Abstract
Human cytomegalovirus (HCMV), one of the most prevalent viruses across the globe, is a common cause of morbidity and mortality for immunocompromised individuals. Recent clinical observations have demonstrated that mixed strain infections are common and may lead to more severe disease progression. This clinical observation illustrates the complexity of the HCMV genome and emphasizes the importance of taking a population-level view of genotypic evolution. Here we review frequently sampled polymorphisms in the glycoproteins of HCMV, comparing the variable regions, and summarizing their corresponding geographic distributions observed to date. The related strain-specific immunity, including neutralization activity and antigen-specific cellular immunity, is also discussed. Given that these glycoproteins are common targets for vaccine design and anti-viral therapies, this observed genetic variation represents an important resource for future efforts to combat HCMV infections.
1. Introduction
Human cytomegalovirus (HCMV) is highly prevalent, with an estimated 83% seroprevalence in the global population [1]. While HCMV-infected immunocompetent individuals are generally asymptomatic, HCMV can cause morbidity and mortality for immunocompromised individuals [2], including in the setting of organ transplant recipients, acquired immunodeficiency syndrome (AIDS), and congenital infection. Infections with multiple HCMV strains are commonly observed among these groups [3,4,5], and strain replacement has additionally been noted [6]. Furthermore, frequent recombination between strains continuously generates novel genotypic combinations [3]. These clinical observations indicate the interesting complexity of the population genetic environment in HCMV [4], and hence elucidating the evolutionary mechanisms dictating observed variation remains as a major research topic in the pursuit of effective vaccines and anti-viral therapies.
2. Complexity of HCMV Genomes and Viral Populations In Vivo
HCMV has long been recognized as being genetically variable. In 1980, Huang et al. observed differences in restriction fragment length polymorphisms (RFLPs) in HCMV samples cultured from congenitally infected infants [5]. Likewise, different laboratory strains were recognized, some of which were utilized in early vaccine trials [7,8]. As DNA sequencing technologies developed, entire genomes of HCMV could be delineated, highlighting nucleotide-level differences between strains on the order of ~2% [9,10,11,12]. Subsequent short read, deep-sequencing studies confirmed genome-wide differences in HCMV consensus sequences and revealed the wide-spread presence of rare alleles, insertions, and deletions in patient samples [13,14,15,16]. Indeed, the significance of this within-host population-level variation in HCMV was quickly recognized [14,17,18,19], with nucleotide diversity approaching that of certain RNA viruses [20]. As with RNA viruses, this diversity allows for populations to be structured and varied, driven by both stochastic (e.g., infection bottleneck) and more deterministic (e.g., natural selection) evolutionary forces [15,21]. The combination of constant purifying selection and episodic positive selection, together with population size change and the related effects of genetic drift, has been found to result in a relatively high-level of neutral and weakly deleterious variation in patient samples [10]. Unlike RNA viruses however, mutation rates tend toward the more modest range of ~2 × 10−7 per site per replication [17]. Other factors contributing to the observed population-level diversity include compartmentalization and gene flow, post-infection population growth rates, reinfection, and recombination [17,19,22,23,24]. Collectively, these features result in a collection of genetic variants in clinical specimens that may influence disease severity, responses to antiviral therapies, and vaccine candidate design.
3. Common Polymorphisms in the Glycoproteins of HCMV
The ~235 kb double-stranded DNA genome of HCMV encodes approximately 230–250 putatively functional open reading frames (ORFs) [12,17,25]. These ORFs include up to 65 unique glycoproteins [9,26], and several of them are responsible for viral attachment to and entry into the host cells. Due to their pivotal role in initiating signaling transduction cascades in target cells and propagating HCMV infection, glycoproteins have been identified as key HCMV vaccine targets. Interestingly, instead of functioning alone, glycoproteins on HCMV virions form complexes, which facilitate the pathogenesis of HCMV infection. These complexes include the glycoprotein B oligomer (gB) [27], glycoprotein M/glycoprotein N (gM/gN) dimer [28], glycoprotein H/glycoprotein L/glycoprotein O (gH/gL/gO) trimer [29], and gH/gL/gO/UL128-130 pentameric complex [30]. Table 1 summarizes the commonly observed polymorphisms in these glycoproteins, including the length and genotypes, as well as the most variable regions described to date. In order to determine the most variable regions among each glycoprotein, multiple sequence alignments were generated using T-coffee version 13.45.0.4846264 [31] (Figure S1). In addition, Table 2 presents the genotypes of several common laboratory-adapted HCMV strains, including the strains Towne, TB40E, AD169, Toledo, VR1814, and Merlin.

Table 1.
Commonly observed glycoprotein polymorphic regions.

Table 2.
Genotyping of common laboratory-adapted HCMV strains.
These highly variable sites in the glycoproteins, together with viral cytokine/chemokine proteins (human cellular homologs), are commonly considered to be key factors for host immunity and HCMV infection [45]. This suggests strain-specific immunity, defined as the discrete immune responses elicited by different variants of the virus genomes. Strain-specific immunity is usually classified by the strain-specific humoral and antigen-specific T-cell responses. With regards to humoral immunity, strain-specific neutralization of HCMV has been identified in human sera [46], and monoclonal antibodies isolated from both humans and rabbits have also demonstrated strain-specific recognition [47]. Strain-specific T-helper-cell response to gH has also been reported when comparing the T-cell proliferative response to antigens from strains Towne and AD169 [48]. Given that strain-specific immunity is associated with observed polymorphisms, we review these commonly variable glycoprotein sites, summarize their current geographic distributions, and present evidence relating this diversity with immune response.
4. gB
HCMV gB is the best-characterized glycoprotein to date and is encoded by UL55. Consisting of ~900 amino acids (NCBI accession number: YP_081514), gB is composed of a large ectodomain region, membrane-proximal region, transmembrane domain, and cytoplasmic domain [49]. The prototypic gB undergoes proteolytic cleavage approximately at codon 460 which generates two gB polypeptides, gp116 and gp55 (also known as gp58), that are covalently linked by disulfide bonds. While most of the gB ectodomain belongs to gp116, gp55 is a type1 transmembrane protein settled on the HCMV envelope. Required for HCMV entry into target cells and HCMV infection via cell-to-cell spread, gB is highly glycosylated and genetically variable [27]. The glycosylation sites of gB include 18 possible N-linked glycosylation sites and two O-linked glycosylation sites [50,51]. In addition, the polymorphisms within gB genotypes occur mostly around the gp55 cleavage site at codon 460 as well as, to a lesser extent, around several coding regions of gp116—an observation which has been associated with homologous recombination [32,33,52,53]. Due to the intragenic variations within the gB sequence, gB genotypes have been defined based on the variations within C-terminus, N-terminus [54,55,56], and the gp55 cleavage site [57] as originally proposed by Chou et al. [32]. Interestingly, samples collected at multiple body sites from the same patient can display different gB genotypes, suggesting distinct cell tropism of HCMV, consistent with the compartmentalization of viral populations observed by deep sequencing [15,17].
To date, five different gB genotypes (gB-1, gB-2, gB-3, gB-4, and gB-5 previously referred to as gB-3′ [34,58,59]) have been identified [32,33]. We here review previous research investigating the correlations between gB genotypes and clinical outcomes, focusing (by and large) on individuals with compromised immune systems (e.g., organ/bone marrow transplant recipients, AIDS patients, and congenitally or perinatally HCMV-infected infants). Figure 1 organizes the gB genotyping studies alphabetically by study continent and country, as well as by patient profile. All five genotypes have been detected in Asia, Europe, and North America, however their geographic distribution differs: gB-1 is the most prevalent genotype in Asia and Egypt [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]; gB-1 and gB-2 are frequently detected in North America [58,100,101,102,103,104,105,106,107,108,109]; gB-2 is the most extensively sampled genotype in Latin America [110,111,112,113,114,115,116,117,118,119,120,121,122,123]; whereas gB-1, gB-2, and gB-3 are commonly observed across Europe (with the exception of Serbia where gB-4 is the most prevalent genotype) [24,55,57,59,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153]. Although the majority of HCMV-positive individuals who participated in these studies carried gB-1, gB-2, and/or gB-3 genotypes, the association between any particular gB genotype and disease severity is inconsistent among studies [24,55,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,83,94,95,96,97,98,99,100,102,103,104,105,106,107,108,109,110,111,112,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,148,149,150,151,152,153,154]. When a gB genotype has been identified as a disease severity marker, it was often simply the most prevalent genotype in the study population. Thus, it is currently difficult to draw a conclusion as to whether there is a specific gB genotype that leads to a more severe disease progression. Compared to a single gB genotype infection, mixed gB genotype infections are frequently reported in organ/bone marrow transplant recipients and AIDS patients [105,118,125,128,129,152]. Multiple studies have also demonstrated that mixed infections are able to be transmitted in utero [63,68,74,75,76,77,109,122,134,137,138,141]. Although there is no established association between single genotype infections and disease outcomes, patients with mixed gB genotype infections have been reported to have faster disease progression or higher viral loads [83,97,102,144]. This observation highlights the importance of targeting diverse HCMV strains in vaccine design and therapeutics.
gB has been identified as one of the major HCMV vaccine targets due to its essential role in virion fusion with the cell and ability to elicit both neutralizing [155] and non-neutralizing antibody responses [156,157]. Currently, the gB subunit protein from the Towne strain (gB-1) with MF59 adjuvant (gB/MF59) is the most effective vaccine tested to date in several phase I and II clinical trials [158,159,160]. gB-1, gB-2, and gB-4 share a greater genetic similarity compared to gB-3 and gB-5, having been described as comprising two “supergroups” [43]. High-throughput sequencing of viruses from infected individuals who were given either a gB/MF59 or a placebo vaccine showed that gB/MF59 vaccinees were more resistant to infection from HCMV from the gB-1, gB-2, and gB-4 genotype supergroup compared to the placebo recipients, suggesting the protective effect of the vaccine was limited to this supergroup [161]. Additionally, a single variant of gB, 275Y of AD169 strain and 585G of VR1814 strain, was shown to impact viral entry, cell fusion, and genome stability via activation of ATM (ataxia-teleangiectasia mutated) -PIDDosome-caspase-2 signaling axis. Thus, this study suggested that even a single nucleotide polymorphism of gB is critical to the pathogenesis of HCMV infection [162]. Interestingly, with 200 HCMV clinical strains analyzed, gB sequences were found to have a greater diversity among strains compared to those of the pentameric complex. This observation highlights the complexity of gB variation, even though gB genotypes exhibit a ~96% mean conserved identity at the amino acid level. This indicates the dominant action of purifying selection in gB, making it more highly conserved than other glycoproteins including gN and gO [163].
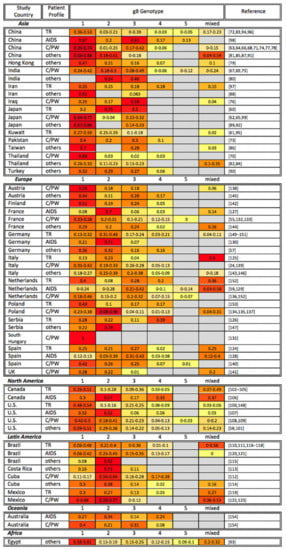
Figure 1.
Global gB genotyping across disease states. gB genotyping studies are organized alphabetically by study continent and country, as well as by patient profile (TR: transplant recipient; AIDS: AIDS patient; C/PW: congenital CMV infection or pregnant woman). The percentage of each gB genotype among study participants is shown, with the most common genotypes highlighted in red, genotypes at intermediate frequency in orange, and less common genotypes in yellow. Gray cells indicate that a particular gB genotype was not identified in the reference studies. Note that the sum of all genotypes may be greater or smaller than 1 if multiple gB genotypes were identified in the same sample or if not all samples in a study were genotyped, respectively.
5. gN
HCMV gN, encoded by UL73, is a type 1 transmembrane protein that links it to gM by disulfide bonds. Although gN is only composed of ~135 amino acids (NCBI accession number: YP_081521), this protein has aroused interest of late due to its considerable post-translational modifications and high-level of polymorphism. The post-translational modifications of gN include 18 potential phosphorylation sites, 33 possible O-linked glycosylation sites [35], and two N-linked glycosylation sites (reviewed in [164]). gN is highly variable particularly in the N-terminal region, outside virus particles, which is believed to be involved in eliciting immune responses and responding to immunological selective pressure. Based on the differences in the N-terminal region, four gN genotypes have been identified. Two subtypes of gN-3 (gN-3a, gN-3b) and three subtypes of gN-4 (gN-4a, gN-4b, gN-4c) have been further distinguished. The similarity of nucleotides within each genotype is around 80–85%, while the nucleotide similarity within each subtype is 96–100% [35,36].
Considering the geographic distribution of gN genotypes, a global study reported that clinical isolates from China, Australia, and Europe unexpectedly demonstrated a similar frequency of gN-1, gN-3, and gN-4, whereas gN-2 was infrequently detected in Europe and not identified in China and Australia. On the other hand, gN-2 is more commonly detected in North America than other regions, though when detected, gN-1, gN-3, and gN-4 remain the more prevalent [36]. However, the findings of the above global study are not entirely consistent with the local gN genotyping studies as shown in Figure 2. All four gN genotypes have been reported in Asia. However, the gN-4 genotype has often been reported as the most prevalent genotype globally, followed by gN-1 or gN-3, and gN-2. In a single study from Egypt, gN-1 was the most prevalent genotype in breast cancer patients [93].
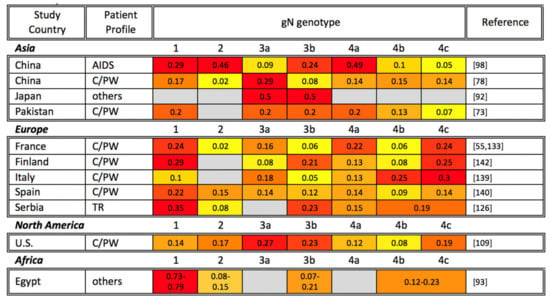
Figure 2.
Global gN genotyping across disease states. gN genotyping studies are organized alphabetically by study continent and country, as well as by patient profile (TR: transplant recipient; AIDS: AIDS patient; C/PW: congenital CMV infection or pregnant woman). The percentage of each gN genotype among study participants is shown, with the most common genotypes highlighted in red, genotypes at intermediate frequency in orange, and less common genotypes in yellow. Gray cells indicate that a particular gN genotype was not identified in the reference studies. Note that the sum of all genotypes may be greater or smaller than 1 if multiple gN genotypes were identified in the same sample or if not all samples in a study were genotyped, respectively.
In terms of the immune responses to gN related to observed genotypic variation, only humoral immunity has been characterized to date. These studies have mainly focused on investigating neutralizing antibody responses against four gN genotypes, and strain-specific neutralizing activity against gN strains has been demonstrated [165,166,167]. It has been shown that anti-gM/gN dimer antibodies possess differential neutralizing activities against AD169, Toledo, and TR strains. Since there have been no gM polymorphisms reported, this study suggested that the neutralizing anti-gM/gN dimer antibody responses against gN might be strain-specific. However, because this study did not isolate gN-specific antibodies and identify neutralizing epitopes lying in the most variable regions of gN, further studies are required to confirm that the neutralization against gN is strain-specific [165]. To investigate the antibody response against gN genotypes more specifically, viruses with four different gN genotypes in the same AD169 virus backbone were constructed. By creating a neutralization assay with these gN-recombinant viruses, strain-specific neutralization was measurable in 30–60% of human sera. Interestingly, human sera collected from subjects in Erlangen (Germany) appeared to neutralize the virus with gN-4 genotype the best, while the cohort from Birmingham (USA) neutralized the virus with gN-2 genotype most potently [166,167]. This observation is consistent with the geographical distribution of HCMV gN genotypes from previous studies [36].
6. gO
HCMV gO, a soluble protein encoded by UL74, is an essential element of the gH/gL/gO trimer. Compared to the pentameric complex, the gH/gL/gO trimer has been reported as an integral component of HCMV entry by promoting fusion with all cell types [168]. gO is composed of 457–472 amino acids (NCBI accession number: YP_081522.1), depending on the number of strain-specific deletions. gO is also highly glycosylated, with variation in glycosylation observed among genotypes. The glycosylation sites of gO include 18 potential N-linked glycosylation sites and a single O-linked glycosylation site [50,51]. Of the polymorphisms most commonly observed in the gO sequence, the major variable region lies in the first 98 codons, with some minor variations between codon 270–313 [37]. Based on these differences among gO sequence, four gO genotypes were identified in AIDS patient in 2002 [38], while the fifth gO genotype was verified in 2005 after detection in renal transplant recipients [39]. By tree-based analysis of HCMV sequences in lung transplant recipient samples, three subtypes of gO-1 (gO-1a, gO-1b, gO-1c), and two subtypes of gO-2 (gO-2a, gO-2b) have been reported [40,41].
Previous gO genotyping studies are organized in Figure 3. The neighbor-joining tree analysis of gO genotypes from several studies found disparate genetic similarities between gO genotypes and their subtypes [41,44,169]. In terms of geographic distribution, five gO genotypes have been detected in Asia, Europe, and Australia, with gO-5 being consistently the least frequently detected. Little correlation has been observed between genotype and disease perhaps due to a paucity of gO genotyping studies. Nonetheless, the gO-1 genotype appears to be the most prevalent in most studies [38,41,98,138,170]. Further, previous work has suggested that the gO-1b genotype is linked to gN-3a and gH-1, while the gO-5 genotype was linked to gN-4c and gH-2. However, the implications of this association, and whether it holds any clinical relevance, remains in need of further study [44].
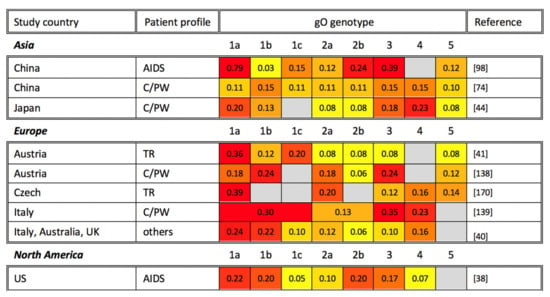
Figure 3.
Global gO genotyping across disease states. gO genotyping studies are organized alphabetically by study continent and country, as well as by patient profile (TR: transplant recipient; AIDS: AIDS patient; C/PW: congenital CMV infection or pregnant woman). The percentage of each gO genotype among study participants is shown, with the most common genotypes highlighted in red, genotypes at intermediate frequency in orange, and less common genotypes in yellow. Gray cells indicate that a particular gO genotype was not identified in the reference studies. Note that the sum of all genotypes may be greater or smaller than 1 if multiple gO genotypes were identified in the same sample or if not all samples in a study were genotyped, respectively.
Finally, the functional difference of gO genotypes have been investigated to some extent. Comparing HCMV reconstituted with two highly variable gO genotypes, gO-1 and gO-4, in the same TB40E backbone, the gO-4 genotype displayed an increasing tropism for epithelial cells compared to the gO-1 genotype [171]. Additionally, there was a different inhibitory effect of soluble HCMV trimer- and pentamer-specific entry receptors observed by comparing reconstituted HCMV with gO-1, gO-2, gO-3, and gO-5 genotypes in the same TB40E backbone [172]. It has also been suggested that different gO genotypes have an impact on neutralizing antibody response to gH epitopes [173].
7. gH
HCMV gH, encoded by UL75, has been identified as an integral component of the gH/gL/gO trimer and the pentameric complex. In addition to gB, the pentameric complex has remained a key vaccine target given that the complex is required for HCMV to enter epithelial, endothelial, and monocytic cells [30,174,175,176]. Belonging to type I transmembrane protein, gH is 742-amino-acids long (NCBI accession number: YP_081523.1), and compared to other glycoproteins described above, gH is moderately glycosylated and less variable. The sequence of gH has been reported to include six potential N-linked glycosylation sites and four O-linked glycosylation sites [50,51]. Based on the variability of gH sequences, the gH genotypes could be divided into two groups, gH-1 and gH-2. The variable regions between the two groups are mostly identified in the first 37 codons near the N-terminal region and minorly between codons 176–181 and 359–365 [42]. The observed geographic distribution of gH genotypes is shown in Figure 4. Moreover, gH-1 and gH-2 genotypes are distributed fairly equally in organ transplant recipients and HCMV-infected children sampled to date.
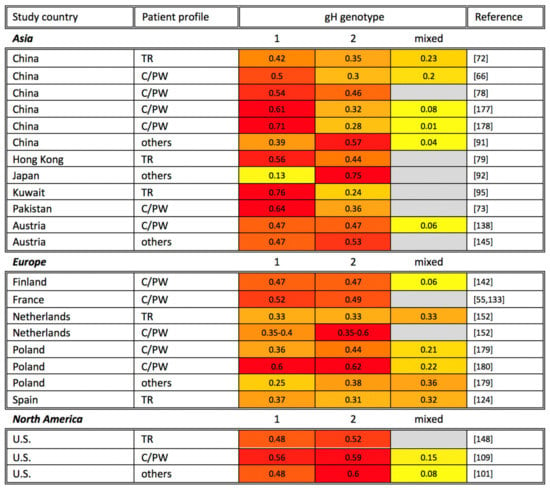
Figure 4.
Global gH genotyping across disease states. gH genotyping studies are organized alphabetically by study continent and country, as well as by patient profile (TR: transplant recipient; AIDS: AIDS patient; C/PW: congenital CMV infection or pregnant woman). The percentage of each gH genotype among study participants is shown, with the most common genotypes highlighted in red, genotypes at intermediate frequency in orange, and less common genotypes in yellow. Gray cells indicate that a particular gH genotype was not identified in the reference studies. Note that the sum of all genotypes may be greater or smaller than 1 if multiple gH genotypes were identified in the same sample or if not all samples in a study were genotyped, respectively.
gH has been identified as one of the major antigens for eliciting neutralizing antibody responses [177,178]. In a recent study, the neutralizing ability of certain gH-specific monoclonal antibodies were shown to be strain-specific in fibroblasts and epithelial cells. The neutralizing epitopes of these antibodies lie at codons 27–48, the most variable region of gH [47]. Additionally, a previous study has compared the outcomes of renal transplant recipients who elicit matched or mismatched strain-specific gH antibody responses after kidney transplantation. The patients with mismatched anti-gH antibodies appear to have more adverse outcomes compared to those with matched anti-gH antibodies, including a higher possibility of acute tissue rejection, HCMV disease-related manifestations, and a higher level of antigenemia. This suggests that the strain-specific gH antibody may be correlated with disease severity [179]. In terms of cellular immunity, it has also been reported that the T-helper-cell response to gH is strain-specific [48].
8. Concluding Remarks
We have reviewed the commonly observed polymorphic sites and regions of HCMV glycoproteins (gB, gN, gO, and gH), which play an essential role in the formation of glycoprotein complexes on the surface of the HCMV virion. Additionally, we have organized data from previous global genotyping studies of HCMV glycoproteins. The dominant gB and gH genotypes across disease states in each continent are summarized in Table 3. The percentage of each genotype in the same country varies by study, and further data will be required to determine whether significant geographic statistical associations exist.

Table 3.
Dominant gB and gH genotypes across disease states in each continent.
We have also reviewed the biological relevance of polymorphisms within viral glycoproteins by presenting evidence relating them with strain-specific immune responses. The biological relevance of specific genotypic variation has been suggested by several studies. For example, gH neutralizing epitopes were shown to lie in the most variable regions of gH [47], suggesting that the polymorphisms of glycoproteins might be an effective strategy for virus immune evasion. Additionally, instead of directly mediating neutralizing antibody responses, the polymorphisms of glycoproteins could indirectly moderate neutralizing antibody responses by blocking neutralizing epitopes of other glycoproteins. For example, it has been reported that polymorphisms within gO protected neutralizing epitopes of gH [173]. In terms of strain-specific T-cell immunity, there are currently no studies at the individual epitope level relating to glycoprotein variation, though one study has reported that T-helper cell response to gH is strain-specific [48]. Further studies are required to fully understand the functional relevance of such glycoprotein variability.
Although considerable data have been accumulated in recent years, many important questions still remain regarding how common polymorphisms in glycoproteins should best be incorporated into CMV vaccine development. Specifically, the nature and degree of associations between genotypic variation in the glycoproteins and functional difference in HCMV, as well as the elicited strain-specific immunity by genotype, should be further studied in relation to protection against CMV acquisition and reinfection. Although the situation is further complicated by the prevalence of mixed-strain infections, the relationship between these mixed infection and disease severity and progression is of growing interest. These emerging results suggest the importance of multivalent vaccine designs, as well as a consideration of glycoprotein variation in future HCMV vaccine design and therapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13061106/s1. Figure S1: Peptide sequence variations that define: (a) five gB genotypes, (b) four gN genotypes, (c) five gO genotypes, and (d) two gH genotypes.
Funding
This research was funded by NIH-NIAID, grant number 5P01AI129859, Immunologic and virologic determinants of congenital Cytomegalovirus transmission and disease in rhesus monkeys.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The sequence alignment figures in Figure 1, Figure 2, Figure 3 and Figure 4 are made by BOXSHADE Server (https://embnet.vital-it.ch/software/BOX_form.html, accessed on May–June 2021).
Conflicts of Interest
S.R.P. is a consultant for Merck, Moderna, and Dynavax vaccine programs. S.M.V. has a sponsored research program with Merck and Moderna. T.F.K. is a consultant to the Moderna vaccine program. All the other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Emery, V.C. Investigation of CMV disease in immunocompromised patients. J. Clin. Pathol. 2001, 54, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.W. Reactivation and recombination of multiple cytomegalovirus strains from individual organ donors. J. Infect. Dis. 1989, 160, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sackman, A.M.; Pfeifer, S.P.; Kowalik, T.F.; Jensen, J.D. On the demographic and selective forces shaping patterns of human cytomegalovirus variation within hosts. Pathogens 2018, 7, 16. [Google Scholar] [CrossRef]
- Huang, E.S.; Huong, S.M.; Tegtmeier, G.E.; Alford, C. Cytomegalovirus: Genetic variation of viral genomes. Ann. N. Y. Acad. Sci. 1980, 354, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Geissler, A.; Winters, M. Inter- and Intragenic Variations Complicate the Molecular Epidemiology of Human Cytomegalovirus. J. Infect. Dis. 2003, 187, 809–819. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Furukawa, T.; Zygraich, N.; Huygelen, C. Candidate cytomegalovirus strain for human vaccination. Infect. Immun. 1975, 12, 521–527. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Friedman, H.M.; Fleisher, G.R.; Dafoe, D.C.; Grossman, R.A.; Lynn Smiley, M.; Starr, S.E.; Wlodaver, C.; Friedman, A.D.; Barker, C.F. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet 1984, 323, 528–530. [Google Scholar] [CrossRef]
- Chee, M.S.; Bankier, A.T.; Beck, S.; Bohni, R.; Brown, C.M.; Cerny, R.; Horsnell, T.; Hutchison, C.A.; Kouzarides, T.; Martignetti, J.A.; et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. In Proceedings of the Cytomegaloviruses; McDougall, J.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 125–169. [Google Scholar]
- Renzette, N.; Pfeifer, S.P.; Matuszewski, S.; Kowalik, T.F.; Jensen, J.D. On the analysis of intrahost and interhost viral populations: Human cytomegalovirus as a case study of pitfalls and expectations. J. Virol. 2017, 91, 1–8. [Google Scholar] [CrossRef]
- Sinzger, C.; Hahn, G.; Digel, M.; Katona, R.; Sampaio, K.L.; Messerle, M.; Hengel, H.; Koszinowski, U.; Brune, W.; Adler, B. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 2008, 89, 359–368. [Google Scholar] [CrossRef]
- Murphy, E.; Yu, D.; Grimwood, J.; Schmutz, J.; Dickson, M.; Jarvis, M.A.; Hahn, G.; Nelson, J.A.; Myers, R.M.; Shenk, T.E. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 2003, 100, 14976–14981. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Gatherer, D.; Hilfrich, B.; Baluchova, K.; Dargan, D.J.; Thomson, M.; Griffiths, P.D.; Wilkinson, G.W.G.; Schulz, T.F.; Davison, A.J. Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J. Gen. Virol. 2010, 91, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Bhattacharjee, B.; Jensen, J.D.; Gibson, L.; Kowalik, T.F. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 2011, 7, e1001344. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Gibson, L.; Bhattacharjee, B.; Fisher, D.; Schleiss, M.R.; Jensen, J.D.; Kowalik, T.F. Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PLoS Genet. 2013, 9, e1003735. [Google Scholar] [CrossRef]
- Sijmons, S.; Thys, K.; Corthout, M.; Van Damme, E.; Van Loock, M.; Bollen, S.; Baguet, S.; Aerssens, J.; Van Ranst, M.; Maes, P. A method enabling high-throughput sequencing of human cytomegalovirus complete genomes from clinical isolates. PLoS ONE 2014, 9, e95501. [Google Scholar] [CrossRef]
- Renzette, N.; Pokalyuk, C.; Gibson, L.; Bhattacharjee, B.; Schleiss, M.R.; Hamprecht, K.; Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Britt, W.J.; Jensen, J.D.; et al. Limits and patterns of cytomegalovirus genomic diversity in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E4120–E4128. [Google Scholar] [CrossRef] [PubMed]
- Suárez, N.M.; Wilkie, G.S.; Hage, E.; Camiolo, S.; Holton, M.; Hughes, J.; Maabar, M.; Vattipally, S.B.; Dhingra, A.; Gompels, U.A.; et al. Human cytomegalovirus genomes sequenced directly from clinical material: Variation, multiple-strain infection, recombination, and gene loss. J. Infect. Dis. 2019, 220, 781–791. [Google Scholar] [CrossRef]
- Cudini, J.; Roy, S.; Houldcroft, C.J.; Bryant, J.M.; Depledge, D.P.; Tutill, H.; Veys, P.; Williams, R.; Worth, A.J.J.; Tamuri, A.U.; et al. Human cytomegalovirus haplotype reconstruction reveals high diversity due to superinfection and evidence of within-host recombination. Proc. Natl. Acad. Sci. USA 2019, 116, 5693–5698. [Google Scholar] [CrossRef]
- Renzette, N.; Gibson, L.; Jensen, J.D.; Kowalik, T.F. Human cytomegalovirus intrahost evolution—A new avenue for understanding and controlling herpesvirus infections. Curr. Opin. Virol. 2014, 8, 109–115. [Google Scholar] [CrossRef]
- Renzette, N.; Kowalik, T.F.; Jensen, J.D. On the relative roles of background selection and genetic hitchhiking in shaping human cytomegalovirus genetic diversity. Mol. Ecol. 2016, 25, 403–413. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Boppana, S.B.; Novak, Z.; Wagatsuma, V.M.; de Frizzo Oliveira, P.; Duarte, G.; Britt, W.J. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am. J. Obstet. Gynecol. 2010, 202, 297.e1–297.e8. [Google Scholar] [CrossRef]
- Pokalyuk, C.; Renzette, N.; Irwin, K.K.; Pfeifer, S.P.; Gibson, L.; Britt, W.J.; Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Kowalik, T.F.; Jensen, J.D. Characterizing human cytomegalovirus reinfection in congenitally infected infants: An evolutionary perspective. Mol. Ecol. 2017, 26, 1980–1990. [Google Scholar] [CrossRef]
- Barbi, M.; Binda, S.; Caroppo, S.; Primache, V.; Didò, P.; Guidotti, P.; Corbetta, C.; Melotti, D. CMV gB genotypes and outcome of vertical transmission: Study on dried blood spots of congenitally infected babies. J. Clin. Virol. 2001, 21, 75–79. [Google Scholar] [CrossRef]
- Murphy, E.; Shenk, T.E. Human Cytomegalovirus Genome; Springer: Berlin/Heidelberg, Germany, 2008; Volume 325, ISBN 9783540773481. [Google Scholar]
- Britt, W.J.; Mach, M. Human cytomegalovirus glycoproteins. Intervirology 1996, 39, 401–412. [Google Scholar] [CrossRef]
- Isaacson, M.K.; Compton, T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J. Virol. 2009, 83, 3891–3903. [Google Scholar] [CrossRef]
- Mach, M.; Kropff, B.; Dal Monte, P.; Britt, W. Complex Formation by Human Cytomegalovirus Glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 2000, 74, 11881–11892. [Google Scholar] [CrossRef]
- Gretch, D.R.; Kari, B.; Rasmussen, L.; Gehrz, R.C.; Stinski, M.F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J. Virol. 1988, 62, 875–881. [Google Scholar] [CrossRef]
- Wang, D.; Shenk, T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 2005, 102, 18153–18158. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Chou, S.; Dennison, K.M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1991, 163, 1229–1234. [Google Scholar] [CrossRef]
- Chou, S. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology 1992, 188, 388–390. [Google Scholar] [CrossRef]
- Shepp, D.H.; Match, M.E.; Lipson, S.M.; Pergolizzi, R.G. A fifth human cytomegalovirus glycoprotein B genotype. Res. Virol. 1998, 149, 109–114. [Google Scholar] [CrossRef]
- Pignatelli, S.; Dal Monte, P.; Landini, M.P. gpUL73 (gN) genomic variants of human cytomegalovirus isolates are clustered into four distinct genotypes. J. Gen. Virol. 2001, 82, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, S.; Dal Monte, P.; Rossini, G.; Chou, S.; Gojobori, T.; Hanada, K.; Guo, J.J.; Rawlinson, W.; Britt, W.; Mach, M.; et al. Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: Identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J. Gen. Virol. 2003, 84, 647–655. [Google Scholar] [CrossRef]
- Paterson, D.A.; Dyer, A.P.; Milne, R.S.B.; Sevilla-Reyes, E.; Gompels, U.A. A role for human cytomegalovirus glycoprotein O (gO) in cell fusion and a new hypervariable locus. Virology 2002, 293, 281–294. [Google Scholar] [CrossRef]
- Rasmussen, L.; Geissler, A.; Cowan, C.; Chase, A.; Winters, M. The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates. J. Virol. 2002, 76, 10841–10848. [Google Scholar] [CrossRef]
- Stanton, R.; Westmoreland, D.; Fox, J.D.; Davison, A.J.; Wilkinson, G.W.G. Stability of Human cytomegalovirus genotypes in persistently infected renal transplant recipients. J. Med. Virol. 2005, 75, 42–46. [Google Scholar] [CrossRef]
- Mattick, C.; Dewin, D.; Polley, S.; Sevilla-Reyes, E.; Pignatelli, S.; Rawlinson, W.; Wilkinson, G.; Dal Monte, P.; Gompels, U.A. Linkage of human cytomegalovirus glycoprotein gO variant groups identified from worldwide clinical isolates with gN genotypes, implications for disease associations and evidence for N-terminal sites of positive selection. Virology 2004, 318, 582–597. [Google Scholar] [CrossRef]
- Görzer, I.; Guelly, C.; Trajanoski, S.; Puchhammer-Stöckl, E. Deep sequencing reveals highly complex dynamics of human cytomegalovirus genotypes in transplant patients over time. J. Virol. 2010, 84, 7195–7203. [Google Scholar] [CrossRef]
- Chou, S. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J. Infect. Dis. 1992, 166, 604–607. [Google Scholar] [CrossRef]
- Murthy, S.; Hayward, G.S.; Wheelan, S.; Forman, M.S.; Ahn, J.H.; Pass, R.F.; Arav-Boger, R. Detection of a single identical cytomegalovirus (CMV) strain in recently seroconverted young women. PLoS ONE 2011, 6, e15949. [Google Scholar] [CrossRef]
- Yan, H.; Koyano, S.; Inami, Y.; Yamamoto, Y.; Suzutani, T.; Mizuguchi, M.; Ushijima, H.; Kurane, I.; Inoue, N. Genetic linkage among human cytomegalovirus glycoprotein N (gN) and gO genes, with evidence for recombination from congenitally and post-natally infected Japanese infants. J. Gen. Virol. 2008, 89, 2275–2279. [Google Scholar] [CrossRef]
- Arav-Boger, R. Strain variation and disease severity in congenital cytomegalovirus infection: In search of a viral marker. Infect. Dis. Clin. N. Am. 2015, 29, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Schoppel, K.; Amvrossiadis, N.; Mach, M. Strain-specific neutralization of human cytomegalovirus isolates by human sera. J. Virol. 1999, 73, 878–886. [Google Scholar] [CrossRef]
- Cui, X.; Freed, D.C.; Wang, D.; Qiu, P.; Li, F.; Fu, T.-M.; Kauvar, L.M.; McVoy, M.A. Impact of antibodies and strain polymorphisms on cytomegalovirus entry and spread in fibroblasts and epithelial cells. J. Virol. 2017, 91, 1–17. [Google Scholar] [CrossRef]
- Beninga, J.; Kalbacher, H.; Mach, M. Analysis of T helper cell response to glycoprotein H (gpUL75) of human cytomegalovirus: Evidence for strain-specific T cell determinants. J. Infect. Dis. 1996, 173, 1051–1061. [Google Scholar] [CrossRef][Green Version]
- Burke, H.G.; Heldwein, E.E. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog. 2015, 11, e1005227. [Google Scholar] [CrossRef]
- Chandramouli, S.; Ciferri, C.; Nikitin, P.A.; Caló, S.; Gerrein, R.; Balabanis, K.; Monroe, J.; Hebner, C.; Lilja, A.E.; Settembre, E.C.; et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat. Commun. 2015, 6, 8176. [Google Scholar] [CrossRef]
- Bagdonaite, I.; Nordén, R.; Joshi, H.J.; King, S.L.; Vakhrushev, S.Y.; Olofsson, S.; Wandall, H.H. Global mapping of o-glycosylation of varicella zoster virus, human cytomegalovirus, and Epstein-Barr virus. J. Biol. Chem. 2016, 291, 12014–12028. [Google Scholar] [CrossRef]
- Spaete, R.R.; Thayer, R.M.; Probert, W.S.; Masiarz, F.R.; Chamberlain, S.H.; Rasmussen, L.; Merigan, T.C.; Pachl, C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 1988, 167, 207–225. [Google Scholar] [CrossRef]
- Haberland, M.; Meyer-König, U.; Hufert, F.T. Variation within the glycoprotein B gene of human cytomegalovirus is due to homologous recombination. J. Gen. Virol. 1999, 80, 1495–1500. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, X.; Chen, X.M.; Gao, H.N.; Yang, M.F.; Zhao, H.; Hu, J.H.; Ma, W.H. Monitoring of human cytomegalovirus glycoprotein B genotypes using real-time quantitative PCR in immunocompromised Chinese patients. J. Virol. Methods 2009, 160, 74–77. [Google Scholar] [CrossRef]
- Grosjean, J.; Hantz, S.; Cotin, S.; Baclet, M.C.; Mengelle, C.; Trapes, L.; Virey, B.; Undreiner, F.; Brosset, P.; Pasquier, C.; et al. Direct genotyping of cytomegalovirus envelope glycoproteins from toddler’s saliva samples. J. Clin. Virol. 2009, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.P.; Gao, H.N.; Yang, M.F.; Zhao, H.; Hu, J.H.; Chen, X.M.; Ma, W.H.; Fan, J. Quantification of cytomegalovirus glycoprotein Bn DNA in hematopoietic stem cell transplant recipients by real-time PCR. PLoS ONE 2012, 7, e51224. [Google Scholar] [CrossRef] [PubMed]
- Meyer-König, U.; Haberland, M.; Von Laer, D.; Haller, O.; Hufert, F.T. Intragenic variability of human cytomegalovirus glycoprotein B in clinical strains. J. Infect. Dis. 1998, 177, 1162–1169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasmussen, L.; Hong, C.; Zipeto, D.; Morris, S.; Sherman, D.; Chou, S.; Miner, R.; Drew, W.L.; Wolitz, R.; Dowling, A.; et al. Cytomegalovirus gB genotype distribution differs in human immunodeficiency virus-infected patients and immunocompromised allograft recipients. J. Infect. Dis. 1997, 175, 179–184. [Google Scholar] [CrossRef]
- Peek, R.; Verbraak, F.; Bruinenberg, M.; Van Der Lelij, A.; Van Den Horn, G.; Kijlstra, A. Cytomegalovirus glycoprotein B genotyping in ocular fluids and blood of AIDS patients with cytomegalovirus retinitis. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1183–1187. [Google Scholar]
- Wada, K.; Mizuno, S.; Kato, K.; Kamiya, T.; Ozawa, K. Cytomegalovirus glycoprotein b sequence variation among japanese bone marrow transplant recipients. Intervirology 1997, 40, 215–219. [Google Scholar] [CrossRef]
- Pacsa, A.S.; Essa, S.; Voevodin, A.; El-Shazly, A.; Kazak, H.; Nampoory, M.R.N.; Johny, K.V.; Said, T.; Al-Nakib, W. Correlation between CMV genotypes, multiple infections with herpesviruses (HHV-6, 7) and development of CMV disease in kidney recipients in Kuwait. FEMS Immunol. Med. Microbiol. 2003, 35, 125–130. [Google Scholar] [CrossRef]
- Tanaka, K.; Numazaki, K.; Tsutsumi, H. Human cytomegalovirus genetic variability in strains isolated from Japanese children during 1983–2003. J. Med. Virol. 2005, 76, 356–360. [Google Scholar] [CrossRef]
- Yu, Z.S.; Zou, C.C.; Zheng, J.Y.; Zhao, Z.Y. Cytomegalovirus gB genotype and clinical features in Chinese infants with congenital infections. Intervirology 2006, 49, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, X.; Li, S. Human cytomegalovirus glycoprotein B genotype correlates with different symptoms of infected infants. Intervirology 2007, 50, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Koyano, S.; Inami, Y.; Yamamoto, Y.; Suzutani, T.; Mizuguchi, M.; Ushijima, H.; Kurane, I.; Inoue, N. Genetic variations in the gB, UL144 and UL149 genes of human cytomegalovirus strains collected from congenitally and postnatally infected Japanese children. Arch. Virol. 2008, 153, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.L.; Cai, T.; Jin, H.M. Human cytomegalovirus glycoprotein genotypes in the genital tract tissue of tubal pregnancy patients. J. Int. Med. Res. 2009, 37, 385–391. [Google Scholar] [CrossRef]
- Mewara, A.; Mishra, B.; Ratho, R.K.; Kumar, P. Cytomegalovirus glycoprotein b gene polymorphism and its association with clinical presentations in infants. Southeast Asian J. Trop. Med. Public Health 2009, 40, 759–764. [Google Scholar]
- Shen, Z.; Shang, S.Q.; Zou, C.C.; Zheng, J.Y.; Yu, Z.S. The detection and clinical features of human cytomegalovirus infection in infants. Fetal Pediatr. Pathol. 2010, 29, 393–400. [Google Scholar] [CrossRef]
- Gandhoke, I.; Hussain, S.A.; Pasha, S.T.; Chauhan, L.S.; Khare, S. Glycoprotein B genotyping in congenital/perinatal cytomegalovirus infection in symptomatic infants. Indian Pediatr. 2013, 50, 663–667. [Google Scholar] [CrossRef]
- Paca-Uccaralertkun, S.; Hiatt, R.; Leecharoen, R.; Tan-Ariya, P.; Mungthin, M.; Pongphong, S. Human cytomegalovirus gB1 genotypes among children who live at the Phayathai Babies’ home in Nonthaburi, Thailand. Southeast Asian J. Trop. Med. Public Health 2013, 44, 636–640. [Google Scholar]
- Ding, Z.Y.; Xu, F.; Chen, D.Z.; Meng, X.N.; Xu, T.S.; Lu, M.D.; Zhuge, H.X. A multifactorial analysis of the pregnancy outcomes in cytomegalovirus-infected women. Gynecol. Obstet. Investig. 2015, 80, 106–112. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, J.; Zheng, S.S.; Ma, W.H. Genetic variation within the glycoprotein B and H genes of human cytomegalovirus in solid organ transplant recipients. Transpl. Infect. Dis. 2007, 9, 73–77. [Google Scholar] [CrossRef]
- Mujtaba, G.; Khurshid, A.; Sharif, S.; Alam, M.M.; Aamir, U.B.; Shaukat, S.; Angez, M.; Rana, M.S.; Umair, M.; Shah, A.A.; et al. Distribution of cytomegalovirus genotypes among neonates born to infected mothers in Islamabad, Pakistan. PLoS ONE 2016, 11, e156049. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Zheng, T.L.; Zhou, T.; Hu, P.W.; Huang, M.J.; Xu, X.; Pei, X.F. Human cytomegalovirus prevalence and distribution of glycoprotein B, O genotypes among hospitalized children with respiratory infections in West China, 2009–2014. Trop. Med. Int. Health 2016, 21, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Das, D.; Ansari, S.; Chatterjee, R.P.; Mishra, L.; Basu, B.; Ghosh, S.K.; Bhattacharyay, M.; Chakraborty, N. Genotypes of glycoprotein B gene among the Indian symptomatic neonates with congenital CMV infection. BMC Pediatr. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Alwan, S.N.; Shamran, H.A.; Ghaib, A.H.; Kadhim, H.S.; Al-Mayah, Q.S.; AL-Saffar, A.J.; Bayati, A.H.; Arif, H.S.; Fu, J.; Wickes, B.L. Genotyping of cytomegalovirus from symptomatic infected neonates in Iraq. Am. J. Trop. Med. Hyg. 2019, 100, 957–963. [Google Scholar] [CrossRef]
- Min, X.; Wang, L.; Cui, A.; Zhang, C.; Wang, D.; Liu, Y.; Li, Z.; Xu, W. The nucleic acid positive rate and genotype distribution of human cytomegalovirus in human milk banks in China. Arch. Virol. 2020, 165, 1099–1107. [Google Scholar] [CrossRef]
- Dong, N.; Cao, L.; Su, L.; Lu, L.; Dong, Z.; Xu, M.; Xu, J. Human cytomegalovirus envelope glycoprotein B, H, and N polymorphisms among infants of Shanghai area in China. J. Med. Virol. 2020, 92, 3674–3681. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lo, C.Y.; Lo, S.K.F.; Siau, H.; Peiris, J.S.M.; Wong, S.S.Y.; Luk, W.K.; Chan, T.M.; Lim, W.W.; Yuen, K.Y. Distinct genotypic distributions of cytomegalovirus (CMV) envelope glycoprotein in bone marrow and renal transplant recipients with CMV disease. Clin. Diagn. Lab. Immunol. 1997, 4, 515–518. [Google Scholar] [CrossRef]
- Madhaven, H.N.; Priya, K. Polymerase chain reaction based restriction fragment length polymorphism for the genotyping of cytomegalovirus (CMV) from patients with CMV disease in Chennai. Indian J. Med. Res. 2002, 130, 556. [Google Scholar]
- Wu, Y.M.; Yan, J.; Ojcius, D.M.; Chen, L.L.; Gu, Z.Y.; Pan, J.P. Correlation between infections with different genotypes of human cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients. J. Clin. Microbiol. 2007, 45, 3665–3670. [Google Scholar] [CrossRef]
- Bhattarakosol, P.; Chantaraarphonkun, S. Prevalence of human cytomegalovirus (HCMV) gB genotypes in Thai patients. Southeast Asian J. Trop. Med. Public Health 2007, 38, 835–840. [Google Scholar] [PubMed]
- Pang, X.; Humar, A.; Preiksaitis, J.K. Concurrent genotyping and quantitation of cytomegalovirus gB genotypes in solid-organ-transplant recipients by use of a real-time PCR assay. J. Clin. Microbiol. 2008, 46, 4004–4010. [Google Scholar] [CrossRef]
- Chantaraarphonkun, S.; Bhattarakosol, P. Intra- and Intergenotypic variations among human cytomegalovirus gB genotypes. Intervirology 2007, 10330, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.S.; Tang, L.F.; Zou, C.C.; Zheng, J.Y.; Zhao, Z.Y. Cytomegalovirus-associated idiopathic thrombocytopenic purpura in Chinese children. Scand. J. Infect. Dis. 2008, 40, 922–927. [Google Scholar] [CrossRef]
- Wu, K.G.; Hung, M.C.; Chang, Y.T.; Chen, C.J.; Yang, S.P.; Liu, C.Y.; Ho, D.M.T.; Chan, Y.J. Occurrence of human cytomegalovirus glycoprotein B genotypes in immunocompetent and immunosuppressed Taiwanese patients. Intervirology 2011, 54, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, J.W.; Liu, Y.M.; Zhong, H.; Wang, L.M.; Deng, F.M.; Qu, Y.Y.; Hui, J.; Cheng, J.; Tang, B.; et al. Human cytomegalovirus infection is associated with essential hypertension in Kazakh and Han Chinese populations. Med. Sci. Monit. 2014, 20, 2508–2519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taherkhani, R.; Farshadpour, F.; Makvandi, M.; Hamidifard, M.; Esmailizadeh, M.; Ahmadi, B.; Heidari, H. Determination of cytomegalovirus prevalence and glycoprotein B genotypes among ulcerative colitis patients in Ahvaz, Iran. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Oka, N.; Suzuki, T.; Inoue, T.; Kobayashi, T.; Ohashi, Y. Polymorphisms in cytomegalovirus genotype in immunocompetent patients with corneal endotheliitis or iridocyclitis. J. Med. Virol. 2015, 87, 1441–1445. [Google Scholar] [CrossRef]
- Eran Daǧlar, D.; Öngüt, G.; Çolak, D.; Özkul, A.; Mutlu, D.; Zeytenoǧlu, A.; Midilli, K.; Gökahmetoǧlu, S.; Günseren, F.; Ögünç, D.; et al. Determination of cytomegalovirus glycoprotein B genotypes in different geographical regions and different patient groups in Turkey. Mikrobiyol. Bul. 2016, 50, 53–62. [Google Scholar] [CrossRef]
- Hu, H.; Cheng, Y.; Peng, Q.; Chen, K. Clinical features, treatment courses, and distribution of cytomegalovirus genotypes among thrombocytopenia patients aged younger than 12 months. Am. J. Perinatol. 2020, 1. [Google Scholar] [CrossRef]
- Nahar, S.; Hokama, A.; Iraha, A.; Ohira, T.; Kinjo, T.; Hirata, T.; Kinjo, T.; Parrott, G.L.; Fujita, J. Distribution of cytomegalovirus genotypes among ulcerative colitis patients in Okinawa, Japan. Intest. Res. 2018, 16, 90–98. [Google Scholar] [CrossRef]
- Mohamed, H.T.; El-Shinawi, M.; Nouh, M.A.; Bashtar, A.R.; Elsayed, E.T.; Schneider, R.J.; Mohamed, M.M. Inflammatory breast cancer: High incidence of detection of mixed human cytomegalovirus genotypes associated with disease pathogenesis. Front. Oncol. 2014, 4, 246. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Xu, Y.; Wu, D.; Sun, A.; Zhu, Z.; Han, Y.; Qiu, H.; Tang, X.; Fu, Z.; et al. Cytomegalovirus glycoprotein B genotype in hematopoietic stem cell transplant patients from China. Biol. Blood Marrow Transplant. 2010, 16, 647–652. [Google Scholar] [CrossRef][Green Version]
- Madi, N.; Al-Nakib, W.; Pacsa, A.; Saeed, T. Cytomegalovirus genotypes gB1 and gH1 Are the most predominant genotypes among renal transplant recipients in Kuwait. Transplant. Proc. 2011, 43, 1634–1637. [Google Scholar] [CrossRef]
- Xia, C.S.; Zhao, X.T.; Sun, Y.Y.; Zhang, Z. Human cytomegalovirus glycoprotein B genotypes in Chinese hematopoietic stem cell transplant recipients. Intervirology 2012, 55, 342–348. [Google Scholar] [CrossRef]
- Soleimani, A.R.; Jafari, M.; Piroozmand, A.; Nikoueinejad, H.; Akbari, H.; Einollahi, B. The Incidence of Cytomegalovirus Glycoprotein B Genotypes in Kidney Transplant Recipients in Iran. Int. J. Organ Transplant. Med. 2018, 9, 173–177. [Google Scholar] [PubMed]
- Jiang, X.J.; Zhang, J.; Xiong, Y.; Jahn, G.; Xiong, H.R.; Yang, Z.Q.; Liu, Y.Y. Human cytomegalovirus glycoprotein polymorphisms and increasing viral load in AIDS patients. PLoS ONE 2017, 12, e0176160. [Google Scholar] [CrossRef] [PubMed]
- Terabe, K.; Sugiyama, K.; Goto, K.; Mizutani, F.; Wada, Y.; Yokoyama, T.; Ando, Y. Relationship between human cytomegalovirus glycoprotein B genotype and serum alanine aminotransferase elevation in infants. Tohoku J. Exp. Med. 2004, 203, 339–344. [Google Scholar] [CrossRef]
- Torok-Storb, B.; Boeckh, M.; Hoy, C.; Leisenring, W.; Myerson, D.; Gooley, T. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood 1997, 90, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Patel, E.U.; Abraham, A.G.; Grabowski, M.K.; Arav-Boger, R.; Avery, R.K.; Quinn, T.C.; Tobian, A.A.R. Prevalence, magnitude, and genotype distribution of urinary cytomegalovirus (CMV) shedding among CMV-seropositive children and adolescents in the United States. Open Forum Infect. Dis. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Sarcinella, L.; Mazzulli, T.; Willey, B.; Humar, A. Cytomegalovirus glycoprotein B genotype does not correlate with outcomes in liver transplant patients. J. Clin. Virol. 2002, 24, 99–105. [Google Scholar] [CrossRef]
- Humar, A.; Kumar, D.; Gilbert, C.; Boivin, G. Cytomegalovirus (CMV) glycoprotein B genotypes and response to antiviral therapy, in solid-organ-transplant recipients with CMV disease. J. Infect. Dis. 2003, 188, 581–584. [Google Scholar] [CrossRef]
- Manuel, O.; Pang, X.L.; Humar, A.; Kumar, D.; Doucette, K.; Preiksaitis, J.K. An assessment of donor-to-recipient transmission patterns of human cytomegalovirus by analysis of viral genomic variants. J. Infect. Dis. 2009, 199, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Manuel, O.; Åsberg, A.; Pang, X.; Rollag, H.; Emery, V.C.; Preiksaitis, J.K.; Kumar, D.; Pescovitz, M.D.; Bignamini, A.A.; Hartmann, A.; et al. Impact of genetic polymorphisms in cytomegalovirus glycoprotein b on outcomes in solid-organ transplant recipients with cytomegalovirus disease. Clin. Infect. Dis. 2009, 49, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Handfield, J.; Toma, E.; Lalonde, R.; Bergeron, M.G.; Boivin, G. Human cytomegalovirus glycoprotein B genotypes in blood of AIDS patients: Lack of association with either the viral DNA load in leukocytes or presence of retinitis. J. Med. Virol. 1999, 59, 98–103. [Google Scholar] [CrossRef]
- Drew, W.L.; Chou, S.; Miner, R.C.; Mohr, B.A.; Busch, M.P.; van der Horst, C.M.; Asmuth, D.M.; Kalish, L.A. Cytomegalovirus glycoprotein B groups in human immunodeficiency virus-infected patients with incident retinitis. J. Infect. Dis. 2002, 186, 114–117. [Google Scholar] [CrossRef][Green Version]
- Bale, J.F.; Murph, J.R.; Demmler, G.J.; Dawson, J.; Miller, J.E.; Petheram, S.J. Intrauterine cytomegalovirus infection and glycoprotein B genotypes. J. Infect. Dis. 2000, 182, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.K.; Pinninti, S.; Novak, Z.; Chowdhury, N.; Patro, R.K.; Fowler, K.; Ross, S.; Boppana, S. Genotypic diversity and mixed infection in newborn disease and hearing loss in congenital cytomegalovirus infection. Pediatr. Infect. Dis. J. 2013, 32, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Aquino, V.H.; Figueiredo, L.T.M. High prevalence of renal transplant recipients infected with more than one cytomegalovirus glycoprotein B genotype. J. Med. Virol. 2000, 61, 138–142. [Google Scholar] [CrossRef]
- Carraro, E.; Granato, C.F.H. Single human cytomegalovirus gB genotype shed in multiple sites at the time of diagnosis in renal transplant recipients. J. Med. Virol. 2003, 70, 240–243. [Google Scholar] [CrossRef]
- Correa, C.; Kourí, V.; Pérez, L.; Soto, Y.; Limia, C. Diagnosis, gB genotype distribution and viral load of symptomatic congenitally infected CMV patients in Cuba. J. Perinatol. 2016, 36, 837–842. [Google Scholar] [CrossRef]
- Ahumada-Ruiz, S.; Taylor-Castillo, L.; Visoná, K.; Luftig, R.B.; Herrero-Uribe, L. Determination of human cytomegalovirus genetic diversity in different patient populations in Costa Rica. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 87–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kourí, V.; González, E.; Martínez, P.; Capó, V.; González, R.; Pérez, L.; Viera, J.; Cardellá, L.; Hengge, U.R. Distinct genotypic distribution of cytomegalovirus (CMV) envelope glycoprotein B (gB) in a Cuban cohort of patients with different CMV diseases. Scand. J. Infect. Dis. 2007, 39, 1038–1044. [Google Scholar] [CrossRef]
- Slavov, S.N.; Kashima, S.; Wagatsuma, V.M.D.; Silva-Pinto, A.C.; Martinez, E.Z.; Favarin, M.D.C.; Covas, D.T. Glycoprotein B genotyping of human cytomegalovirus strains isolated from Brazilian patients with sickle cell disease and beta-thalassemia major. Viral Immunol. 2015, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Ozaki, K.S.; Tomiyama, H.; Câmara, N.O.S.; Granato, C.F.H. Clinical correlations of human cytomegalovirus strains and viral load in kidney transplant recipients. Int. Immunopharmacol. 2009, 9, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Dieamant, D.D.C.; Bonon, S.H.A.; Prates, L.C.; Belangelo, V.M.S.; Pontes, E.R.; Costa, S.C.B. Active human cytomegalovirus infection and glycoprotein B genotypes in Brazilian pediatric renal or hematopoietic stem cell transplantation patients. Braz. J. Microbiol. 2009, 53, 1689–1699. [Google Scholar] [CrossRef]
- Correia-Silva, J.F.; Resende, R.G.; Arão, T.C.; Abreu, M.; Teixeira, M.M.; Bittencourt, H.; Silva, T.A.; Gomez, R.S. HCMV gB genotype and its association with cytokine levels in hematopoietic stem cell transplantation. Oral Dis. 2011, 17, 530–537. [Google Scholar] [CrossRef] [PubMed]
- González-Ramírez, J.; Uribe-Gutiérrez, G.; Jiménez-Hernandez, E.; Velázquez-Guadarrama, N.; Bello-González, A.; Vazquez-Meraz, E.; Arellano-Galindo, J. Cytomegalovirus gB genotype distribution in Mexican children undergoing allogeneic bone marrow transplantation. Intervirology 2012, 55, 318–320. [Google Scholar] [CrossRef]
- Vilas Boas, L.S.; De Souza, V.A.U.F.; De Oliveira, A.C.P.; Rodriguez Viso, A.T.; Nascimento Filho, A.M.; Nascimento, M.C.; Pannuti, C.S. Cytomegalovirus glycoprotein B genotypes and central nervous system disease in AIDS patients. J. Med. Virol. 2003, 71, 404–407. [Google Scholar] [CrossRef]
- Cunha, A.A.; Aquino, V.H.; Mariguela, V.; Nogueira, M.L.; Figueiredo, L.T.M. Evaluation of glycoprotein B genotypes and load of CMV infecting blood leukocytes on prognosis of AIDS patients. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 83–88. [Google Scholar] [CrossRef]
- Arellano-Galindo, J.; Villanueva-García, D.; Cruz-Ramírez, J.L.; Yalaupari-Mejía, J.P.; Uribe-Gutiérrez, G.; Velazquez-Guadarrama, N.; Nava-Frías, M.; Muñoz-Hernández, O.; Mejía-Aranguré, J.M. Detection and gB genotyping of CMV in Mexican preterm infants in the context of maternal seropositivity. J. Infect. Dev. Ctries. 2014, 8, 758–767. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Sanchez, H.M.; Alvarado-Hernandez, D.L.; Guerra-Palomares, S.; Garcia-Sepulveda, C.A.; Noyola, D.E. Cytomegalovirus glycoprotein B genotypes in Mexican children and women. Intervirology 2015, 58, 115–121. [Google Scholar] [CrossRef]
- Barrado, L.; Prieto, C.; Hernando, S.; Folgueira, L. Detection of glycoproteins B and H genotypes to predict the development of Cytomegalovirus disease in solid organ transplant recipients. J. Clin. Virol. 2018, 109, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Cella, E.; Rittà, M.; Ciccozzi, M.; Cavallo, R.; Perno, C.F.; Costa, C. Cytomegalovirus glycoprotein B genotype distribution in Italian transplant patients. Intervirology 2018, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Janković, M.; Ćupić, M.; Knežević, A.; Vujić, D.; Soldatović, I.; Zečević, Ž.; Gobeljić, B.; Jovanović, T. Cytomegalovirus glycoprotein B and N genotypes in pediatric recipients of the hematopoietic stem cell transplant. Virology 2020, 548, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Fidouh-Houhou, N.; Duval, X.; Bissuel, F.; Bourbonneux, V.; Flandre, P.; Ecobichon, J.L.; Jordan, M.C.; Vildé, J.L.; Brun-Vézinet, F.; Leport, C. Salivary cytomegalovirus (CMV) shedding, glycoprotein b genotype distribution, and CMV disease in human immunodeficiency virus-seropositive patients. Clin. Infect. Dis. 2001, 33, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Tarragó, D.; Quereda, C.; Tenorio, A. Different cytomegalovirus glycoprotein B genotype distribution in serum and cerebrospinal fluid specimens determined by a novel multiplex nested PCR. J. Clin. Microbiol. 2003, 41, 2872–2877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goossens, V.J.; Wolffs, P.F.; Van Loo, I.H.; Bruggeman, C.A.; Verbon, A. CMV DNA levels and CMV gB subtypes in ART-naive HAART-treated patients: A 2-year follow-up study in the Netherlands. Aids 2009, 23, 1425–1429. [Google Scholar] [CrossRef]
- Vogel, J.U.; Otte, J.; Koch, F.; Gümbel, H.; Doerr, H.W.; Cinatl, J. Role of human cytomegalovirus genotype polymorphisms in AIDS patients with cytomegalovirus retinitis. Med. Microbiol. Immunol. 2013, 202, 37–47. [Google Scholar] [CrossRef]
- Lukacsi, A.; Tarodi, B.; Endreffy, E.; Babinszki, A.; Pal, A.; Pusztai, R. Human cytomegalovirus gB genotype 1 is dominant in congenital infections in South Hungary. J. Med. Virol. 2001, 65, 537–542. [Google Scholar] [CrossRef]
- Picone, O.; Costa, J.M.; Leruez-Ville, M.; Ernault, P.; Olivi, M.; Ville, Y. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat. Diagn. 2004, 24, 1001–1006. [Google Scholar] [CrossRef]
- Grosjean, J.; Trapes, L.; Hantz, S.; Mengelle, C.; Virey, B.; Undreiner, F.; Messager, V.; Denis, F.; Marin, B.; Alain, S. Human cytomegalovirus quantification in toddlers saliva from day care centers and emergency unit: A feasibility study. J. Clin. Virol. 2014, 61, 371–377. [Google Scholar] [CrossRef]
- Zawilińska, B.; Szostek, S.; Kopeć, J.; Koprynia, M.; Kosz-Vnenchak, M. UL55 genotype diversity of cytomegalovirus strains isolated from newborns and infants hospitalized in southern Poland. Przegląd Epidemiol. 2011, 65, 409–413. [Google Scholar]
- Paradowska, E.; Studzińska, M.; Nowakowska, D.; Wilczyński, J.; Rycel, M.; Suski, P.; Gaj, Z.; Kaczmarek, B.; Zbróg, Z.; Leśnikowski, Z.J. Distribution of UL144, US28 and UL55 genotypes in Polish newborns with congenital cytomegalovirus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Nijman, J.; Mandemaker, F.S.; Verboon-Maciolek, M.A.; Aitken, S.C.; Van Loon, A.M.; De Vries, L.S.; Schuurman, R. Genotype distribution, viral load and clinical characteristics of infants with postnatal or congenital cytomegalovirus infection. PLoS ONE 2014, 9, e108018. [Google Scholar] [CrossRef][Green Version]
- Paradowska, E.; Studzińska, M.; Suski, P.; Kasztelewicz, B.; Wisniewska-Ligier, M.; Zawilinska, B.; Gaj, Z.; Nowakowska, D. Human cytomegalovirus UL55, UL144, and US28 genotype distribution in infants infected congenitally or postnatally. J. Med. Virol. 2015, 87, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Trajanoski, S.; Popow-Kraupp, T.; Puchhammer-Stöckl, E. Analysis of human cytomegalovirus strain populations in urine samples of newborns by ultra deep sequencing. J. Clin. Virol. 2015, 73, 101–104. [Google Scholar] [CrossRef]
- Arcangeletti, M.C.; Simone, R.V.; Rodighiero, I.; De Conto, F.; Medici, M.C.; Martorana, D.; Chezzi, C.; Calderaro, A. Combined genetic variants of human cytomegalovirus envelope glycoproteins as congenital infection markers. Virol. J. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Brañas, P.; Blázquez-Gamero, D.; Galindo, A.; Prieto, C.; Olabafrieta, I.; Cuadrado, I.; Folgueria, L. Cytomegalovirus genotype distribution among congenitally and postnatally infected patients: Association of particular glycoprotein (g)B and gN types with symptomatic disease. Open Forum Infect. Dis. 2015, 2, 2633851. [Google Scholar] [CrossRef]
- Kadambari, S.; Atkinson, C.; Luck, S.; Macartney, M.; Conibear, T.; Harrison, I.; Booth, C.; Sharland, M.; Griffiths, P.D. Characterising variation in five fenetic loci of cytomegalovirus during treatment for congenital infection. J. Med. Virol. 2017, 89, 502–507. [Google Scholar] [CrossRef]
- Puhakka, L.; Pati, S.; Lappalainen, M.; Lönnqvist, T.; Niemensivu, R.; Lindahl, P.; Nieminen, T.; Seuri, R.; Nupponen, I.; Boppana, S.; et al. Viral shedding, and distribution of cytomegalovirus glycoprotein H (UL75), glycoprotein B (UL55), and glycoprotein N (UL73) genotypes in congenital cytomegalovirus infection. J. Clin. Virol. 2020, 125, 104287. [Google Scholar] [CrossRef]
- Arista, S.; De Grazia, S.; Giammanco, G.M.; Di Carlo, P.; Iannitto, E. Human cytomegalovirus glycoprotein B genotypes in immunocompetent, immunocompromised, and congenitally infected Italian populations. Arch. Virol. 2003, 148, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Coaquette, A.; Bourgeois, A.; Dirand, C.; Varin, A.; Chen, W.; Herbein, G. Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin. Infect. Dis. 2004, 39, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Kerschner, H.; Redlberger-Fritz, M.; Puchhammer-Stöckl, E. Human cytomegalovirus (HCMV) genotype populations in immunocompetent individuals during primary HCMV infection. J. Clin. Virol. 2010, 48, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Costa, C.; Gambarino, S.; Tornicelli, A.; Astegiano, S.; Terlizzi, M.E.; Solidoro, P.; Cavallo, R. Human cytomegalovirus glycoprotein B genotyping from bronchoalveolar lavage specimens. Can. J. Microbiol. 2011, 57, 273–277. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Andric, M.; Knezevic, A.; Soldatovic, I.; Nikolic, N.; Karalic, D.; Milasin, J. Human cytomegalovirus and Epstein-Barr virus genotypes in apical periodontitis lesions. J. Endod. 2015, 41, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Fries, B.C.; Chon, S.; Boeckh, M.; Torok-Storb, B. Frequency distribution of cytomegalovirus envelope glycoprotein genotypes in bone marrow transplant recipients. J. Infect. Dis. 1994, 169, 769–774. [Google Scholar] [CrossRef]
- Vogelberg, C.; Meyer-König, U.; Hufert, F.T.; Kirste, G.; Von Laer, D. Human cytomegalovirus glycoprotein B genotypes in renal transplant recipients. J. Med. Virol. 1996, 50, 31–34. [Google Scholar] [CrossRef]
- Hebart, H.; Greif, M.; Krause, H.; Kanz, L.; Jahn, G.; Müller, C.A.; Einsele, H. Interstrain variation of immediate early DNA sequences and glycoprotein B genotypes in cytomegalovirus clinical isolates. Med. Microbiol. Immunol. 1997, 186, 135–138. [Google Scholar] [CrossRef]
- Meyer-König, U.; Vogelberg, C.; Bongarts, A.; Kampa, D.; Delbrück, R.; Wolff-Vorbeck, G.; Kirste, G.; Haberland, M.; Hufert, F.T.; Von Laer, D. Glycoprotein B genotype correlates with cell tropism in vivo of human cytomegalovirus infection. J. Med. Virol. 1998, 55, 75–81. [Google Scholar] [CrossRef]
- De Vries, J.J.C.; Wessels, E.; Korver, A.M.H.; Van Der Eijk, A.A.; Rusman, L.G.; Kroes, A.C.M.; Vossen, A.C.T.M. Rapid genotyping of cytomegalovirus in dried blood spots by multiplex real-time PCR assays targeting the envelope glycoprotein gB and gH genes. J. Clin. Microbiol. 2012, 50, 232–237. [Google Scholar] [CrossRef][Green Version]
- Zawilinska, B.; Szostek, S.; Kopec, J.; Piatkowska-Jakubas, B.; Kosz-Vnenchak, M. Multiplex real-time PCR to identify a possible reinfection with different strains of human cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Acta Biochim. Pol. 2016, 63, 161–166. [Google Scholar] [CrossRef]
- Trincado, D.E.; Scott, G.M.; White, P.A.; Hunt, C.; Rasmussen, L.; Rawlinson, W.D. Human cytomegalovirus strains associated with congenital and perinatal infections. J. Med. Virol. 2000, 61, 481–487. [Google Scholar] [CrossRef]
- Cranage, M.P.; Kouzarides, T.; Bankier, A.T.; Satchwell, S.; Weston, K.; Tomlinson, P.; Barrell, B.; Hart, H.; Bell, S.E.; Minson, A.C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986, 5, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.S.; Huffman, T.; Jenks, J.A.; De La Rosa, E.C.; Xie, G.; Vandergrift, N.; Pass, R.F.; Pollara, J.; Permar, S.R. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 2018, 115, 6267–6272. [Google Scholar] [CrossRef] [PubMed]
- Bootz, A.; Karbach, A.; Spindler, J.; Kropff, B.; Reuter, N.; Sticht, H.; Winkler, T.H.; Britt, W.J.; Mach, M. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog. 2017, 13, e1006601. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, I.; Gomes, A.C.; Sodi, I.; Langstone, T.; Rothwell, E.; Atkinson, C.; Pichon, S.; Piras-Douce, F.; Griffiths, P.D.; Reeves, M.B. Seronegative patients vaccinated with cytomegalovirus gB-MF59 vaccine have evidence of neutralising antibody responses against gB early post-transplantation. EBioMedicine 2019, 50, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016, 34, 313–319. [Google Scholar] [CrossRef]
- Zhang, C.; Pass, R.F. Detection of cytomegalovirus infection during clinical trials of glycoprotein B vaccine. Vaccine 2004, 23, 507–510. [Google Scholar] [CrossRef]
- Nelson, C.S.; Vera Cruz, D.; Su, M.; Xie, G.; Vandergrift, N.; Pass, R.F.; Forman, M.; Diener-West, M.; Koelle, K.; Arav-Boger, R.; et al. Intrahost dynamics of human cytomegalovirus variants acquired by seronegative glycoprotein B vaccinees. J. Virol. 2018, 93, 1–17. [Google Scholar] [CrossRef]
- Tang, J.; Frascaroli, G.; Lebbink, R.J.; Ostermann, E.; Brune, W. Human cytomegalovirus glycoprotein B variants affect viral entry, cell fusion, and genome stability. Proc. Natl. Acad. Sci. USA 2019, 116, 18021–18030. [Google Scholar] [CrossRef]
- Foglierini, M.; Marcandalli, J.; Perez, L. HCMV envelope glycoprotein diversity demystified. Front. Microbiol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Gardner, T.J.; Tortorella, D. Virion glycoprotein-mediated immune evasion by human cytomegalovirus: A sticky virus makes a slick getaway. Microbiol. Mol. Biol. Rev. 2016, 80, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Mach, M.; Britt, W.J. Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response. J. Virol. 2006, 80, 4591–4600. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, C.; Himmelein, S.; Britt, W.; Winkler, T.; Mach, M. Glycoprotein N subtypes of human cytomegalovirus induce a strain-specific antibody response during natural infection. J. Gen. Virol. 2009, 90, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.K.; Novak, Z.; Purser, M.; Arora, N.; Mach, M.; Britt, W.J.; Boppana, S.B. Strain-specific neutralizing antibody responses against human cytomegalovirus envelope glycoprotein N. Clin. Vaccine Immunol. 2012, 19, 909–913. [Google Scholar] [CrossRef]
- Zhou, M.; Lanchy, J.-M.; Ryckman, B.J. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J. Virol. 2015, 89, 8999–9009. [Google Scholar] [CrossRef]
- Puchhammer-Stöckl, E.; Görzer, I.; Zoufaly, A.; Jaksch, P.; Bauer, C.C.; Klepetko, W.; Popow-Kraupp, T. Emergence of multiple cytomegalovirus strains in blood and lung of lung transplant recipients. Transplantation 2006, 81, 187–194. [Google Scholar] [CrossRef]
- Roubalova, K.; Strunecky, O.; Vitek, A.; Zufanova, S.; Prochazka, B. Genetic variability of cytomegalovirus glycoprotein O in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2011, 13, 237–243. [Google Scholar] [CrossRef]
- Kalser, J.; Adler, B.; Mach, M.; Kropff, B.; Puchhammer-Stöckl, E.; Görzer, I. Differences in growth properties among two human cytomegalovirus glycoprotein O genotypes. Front. Microbiol. 2017, 8, 1609. [Google Scholar] [CrossRef]
- Brait, N.; Stögerer, T.; Kalser, J.; Adler, B.; Kunz, I.; Benesch, M.; Kropff, B.; Mach, M.; Puchhammer-Stöckl, E.; Görzer, I. Influence of human cytomegalovirus glycoprotein O polymorphism on the inhibitory effect of soluble forms of trimer- and pentamer-specific entry receptors. J. Virol. 2020, 94, 1–17. [Google Scholar] [CrossRef]
- Day, L.Z.; Stegmann, C.; Schultz, E.P.; Lanchy, J.-M.; Yu, Q.; Ryckman, B.J. Polymorphisms in human cytomegalovirus glycoprotein O (gO) exert epistatic influences on cell-free and cell-to-cell spread and antibody neutralization on gH epitopes. J. Virol. 2020, 94, e02051-19. [Google Scholar] [CrossRef]
- Adler, B.; Scrivano, L.; Ruzcics, Z.; Rupp, B.; Sinzger, C.; Koszinowski, U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 2006, 87, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Straschewski, S.; Patrone, M.; Walther, P.; Gallina, A.; Mertens, T.; Frascaroli, G. Protein pUL128 of human cytomegalovirus Is necessary for monocyte infection and blocking of migration. J. Virol. 2011, 85, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Gerna, G.; Percivalle, E.; Lilleri, D.; Lozza, L.; Fornara, C.; Hahn, G.; Baldanti, F.; Revello, M.G. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 2005, 86, 275–284. [Google Scholar] [CrossRef]
- Urban, M.; Klein, M.; Britt, W.J.; Haßfurther, E.; Mach, M. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 1996, 77, 1537–1547. [Google Scholar] [CrossRef]
- Freed, D.C.; Tang, Q.; Tang, A.; Li, F.; He, X.; Huang, Z.; Meng, W.; Xia, L.; Finnefrock, A.C.; Durr, E.; et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc. Natl. Acad. Sci. USA 2013, 110, 4997–5005. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tokumoto, T.; Tanabe, K.; Shirakawa, H.; Hashimoto, K.; Kushida, N.; Yanagida, T.; Inoue, N.; Yamaguchi, O.; Toma, H.; et al. Association of the outcome of renal transplantation with antibody response to cytomegalovirus strain--specific glycoprotein H epitopes. Clin. Infect. Dis. 2007, 45, 60–67. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Tao, R.; Zheng, X.; Li, H.; Shang, S. Epidemiological characteristics of human cytomegalovirus infection and glycoprotein H genotype in Chinese children. Pediatr. Neonatol. 2020, 61, 63–67. [Google Scholar] [CrossRef]
- Li, W.; Tao, R.; Zhang, X.; Shu, Q.; Gao, H.H.; Shang, S.Q.; Peng, Z.Y.; Li, H.M. Rapid and sensitive identification of glycoprotein H genotypes in clinical human cytomegalovirus samples. Jpn. J. Infect. Dis. 2015, 68, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Jabłońska, A.; Studzińska, M.; Kasztelewicz, B.; Zawilińska, B.; Wiśniewska-Ligier, M.; Dzierżanowska-Fangrat, K.; Woźniakowska-Gęsicka, T.; Kosz-Vnenchak, M.; Leśnikowski, Z.J. Cytomegalovirus glycoprotein H genotype distribution and the relationship with hearing loss in children. J. Med. Virol. 2014, 86, 1421–1427. [Google Scholar] [CrossRef]
- Paradowska, E.; Jabłońska, A.; Studzińska, M.; Kasztelewicz, B.; Wiśniewska-Ligier, M.; Dzierżanowska-Fangrat, K.; Woźniakowska-Gęsicka, T.; Czech-Kowalska, J. Distribution of the CMV glycoprotein gH/gL/gO and gH/gL/pUL128/pUL130/pUL131A complex variants and associated clinical manifestations in infants infected congenitally or postnatally. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).