | 0.4 | 2.5 | - | - | 0.2 | 0.8 | - | - | 0.5 | 3.1 | - | yes |
| 08CMNYU707 | 2008 | - | 53.6 | 87.3 | - | - | 25.6 | 35.2 | - | - | 274.7 | 479.1 | - | yes |
| 08CMNYU871 | 2008 | - | 100.5 | 52.8 | - | - | 25.2 | 18 | - | - | 53.2 | 36.1 | - | yes |
| 08CMNYU997 | 2008 | - | 1.5 | 1.8 | - | - | 0.3 | 0.5 | - | - | 1.4 | 2 | - | yes |
| 08CMNYU1003 | 2008 | - | 37.3 | 47.9 | - | - | 19.6 | 24.6 | - | - | 0 | 70.4 | - | yes |
| 10CMLB008 | 2010 | - | - | 41.3 | 14.1 | - | - | 21.1 | 9.1 | - | - | 70.6 | 10.9 | yes |
| 10CMLB022 | 2010 | - | - | 100.6 | 0 | - | - | 131.1 | 37.7 | - | - | 275.1 | 40.7 | yes |
| 10CMLB030 | 2010 | - | - | 83.6 | 85.4 | - | - | 18.6 | 23.4 | - | - | 48.7 | 56.4 | yes |
| 10CMLB031 | 2010 | - | - | 180.8 | 52.8 | - | - | 107 | 32.6 | - | - | 217.5 | 60.9 | yes |
| 10CMLB040 | 2010 | - | - | 162.6 | 48.1 | - | - | 120.9 | 6.1 | - | - | 228.5 | 0 | yes |
| 10CMLB070 | 2010 | - | - | 168.4 | 35.1 | - | - | 133.4 | 39.6 | - | - | 323 | 140.4 | yes |
| 10CMLB075 | 2010 | - | - | 106.4 | 0 | - | - | 98.9 | 32.7 | - | - | 261.4 | 108.9 | yes |
| 10CMLB092 | 2010 | - | - | N.D. | N.D. | - | - | 39.8 | 12.5 | - | - | 271.4 | 41.6 | yes |
| 10CMLB097 | 2010 | - | - | N.D. | N.D. | - | - | 4.2 | 1.7 | - | - | 66.1 | 22.9 | yes |
| 10CMLB034 | 2010 | - | - | 73.6 | - | - | - | 27.8 | - | - | - | 246.9 | - | |
| 10CMLB011 | 2010 | - | - | 17.3 | - | - | - | 16.3 | - | - | - | 414.3 | - | |
| 10CMLB012 | 2010 | - | - | 41.5 | - | - | - | 10.5 | - | - | - | 82.5 | - | |
| 10CMLB029 | 2010 | - | - | 298.6 | - | - | - | 173.2 | - | - | - | 318.8 | - | |
| 10CMLB068 | 2010 | - | - | 78 | - | - | - | 92.9 | - | - | - | 189 | - | |
| 10CMNYU488 | 2010 | - | - | 163.7 | - | - | - | 73.2 | - | - | - | 207.5 | - | |
| 08CMARC092 | 2008 | - | - | 111.7 | - | - | - | 27.7 | - | - | - | 288.4 | - | |
| 08CMBDSH132 | 2008 | - | - | 51.4 | - | - | - | 22.8 | - | - | - | 65.6 | - | |
| 08CMBDSH138 | 2008 | - | - | 20.3 | - | - | - | 15.2 | - | - | - | 68 | - | |
| 08CMBDSH139 | 2008 | - | - | 83.6 | - | - | - | 30.6 | - | - | - | 199.6 | - | |
| 08CMBDSH25 | 2008 | - | - | 398.9 | - | - | - | 85.4 | - | - | - | 580.6 | - | |
| 08CMBDSH9 | 2008 | - | - | 44.2 | - | - | - | 23.8 | - | - | - | 50.9 | - | |
| Patient ID | Year | Cultured (Day) | Size (bp) | Identity (%) | Genotype | GB Acc. No |
|---|---|---|---|---|---|---|
| 06CMARC01 | 2006 | 21 | 9083 | 84.2 | 2 | MN153477 |
| 08CMNYU707 | 2008 | 21 | 8855 | 83.2 | 2 | MN153478 |
| 06CMARC10 | 2006 | 21 | 8677 | 82.1 | 2 | MN153479 |
| 06CMARC11 | 2006 | 22 | 8865 | 85.1 | 2 | MN153480 |
| 06CMARC13 | 2006 | 22 | 8857 | 83.6 | 6 | MN153481 |
| 06CMBDSH13 | 2006 | 14 | 8933 | 83.7 | 02,U | MN153482 |
| 08CMBDSH131 | 2008 | 21 | 8862 | 84 | 2 | MN153486 |
| 08CMBDSH132 | 2008 | 21 | 8853 | 86.1 | F2 | MN153483 |
| 08CMBDSH138 | 2008 | 21 | 8868 | 83.7 | 22 | MN153487 |
| 05CMBDSH19 | 2005 | 22 | 9017 | 83.3 | G | MN153484 |
| 08CMBDSH24 | 2008 | 22 | 8855 | 84.7 | F2 | MN153485 |
| 08CMBDSH25 | 2008 | 21 | 8936 | 87.6 | D | MN153488 |
| 08CMARC092 | 2008 | 21 | 8726 | 85.4 | 02,D,U | MN153489 |
| 08CMNYU997 | 2008 | 21 | 8906 | 84.3 | 02,A1 | MN153490 |
| 06CMLPH19C17 | 2006 | 21 | 8915 | 84.3 | A1 | MN153491 |
| 06CMARC076 | 2006 | 20 | 8690 | 84 | 02,F2,U | MN153492 |
| 06CMARC09 | 2006 | 21 | 8777 | 83.6 | 02,36,U | MN153493 |
| 08CMBDSH9 | 2008 | 21 | 8923 | 84 | 2 | MN153494 |
| 08CMNYU1003 | 2008 | 21 | 8916 | 84.1 | 2 | MN153495 |
| 06CMARC055 | 2006 | 8 | 8893 | 84.7 | 02,A1 | MN153496 |
| 10CMLB008 | 2010 | 21 | 8910 | 83.1 | 02,18 | MN153497 |
| 10CMLB018 | 2010 | 21 | 8950 | 85 | 18 | MN153475 |
| 10CMLB030 | 2010 | 21 | 8929 | 83.2 | G | MT349406 |
| 10CMLB040 | 2010 | 21 | 9015 | 83.3 | G,BG | MT349407 |
| 10CMLB088 | 2010 | 21 | 8963 | 84.4 | 2 | MT349408 |
| 10CMLB070 | 2010 | 21 | 9103 | 83.6 | 2 | MT349409 |
| 10CMLB071 | 2010 | 21 | 8822 | 83.5 | 22 | MT349410 |
| 10CMLB075 | 2010 | 21 | 8531 | 84.1 | 2 | MT349411 |
| 10CMLB077 | 2010 | 21 | 8886 | 83.9 | 2 | MT349412 |
| 10CMLB092 | 2010 | 21 | 8247 | 82.6 | 22 | MT349413 |
| 10CMLB097 | 2010 | 21 | 8919 | 84.2 | 2 | MT349414 |
| 11CMLB115 | 2011 | 28 | 8868 | 84.7 | 2 | MT349415 |
| 11CMMDC211 | 2011 | 24 | 8888 | 84.2 | 22 | MT349416 |
| 11CMMDC224 | 2011 | 24 | 8854 | 84.4 | F2 | MT349417 |
| 11CMMDC241 | 2011 | 24 | 8768 | 83.4 | A1,U | MT349418 |
| 06CMLPH19AM | 2006 | 21 | 8913 | 84.9 | 2 | MT349419 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, H.; Lee, S.; Ragupathy, V.; Biswas, S.; Mbondji-wonje, C.; Wang, X.; Jiang, A.; Hewlett, I. Identification, Genetic Characterization and Validation of Highly Diverse HIV-1 Viruses for Reference Panel Development. Viruses 2021, 13, 1417. https://doi.org/10.3390/v13071417
Zhao J, Huang H, Lee S, Ragupathy V, Biswas S, Mbondji-wonje C, Wang X, Jiang A, Hewlett I. Identification, Genetic Characterization and Validation of Highly Diverse HIV-1 Viruses for Reference Panel Development. Viruses. 2021; 13(7):1417. https://doi.org/10.3390/v13071417
Chicago/Turabian StyleZhao, Jiangqin, Hanxia Huang, Sherwin Lee, Viswanath Ragupathy, Santanu Biswas, Christelle Mbondji-wonje, Xue Wang, Alex Jiang, and Indira Hewlett. 2021. "Identification, Genetic Characterization and Validation of Highly Diverse HIV-1 Viruses for Reference Panel Development" Viruses 13, no. 7: 1417. https://doi.org/10.3390/v13071417
APA StyleZhao, J., Huang, H., Lee, S., Ragupathy, V., Biswas, S., Mbondji-wonje, C., Wang, X., Jiang, A., & Hewlett, I. (2021). Identification, Genetic Characterization and Validation of Highly Diverse HIV-1 Viruses for Reference Panel Development. Viruses, 13(7), 1417. https://doi.org/10.3390/v13071417






