The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus, Plants, and Growth Conditions
2.2. Protein Extraction, Trypsin Digestion and Isobaric Tag for Relative and Absolute Quantitation (Itraq) Analysis
2.3. LC-MS/MS Analysis and Protein Identification and Quantification
2.4. Bioinformatic Analysis
2.5. RNA Extraction and Real Time Quantitative RT-PCR (RT-qPCR)
2.6. Analysis of MTC-Related Metabolites
2.7. Statistics
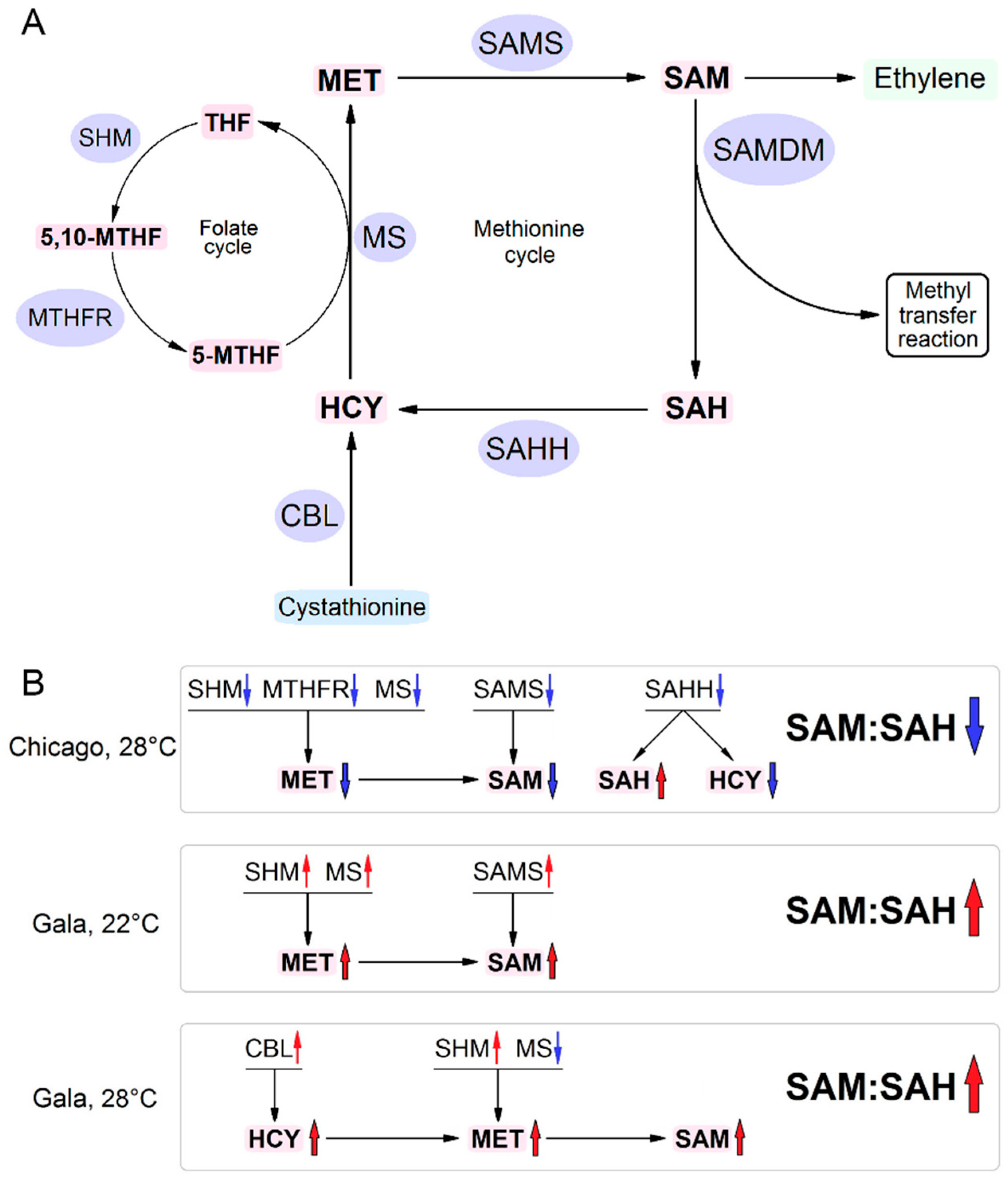
3. Results
3.1. Protein Profiles of PVY-Infected Gala Plants at Normal and High Temperature
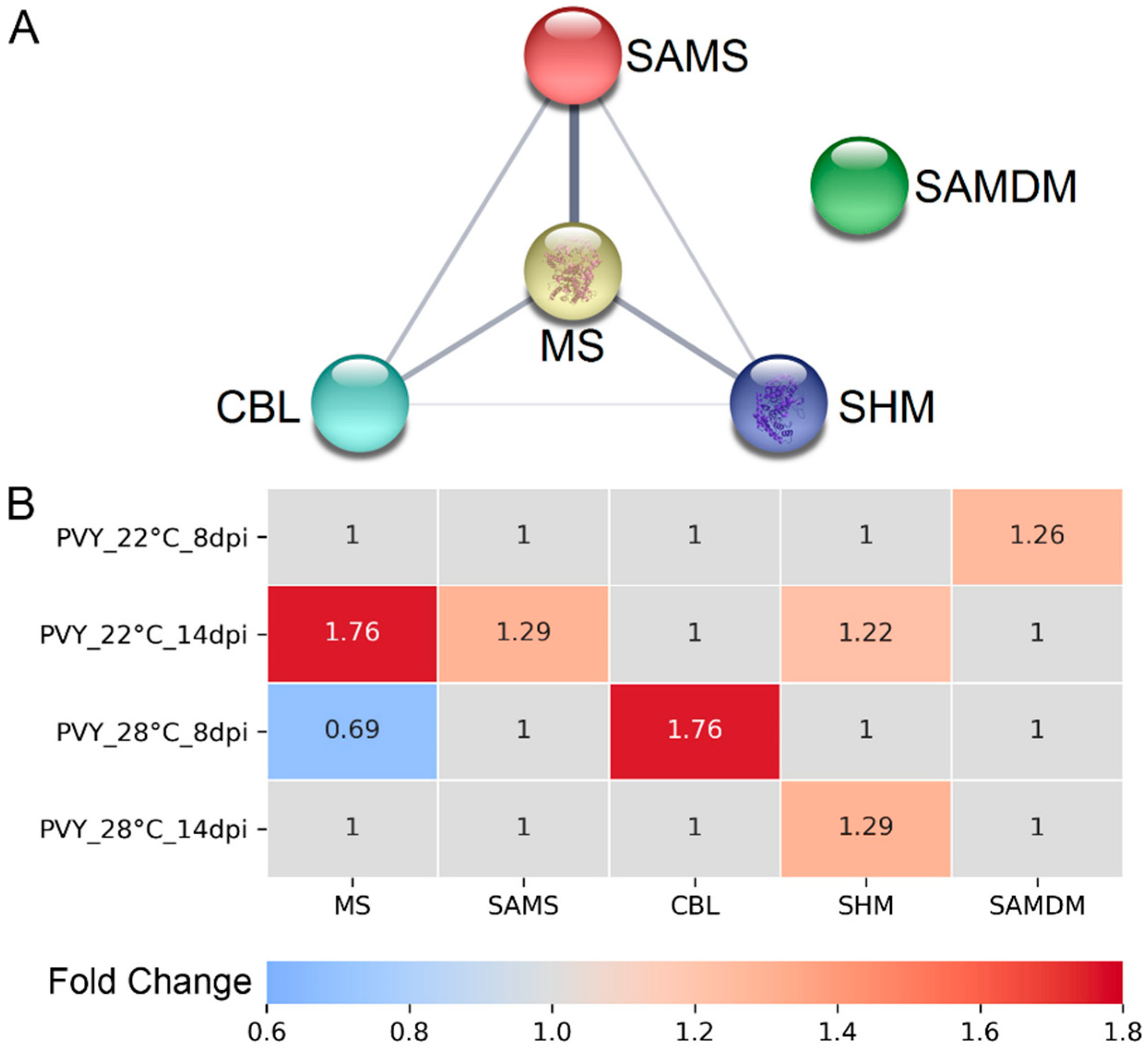
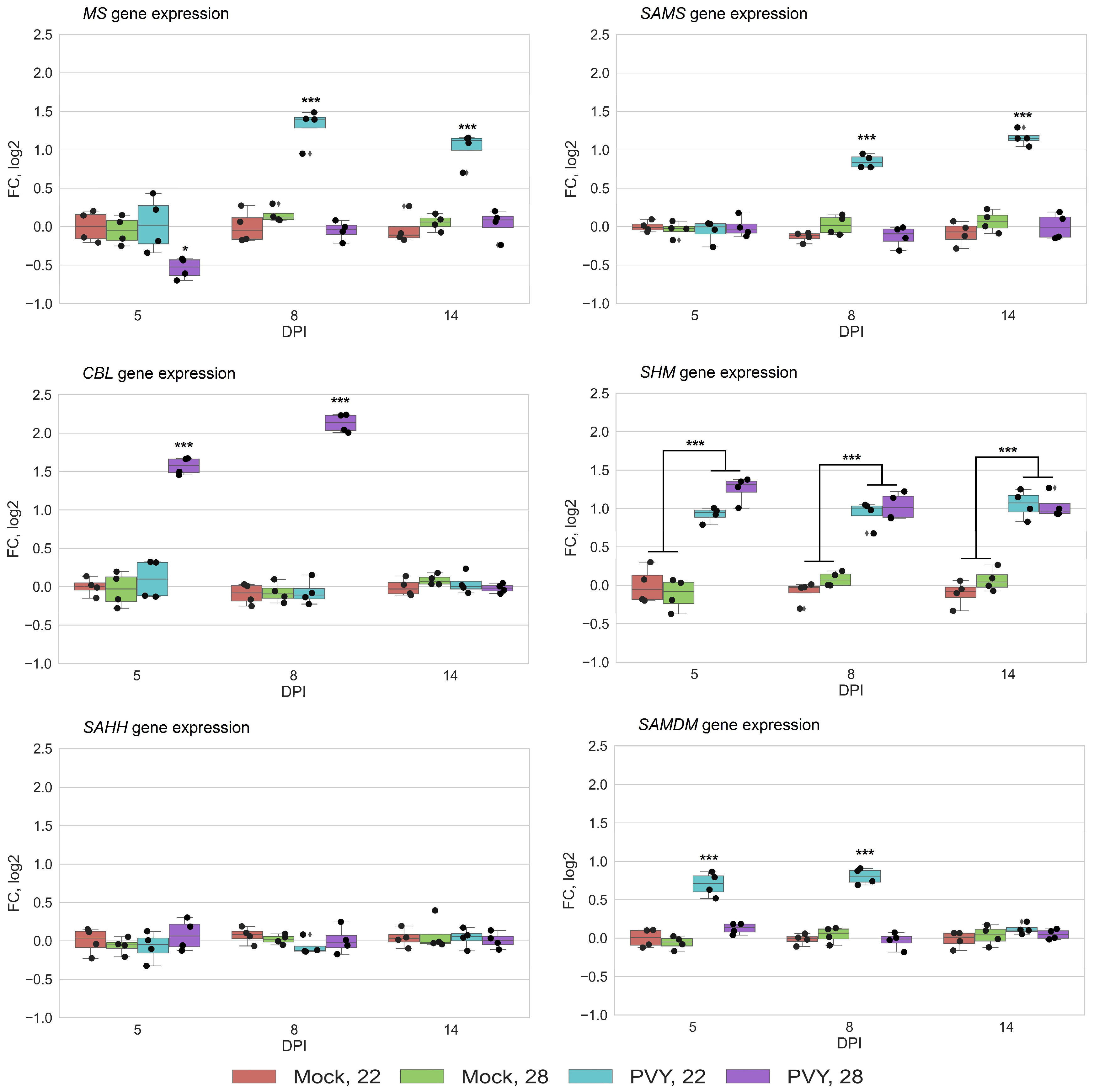
3.2. RNA Expression Levels of Key MTC-Related Genes
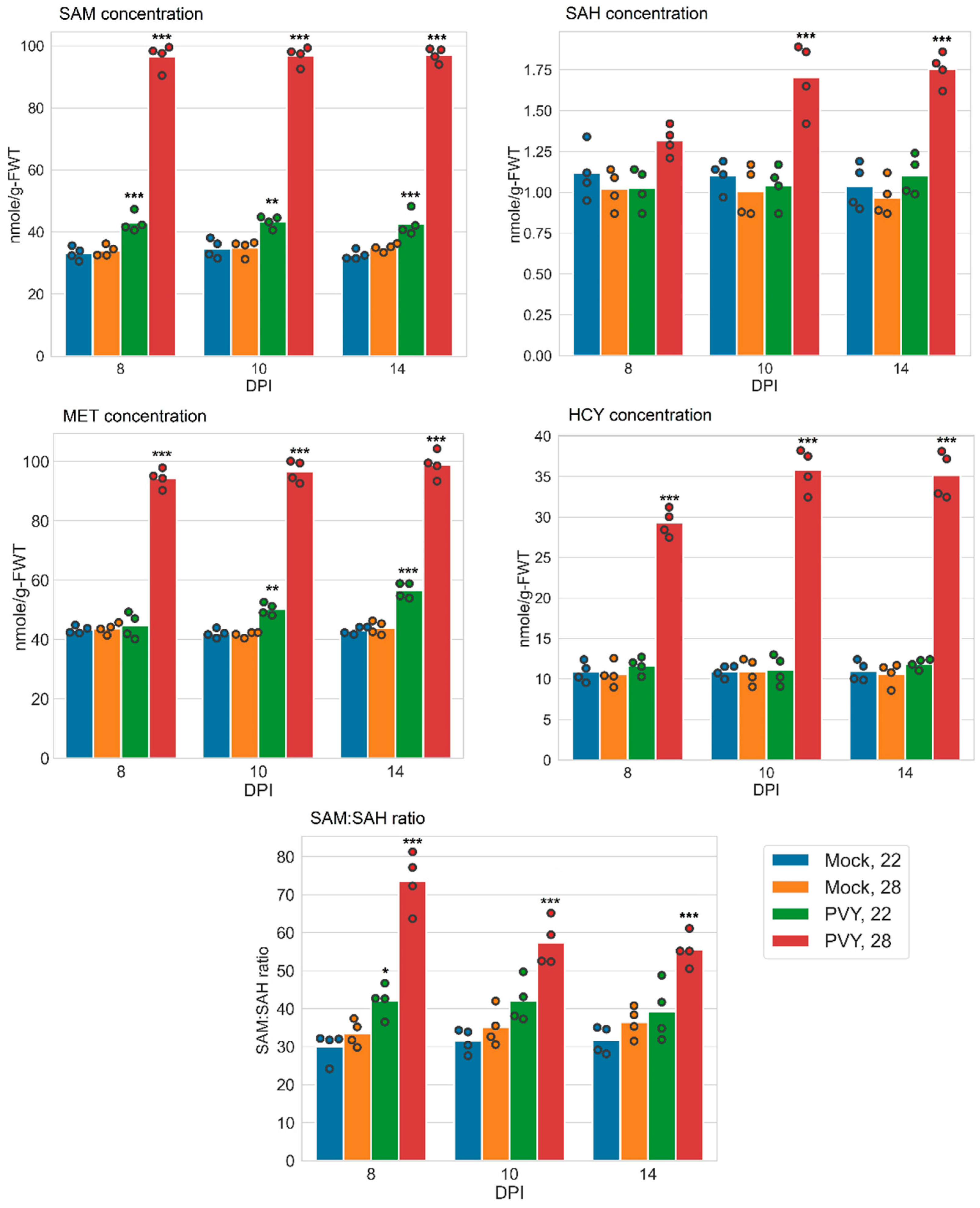
3.3. Accumulation of MTC Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elena, S.F.; Fraile, A.; García-Arenal, F. Chapter Three—Evolution and Emergence of Plant Viruses. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 88, pp. 161–191. [Google Scholar] [CrossRef]
- Syller, J.; Grupa, A. Antagonistic within-host interactions between plant viruses: Molecular basis and impact on viral and host fitness. Mol. Plant Pathol. 2016, 17, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Llave, C. Dynamic cross-talk between host primary metabolism and viruses during infections in plants. Curr. Opin. Virol. 2016, 19, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.; De, S. The significance of methionine cycle enzymes in plant virus infections. Curr. Opin. Plant Biol. 2019, 50, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, I.; Spechenkova, N.; Mamaeva, A.; Makhotenko, A.V.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y. Mol. Plant Pathol. 2021, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. RNA silencing. Trends Biochem. Sci. 2005, 30, 290–293. [Google Scholar] [CrossRef]
- Ding, S.-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef]
- Mlotshwa, S.; Pruss, G.J.; Vance, V. Small RNAs in viral infection and host defense. Trends Plant Sci. 2008, 13, 375–382. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y. Dissection of RNAi-based antiviral immunity in plants. Curr. Opin. Virol. 2018, 32, 88–99. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Yu, B.; Liu, J.; Chen, X. Methylation Protects miRNAs and siRNAs from a 3′-End Uridylation Activity in Arabidopsis. Curr. Biol. 2005, 15, 1501–1507. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Eskelin, K.; Bašić, M.; De, S.; Lõhmus, A.; Varjosalo, M.; Mäkinen, K. Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J. 2016, 85, 30–45. [Google Scholar] [CrossRef]
- Cañizares, M.C.; Lozano-Durán, R.; Canto, T.; Bejarano, E.R.; Bisaro, D.M.; Navas-Castillo, J.; Moriones, E. Effects of the Crinivirus Coat Protein–Interacting Plant Protein SAHH on Post-Transcriptional RNA Silencing and Its Suppression. Mol. Plant-Microbe Interact. 2013, 26, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Talianksy, M.E. Potato Virus Y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection with Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Gibson, R.W.; Pehu, E.; Woods, R.D.; Jones, M.G.K. Resistance to potato virus Y and potato virus X in Solanurn brevidens. Ann. Appl. Biol. 1990, 116, 151–156. [Google Scholar] [CrossRef]
- Faurobert, M.; Pelpoir, E.; Chaïb, J. Phenol Extraction of Proteins for Proteomic Studies of Recalcitrant Plant Tissues. In Plant Proteomics: Methods and Protocols; Thiellement, H., Zivy, M., Damerval, C., Méchin, V., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2007; pp. 9–14. [Google Scholar] [CrossRef]
- Ow, S.Y.; Salim, M.; Noirel, J.; Evans, C.; Rehman, I.; Wright, P.C. iTRAQ Underestimation in Simple and Complex Mixtures: “The Good, the Bad and the Ugly”. J. Proteome Res. 2009, 8, 5347–5355. [Google Scholar] [CrossRef]
- STRING: Functional Protein Association Networks. Available online: https://string-db.org/cgi/input?sessionId=bqGWh48lnqym&input_page_show_search=on (accessed on 12 April 2021).
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- g:Profiler—A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists. Available online: https://biit.cs.ut.ee/gprofiler/gost (accessed on 12 April 2021).
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Phytozome v12.1: Home. Available online: https://phytozome.jgi.doe.gov/pz/portal.html (accessed on 12 April 2021).
- Nicot, N.; Hausman, J.-F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Stare, K.; Kovač, M.; Blejec, A.; Prezelj, N.; Stare, T.; Kogovšek, P.; Pompe-Novak, M.; Rosahl, S.; Ravnikar, M.; et al. Dynamics of Responses in Compatible Potato—Potato virus Y Interaction Are Modulated by Salicylic Acid. PLoS ONE 2011, 6, e29009. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G. Python Tutorial, Technical Report CS-R9526; Centrum voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pandey, A.; Pandey, G.K. β-catenin in plants and animals: Common players but different pathways. Front. Plant Sci. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Ren, H. Profilin as a regulator of the membrane-actin cytoskeleton interface in plant cells. Front. Plant Sci. 2013, 4, 512. [Google Scholar] [CrossRef]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2018, 69, 117–132. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Suarez, M.F.; Filonova, L.H.; Daniel, G.; Zamyatnin, A.A.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Smertenko, A. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 14463–14468. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Droux, M.; Ravanel, S.; Douce, R. Methionine Biosynthesis in Higher Plants.II. Purification and Characterization of Cystathionine β-Lyase from Spinach Chloroplasts. Arch. Biochem. Biophys. 1995, 316, 585–595. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Piquerez, S.J.M.; Bendahmane, A.; Hirt, H.; Raynaud, C.; Benhamed, M. Modify the Histone to Win the Battle: Chromatin Dynamics in Plant–Pathogen Interactions. Front. Plant Sci. 2018, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, M.V.; Chen, H.; Gladon, R.J.; Braun, E.; Hannapel, D.J. A Leaf Lipoxygenase of Potato Induced Specifically by Pathogen Infection. Plant Physiol. 2000, 124, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, B.A.; Weretilnyk, E.A. SustainingS-adenosyl-l-methionine-dependent methyltransferase activity in plant cells. Physiol. Plant. 2001, 113, 435–442. [Google Scholar] [CrossRef]
- Bernardo, P.; Charles-Dominique, T.; Barakat, M.; Ortet, P.; Fernandez, E.; Filloux, D.; Hartnady, P.; Rebelo, T.A.; Cousins, S.R.; Mesleard, F.; et al. Geometagenomics illuminates the impact of agriculture on the distribution and prevalence of plant viruses at the ecosystem scale. ISME J. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, J.P.T. Chapter 28—Viruses: Economical Losses and Biotechnological Potential. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A., Ross, H.A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 619–641. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Nolte, P.; McIntosh, C.; Davidson, R. Effect of Potato virus Y on Yield of Three Potato Cultivars Grown under Different Nitrogen Levels. Plant Dis. 2006, 90, 73–76. [Google Scholar] [CrossRef][Green Version]
- Solomon-Blackburn, R.M.; Bradshaw, J.E. Resistance to Potato virus Y in a Multitrait Potato Breeding Scheme without Direct Selection in Each Generation. Potato Res. 2007, 50, 87–95. [Google Scholar] [CrossRef]
- Foong, S.-L.; Paek, K.-H. Capsicum annum Hsp26.5 promotes defense responses against RNA viruses via ATAF2 but is hijacked as a chaperone for tobamovirus movement protein. J. Exp. Bot. 2020, 71, 6142–6158. [Google Scholar] [CrossRef]
- Corrêa, R.L.; Sanz-Carbonell, A.; Kogej, Z.; Müller, S.Y.; Ambrós, S.; López-Gomollón, S.; Gómez, G.; Baulcombe, D.C.; Elena, S.F. Viral Fitness Determines the Magnitude of Transcriptomic and Epigenomic Reprograming of Defense Responses in Plants. Mol. Biol. Evol. 2020, 37, 1866–1881. [Google Scholar] [CrossRef]
- Kuźnicki, D.; Meller, B.; Arasimowicz-Jelonek, M.; Braszewska-Zalewska, A.; Drozda, A.; Floryszak-Wieczorek, J. BABA-Induced DNA Methylome Adjustment to Intergenerational Defense Priming in Potato to Phytophthora infestans. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef]
| Identifications | PVY 22 °C 8 dpi | PVY 28 °C 8 dpi | PVY 22 °C 14 dpi | PVY 28 °C 14 dpi |
|---|---|---|---|---|
| Peptides | 11,353 | 14,106 | 12,893 | 13,703 |
| Protein groups | 2386 | 2693 | 2722 | 2619 |
| Samples | SAM (nmol/g-FWT) | SAM:SAH | ||
|---|---|---|---|---|
| Gala | Chicago | Gala | Chicago | |
| Mock 22 °C | 33.15 ± 1.07 | 27.94 ± 0.36 | 30.13 ± 1.98 | 33.32 ± 0.63 |
| Mock 28 °C | 33.95 ± 0.89 | 27.16 ± 0.61 | 33.56 ± 1.70 | 29.78 ± 0.82 |
| PVY 22 °C | 42.99 ± 1.49 ** | 28.02 ± 0.34 | 42.16 ± 2.10 * | 30.98 ± 0.61 |
| PVY 28 °C | 96.349 ± 2.05 *** | 10.96 ± 0.51 | 73.61 ± 3.78 *** | 3.62 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spechenkova, N.; Fesenko, I.A.; Mamaeva, A.; Suprunova, T.P.; Kalinina, N.O.; Love, A.J.; Taliansky, M. The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses 2021, 13, 955. https://doi.org/10.3390/v13060955
Spechenkova N, Fesenko IA, Mamaeva A, Suprunova TP, Kalinina NO, Love AJ, Taliansky M. The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses. 2021; 13(6):955. https://doi.org/10.3390/v13060955
Chicago/Turabian StyleSpechenkova, Nadezhda, Igor A. Fesenko, Anna Mamaeva, Tatyana P. Suprunova, Natalia O. Kalinina, Andrew J. Love, and Michael Taliansky. 2021. "The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index" Viruses 13, no. 6: 955. https://doi.org/10.3390/v13060955
APA StyleSpechenkova, N., Fesenko, I. A., Mamaeva, A., Suprunova, T. P., Kalinina, N. O., Love, A. J., & Taliansky, M. (2021). The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index. Viruses, 13(6), 955. https://doi.org/10.3390/v13060955







