Revisiting the Role of γδ T Cells in Anti-CMV Immune Response after Transplantation
Abstract
1. Introduction
2. Receptors and Activating Ligands Potentially Involved in CMV Immune Response
3. CMV-Induced Changes in γδ T Cells
3.1. Composition Changes
3.2. Phenotypic Changes
3.3. TCR Repertoire Changes
4. Adaptive-Like Immune Response to CMV Infection
5. Effects of CMV Reactive γδ T Cells after Transplantation
5.1. Favorable Effects
5.1.1. Anti-CMV Protective Role
5.1.2. Anti-Tumor Role
5.2. Unfavorable Effects
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lachmann, R.; Loenenbach, A.; Waterboer, T.; Brenner, N.; Pawlita, M.; Michel, A.; Thamm, M.; Poethko-Müller, C.; Wichmann, O.; Wiese-Posselt, M. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS ONE 2018, 13, e0200267. [Google Scholar] [CrossRef]
- Khairallah, C.; Dechanet-Meraville, J.; Capone, M. gammadelta T Cell-Mediated Immunity to Cytomegalovirus Infection. Front. Immunol. 2017, 8, 105. [Google Scholar] [CrossRef]
- Lafarge, X.; Merville, P.; Cazin, M.C.; Berge, F.; Potaux, L.; Moreau, J.F.; Dechanet-Merville, J. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J. Infect Dis. 2001, 184, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Broers, A.E.; van Der Holt, R.; van Esser, J.W.; Gratama, J.W.; Henzen-Logmans, S.; Kuenen-Boumeester, V.; Lowenberg, B.; Cornelissen, J.J. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000, 95, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Szydlo, R.M.; Dazzi, F.; Olavarria, E.; Cwynarski, K.; Yong, A.; Brookes, P.; de la Fuente, J.; Kanfer, E.; Apperley, J.F.; et al. Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: The case for tailored graft-versus-host disease prophylaxis. Br. J. Haematol. 2001, 112, 228–236. [Google Scholar] [CrossRef]
- Schmidt-Hieber, M.; Labopin, M.; Beelen, D.; Volin, L.; Ehninger, G.; Finke, J.; Socie, G.; Schwerdtfeger, R.; Kroger, N.; Ganser, A.; et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: A report from the Acute Leukemia Working Party of EBMT. Blood 2013, 122, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Reinke, P.; Lippert, J.; Ewert, R.; Fietze, E.; Ode-Hakim, S.; Volk, H.D.; Prösch, S. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet 1994, 344, 1737–1738. [Google Scholar] [CrossRef]
- Basgoz, N.; Preiksaitis, J.K. Post-transplant lymphoproliferative disorder. Infect Dis. Clin. N. Am. 1995, 9, 901–923. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Y.; Lau, Y.L.; Tu, W. gammadelta-T cells: An unpolished sword in human anti-infection immunity. Cell Mol. Immunol. 2013, 10, 50–57. [Google Scholar] [CrossRef]
- Lamb, L.S., Jr.; Lopez, R.D. gammadelta T cells: A new frontier for immunotherapy? Biol. Blood Marrow Transpl. 2005, 11, 161–168. [Google Scholar] [CrossRef]
- Lafont, V.; Sanchez, F.; Laprevotte, E.; Michaud, H.A.; Gros, L.; Eliaou, J.F.; Bonnefoy, N. Plasticity of gammadelta T Cells: Impact on the Anti-Tumor Response. Front. Immunol. 2014, 5, 622. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. gammadelta T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, Y.L.; Huang, J.; Newell, E.W.; Yu, H.; Kidd, B.A.; Kuhns, M.S.; Waters, R.W.; Davis, M.M.; Weaver, C.T.; et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 2012, 37, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ly, D.; Castro, C.D.; Li, N.S.; Hawk, A.J.; Altman, J.D.; Meredith, S.C.; Piccirilli, J.A.; Moody, D.B.; Adams, E.J. Molecular Analysis of Lipid-Reactive Vdelta1 gammadelta T Cells Identified by CD1c Tetramers. J. Immunol. 2016, 196, 1933–1942. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-lipid antigen recognition by the gammadelta TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Pizarro, J.C.; Holmes, M.A.; McBeth, C.; Groh, V.; Spies, T.; Strong, R.K. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl. Acad. Sci. USA 2011, 108, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; et al. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Willcox, C.R.; Pitard, V.; Netzer, S.; Couzi, L.; Salim, M.; Silberzahn, T.; Moreau, J.F.; Hayday, A.C.; Willcox, B.E.; Dechanet-Merville, J. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012, 13, 872–879. [Google Scholar] [CrossRef]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. gammadelta T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef]
- Stankovic, S.; Davey, M.S.; Shaw, E.M.; von Borstel, A.; Cristiano, Y.; Levvey, B.J.; Rossjohn, J.; Westall, G.P.; Snell, G.I.; Brooks, A.G.; et al. Cytomegalovirus replication is associated with enrichment of distinct gammadelta T cell subsets following lung transplantation: A novel therapeutic approach? J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Dolstra, H.; Fredrix, H.; van der Meer, A.; de Witte, T.; Figdor, C.; van de Wiel-van Kemenade, E. TCRγδ cytotoxic T lymphocytes expressing the killer cell-inhibitory receptor p58.2 (CD158b) selectively lyse acute myeloid leukemia cells. Bone Marrow Transplant. 2001, 27, 1087–1093. [Google Scholar] [CrossRef]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, Z.; Prinz, I.; Dechanet-Merville, J.; Silva-Santos, B.; Kuball, J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2020, 19, 169–184. [Google Scholar] [CrossRef]
- Serrano, R.; Wesch, D.; Kabelitz, D. Activation of Human gammadelta T Cells: Modulation by Toll-Like Receptor 8 Ligands and Role of Monocytes. Cells 2020, 9, 713. [Google Scholar] [CrossRef]
- Hoeres, T.; Smetak, M.; Pretscher, D.; Wilhelm, M. Improving the Efficiency of Vgamma9Vdelta2 T-Cell Immunotherapy in Cancer. Front. Immunol. 2018, 9, 800. [Google Scholar] [CrossRef]
- Couzi, L.; Pitard, V.; Moreau, J.-F.; Merville, P.; Déchanet-Merville, J. Direct and Indirect Effects of Cytomegalovirus-Induced γδ T Cells after Kidney Transplantation. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Dechanet, J.; Merville, P.; Berge, F.; Bone-Mane, G.; Taupin, J.L.; Michel, P.; Joly, P.; Bonneville, M.; Potaux, L.; Moreau, J.F. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J. Infect Dis. 1999, 179, 1–8. [Google Scholar] [CrossRef]
- Dechanet, J.; Merville, P.; Lim, A.; Retiere, C.; Pitard, V.; Lafarge, X.; Michelson, S.; Meric, C.; Hallet, M.M.; Kourilsky, P.; et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J. Clin. Investig. 1999, 103, 1437–1449. [Google Scholar] [CrossRef]
- Pitard, V.; Roumanes, D.; Lafarge, X.; Couzi, L.; Garrigue, I.; Lafon, M.E.; Merville, P.; Moreau, J.F.; Dechanet-Merville, J. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 2008, 112, 1317–1324. [Google Scholar] [CrossRef]
- Puig-Pey, I.; Bohne, F.; Benitez, C.; Lopez, M.; Martinez-Llordella, M.; Oppenheimer, F.; Lozano, J.J.; Gonzalez-Abraldes, J.; Tisone, G.; Rimola, A.; et al. Characterization of gammadelta T cell subsets in organ transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2010, 23, 1045–1055. [Google Scholar] [CrossRef]
- D’Offizi, G.; Gioia, C.; Martini, F.; Volpi, I.; Solmone, M.; Poccia, F.; Narciso, P.; Vennarecci, G.; Ettore, G.M.; Antonini, M.; et al. Gamma delta T cells and resolution of cytomegalovirus infection in an HIV/HCV coinfected patient after liver transplantation. Transplantation 2005, 80, 1523–1524. [Google Scholar] [CrossRef]
- Kaminski, H.; Menard, C.; El Hayani, B.; Adjibabi, A.N.; Marseres, G.; Courant, M.; Zouine, A.; Pitard, V.; Garrigue, I.; Burrel, S.; et al. Characterization of a Unique gammadelta T-Cell Subset as a Specific Marker of Cytomegalovirus Infection Severity. J Infect Dis. 2021, 223, 655–666. [Google Scholar] [CrossRef]
- Kaminski, H.; Garrigue, I.; Couzi, L.; Taton, B.; Bachelet, T.; Moreau, J.F.; Dechanet-Merville, J.; Thiebaut, R.; Merville, P. Surveillance of gammadelta T Cells Predicts Cytomegalovirus Infection Resolution in Kidney Transplants. J. Am. Soc. Nephrol. JASN 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Lee, S.; Affandi, J.S.; Irish, A.B.; Price, P. Cytomegalovirus infection alters phenotypes of different gammadelta T-cell subsets in renal transplant recipients with long-term stable graft function. J. Med. Virol. 2017, 89, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Pitard, V.; Sicard, X.; Garrigue, I.; Hawchar, O.; Merville, P.; Moreau, J.-F.; Déchanet-Merville, J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 2012, 119, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Pitard, V.; Netzer, S.; Garrigue, I.; Lafon, M.E.; Moreau, J.F.; Taupin, J.L.; Merville, P.; Dechanet-Merville, J. Common features of gammadelta T cells and CD8(+) alphabeta T cells responding to human cytomegalovirus infection in kidney transplant recipients. J Infect Dis. 2009, 200, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Levaillant, Y.; Jamai, A.; Pitard, V.; Lassalle, R.; Martin, K.; Garrigue, I.; Hawchar, O.; Siberchicot, F.; Moore, N.; et al. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J. Am. Soc. Nephrol. JASN 2010, 21, 181–188. [Google Scholar] [CrossRef]
- Couzi, L.; Lafarge, X.; Pitard, V.; Neau-Cransac, M.; Dromer, C.; Billes, M.-A.; Lacaille, F.; Moreau, J.-F.; Merville, P.; Déchanet-Merville, J. Gamma-delta T cell expansion is closely associated with cytomegalovirus infection in all solid organ transplant recipients. Transpl. Int. 2011, 24, e40–e42. [Google Scholar] [CrossRef]
- Prinz, I.; Thamm, K.; Port, M.; Weissinger, E.M.; Stadler, M.; Gabaev, I.; Jacobs, R.; Ganser, A.; Koenecke, C. Donor Vδ1+ γδ T cells expand after allogeneic hematopoietic stem cell transplantation and show reactivity against CMV-infected cells but not against progressing B-CLL. Exp. Hematol. Oncol. 2013, 2, 14. [Google Scholar] [CrossRef]
- Scheper, W.; van Dorp, S.; Kersting, S.; Pietersma, F.; Lindemans, C.; Hol, S.; Heijhuurs, S.; Sebestyen, Z.; Gründer, C.; Marcu-Malina, V.; et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013, 27, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Madrigal, A.J.; Grace, S.; Sivakumaran, J.; Kottaridis, P.; Mackinnon, S.; Travers, P.J.; Lowdell, M.W. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010, 116, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Baker, A.T.; Hunter, S.; Willcox, B.E. Recasting Human Vdelta1 Lymphocytes in an Adaptive Role. Trends Immunol. 2018, 39, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salim, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengelov, H. The Role of Gamma Delta T Cells in Haematopoietic Stem Cell Transplantation. Scand. J. Immunol. 2015, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdorfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Human gamma delta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017, 18, 393–401. [Google Scholar] [CrossRef]
- Arruda, L.C.M.; Gaballa, A.; Uhlin, M. Graft gammadelta TCR Sequencing Identifies Public Clonotypes Associated with Hematopoietic Stem Cell Transplantation Efficacy in Acute Myeloid Leukemia Patients and Unravels Cytomegalovirus Impact on Repertoire Distribution. J. Immunol. 2019, 202, 1859–1870. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Oo, Y.H.; Willcox, B.E. Vdelta2(+) T Cells-Two Subsets for the Price of One. Front. Immunol. 2018, 9, 2106. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, A.; Arruda, L.C.M.; Radestad, E.; Uhlin, M. CD8(+) gammadelta T Cells Are More Frequent in CMV Seropositive Bone Marrow Grafts and Display Phenotype of an Adaptive Immune Response. Stem Cells Int. 2019, 2019, 6348060. [Google Scholar] [CrossRef]
- Hammer, Q.; Rückert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef]
- Arruda, L.C.M.; Gaballa, A.; Uhlin, M. Impact of gammadelta T cells on clinical outcome of hematopoietic stem cell transplantation: Systematic review and meta-analysis. Blood Adv. 2019, 3, 3436–3448. [Google Scholar] [CrossRef] [PubMed]
- Daguzan, C.; Moulin, M.; Kulyk-Barbier, H.; Davrinche, C.; Peyrottes, S.; Champagne, E. Aminobisphosphonates Synergize with Human Cytomegalovirus To Activate the Antiviral Activity of Vgamma9Vdelta2 Cells. J. Immunol. 2016, 196, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Lonnqvist, B.; Ringden, O.; Ljungman, P.; Wahren, B.; Gahrton, G. Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br. J. Haematol. 1986, 63, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Teira, P.; Battiwalla, M.; Ramanathan, M.; Barrett, A.J.; Ahn, K.W.; Chen, M.; Green, J.S.; Saad, A.; Antin, J.H.; Savani, B.N.; et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 2016, 127, 2427–2438. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Koldehoff, M. Cytomegalovirus replication reduces the relapse incidence in patients with acute myeloid leukemia. Blood 2016, 128, 456–459. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Steckel, N.K.; Koldehoff, M.; Hegerfeldt, Y.; Trenschel, R.; Ditschkowski, M.; Christoph, S.; Gromke, T.; Kordelas, L.; Ottinger, H.D.; et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: Evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011, 118, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Leisenring, W.M.; Xie, H.; Walter, R.B.; Mielcarek, M.; Sandmaier, B.M.; Riddell, S.R.; Boeckh, M. CMV reactivation after allogeneic HCT and relapse risk: Evidence for early protection in acute myeloid leukemia. Blood 2013, 122, 1316–1324. [Google Scholar] [CrossRef]
- Takenaka, K.; Nishida, T.; Asano-Mori, Y.; Oshima, K.; Ohashi, K.; Mori, T.; Kanamori, H.; Miyamura, K.; Kato, C.; Kobayashi, N.; et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol. Blood Marrow Transpl. 2015, 21, 2008–2016. [Google Scholar] [CrossRef]
- Jang, J.E.; Kim, S.J.; Cheong, J.W.; Hyun, S.Y.; Kim, Y.D.; Kim, Y.R.; Kim, J.S.; Min, Y.H. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann. Hematol. 2015, 94, 275–282. [Google Scholar] [CrossRef]
- Ito, S.; Pophali, P.; Co, W.; Koklanaris, E.K.; Superata, J.; Fahle, G.A.; Childs, R.; Battiwalla, M.; Barrett, A.J. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transpl. 2013, 48, 1313–1316. [Google Scholar] [CrossRef][Green Version]
- Manjappa, S.; Bhamidipati, P.K.; Stokerl-Goldstein, K.E.; DiPersio, J.F.; Uy, G.L.; Westervelt, P.; Liu, J.; Schroeder, M.A.; Vij, R.; Abboud, C.N.; et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol. Blood Marrow Transpl. 2014, 20, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Miller, J.S.; Anderson, S.K.; Bryceson, Y.T. Epigenetic regulation of NK cell differentiation and effector functions. Front. Immunol. 2013, 4, 55. [Google Scholar] [CrossRef]
- Solano, C.; Vazquez, L.; Gimenez, E.; de la Camara, R.; Albert, E.; Rovira, M.; Espigado, I.; Calvo, C.M.; Lopez-Jimenez, J.; Suarez-Lledo, M.; et al. Cytomegalovirus DNAemia and risk of mortality in allogeneic hematopoietic stem cell transplantation: Analysis from the Spanish Hematopoietic Transplantation and Cell Therapy Group. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Leserer, S.; Bayraktar, E.; Trilling, M.; Bogdanov, R.; Arrieta-Bolanos, E.; Tsachakis-Muck, N.; Crivello, P.; Koldehoff, M.; Maassen, F.; Ross, R.S.; et al. Cytomegalovirus kinetics after hematopoietic cell transplantation reveal peak titers with differential impact on mortality, relapse and immune reconstitution. Am. J. Hematol. 2021, 96, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Goldmacher, V.S. Cell death suppression by cytomegaloviruses. Apoptosis 2005, 10, 251–265. [Google Scholar] [CrossRef]
- Koldehoff, M.; Lindemann, M.; Opalka, B.; Bauer, S.; Ross, R.S.; Elmaagacli, A.H. Cytomegalovirus induces apoptosis in acute leukemia cells as a virus-versus-leukemia function. Leuk Lymphoma 2015, 56, 3189–3197. [Google Scholar] [CrossRef]
- Thomson, K.J.; Mackinnon, S.; Peggs, K.S. CMV-specific cellular therapy for acute myeloid leukemia? Blood 2012, 119, 1088–1090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elmaagacli, A.H.; Koldehoff, M.; Lindemann, M.; Sonius, M.; Ditschkowski, M.; Steckel, N.; Beelen, D.W. Response: T cells are required for the CMV-induced antileukemia effect after transplant. Blood 2012, 119, 1090–1091. [Google Scholar] [CrossRef][Green Version]
- Bigley, A.B.; Baker, F.L.; Simpson, R.J. Cytomegalovirus: An unlikely ally in the fight against blood cancers? Clin. Exp. Immunol. 2018, 193, 265–274. [Google Scholar] [CrossRef]
- Litjens, N.H.R.; van der Wagen, L.; Kuball, J.; Kwekkeboom, J. Potential Beneficial Effects of Cytomegalovirus Infection after Transplantation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Halary, F.; Pitard, V.; Dlubek, D.; Krzysiek, R.; de la Salle, H.; Merville, P.; Dromer, C.; Emilie, D.; Moreau, J.-F.; Déchanet-Merville, J. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005, 201, 1567–1578. [Google Scholar] [CrossRef]
- Godder, K.T.; Henslee-Downey, P.J.; Mehta, J.; Park, B.S.; Chiang, K.Y.; Abhyankar, S.; Lamb, L.S. Long term disease-free survival in acute leukemia patients recovering with increased γδ T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007, 39, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Arnouk, H.; Britt, W.; Gillespie, G.Y.; Cloud, G.A.; Harkins, L.; Su, Y.; Lowdell, M.W.; Lamb, L.S. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vdelta1+ gammadelta T cells. PLoS ONE 2013, 8, e68729. [Google Scholar] [CrossRef]
- Cantoni, N.; Hirsch, H.H.; Khanna, N.; Gerull, S.; Buser, A.; Bucher, C.; Halter, J.; Heim, D.; Tichelli, A.; Gratwohl, A.; et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol. Blood Marrow Transpl. 2010, 16, 1309–1314. [Google Scholar] [CrossRef]
- Einsele, H.; Ehninger, G.; Hebart, H.; Weber, P.; Dette, S.; Link, H.; Horny, H.P.; Meuter, V.; Wagner, S.; Waller, H.D.; et al. Incidence of local CMV infection and acute intestinal GVHD in marrow transplant recipients with severe diarrhoea. Bone Marrow Transpl. 1994, 14, 955–963. [Google Scholar]
- Takemoto, Y.; Takatsuka, H.; Wada, H.; Mori, A.; Saheki, K.; Okada, M.; Tamura, S.; Fujimori, Y.; Okamoto, T.; Kakishita, E.; et al. Evaluation of CMV/human herpes virus-6 positivity in bronchoalveolar lavage fluids as early detection of acute GVHD following BMT: Evidence of a significant relationship. Bone Marrow Transpl. 2000, 26, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaballa, A.; Stikvoort, A.; Onfelt, B.; Mattsson, J.; Sundin, M.; Watz, E.; Uhlin, M. T-cell frequencies of CD8(+) gammadelta and CD27(+) gammadelta cells in the stem cell graft predict the outcome after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2019, 54, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Erdbruegger, U.; Scheffner, I.; Mengel, M.; Schwarz, A.; Verhagen, W.; Haller, H.; Gwinner, W. Impact of CMV infection on acute rejection and long-term renal allograft function: A systematic analysis in patients with protocol biopsies and indicated biopsies. Nephrol. Dial. Transplant. 2011, 27, 435–443. [Google Scholar] [CrossRef]
- Heutinck, K.M.; Yong, S.L.; Tonneijck, L.; van den Heuvel, H.; van der Weerd, N.C.; van der Pant, K.A.M.I.; Bemelman, F.J.; Claas, F.H.J.; ten Berge, I.J.M. Virus-Specific CD8+ T Cells Cross-Reactive to Donor-Alloantigen Are Transiently Present in the Circulation of Kidney Transplant Recipients Infected with CMV and/or EBV. Am. J. Transplant. 2016, 16, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Nickel, P.; Bold, G.; Presber, F.; Biti, D.; Babel, N.; Kreutzer, S.; Pratschke, J.; Schönemann, C.; Kern, F.; Volk, H.-D.; et al. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl. Immunol. 2009, 20, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Potena, L.; Stepick-Biek, P.; Liu, L.; Dionis, K.Y.; Luikart, H.; Fearon, W.F.; Holmes, T.H.; Chin, C.; Cooke, J.P.; et al. T-Cell Immunity to Subclinical Cytomegalovirus Infection Reduces Cardiac Allograft Disease. Circulation 2006, 114, 1608–1615. [Google Scholar] [CrossRef][Green Version]
- Martínez-Llordella, M.; Puig-Pey, I.; Orlando, G.; Ramoni, M.; Tisone, G.; Rimola, A.; Lerut, J.; Latinne, D.; Margarit, C.; Bilbao, I.; et al. Multiparameter Immune Profiling of Operational Tolerance in Liver Transplantation. Am. J. Transplant. 2007, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Ohe, H.; Nafady-Hego, H.; Uemoto, S.; Bishop, G.A.; Koshiba, T. Intragraft Vδ1 γδ T Cells with a Unique T-Cell Receptor Are Closely Associated With Pediatric Semiallogeneic Liver Transplant Tolerance. Transplantation 2013, 95, 192–202. [Google Scholar] [CrossRef]
- Bachelet, T.; Couzi, L.; Pitard, V.; Sicard, X.; Rigothier, C.; Lepreux, S.; Moreau, J.-F.; Taupin, J.-L.; Merville, P.; Déchanet-Merville, J. Cytomegalovirus-Responsive γδ T Cells: Novel Effector Cells in Antibody-Mediated Kidney Allograft Microcirculation Lesions. J. Am. Soc. Nephrol. 2014, 25, 2471–2482. [Google Scholar] [CrossRef]
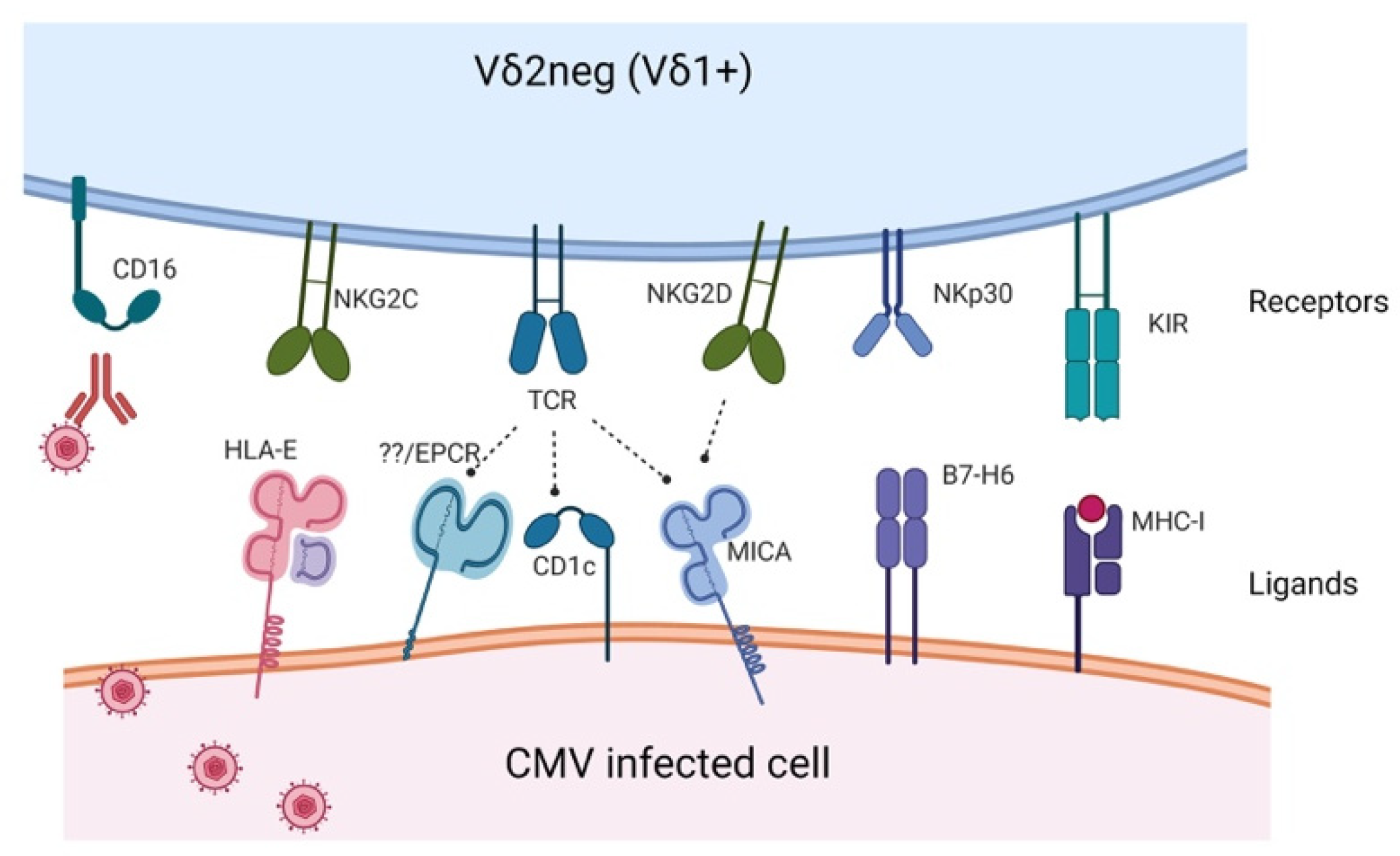

| Receptor | Ligand | Study |
|---|---|---|
| Vδ1 TCR | CD1c, CD1d, MICA, PE, | Zeng et al. [14] Roy et al. [15] Uldrich et al. [16] Xu et al. [17] |
| Vγ4Vδ1 TCR | BTNL3-BTNL8 | Melandri et al. [18] |
| Vγ4Vδ5 TCR | EPCR | Willcox et al. [19] |
| Vγ8Vδ3 TCR | Annexin A2 | Marlin et al. [20] |
| NKG2D | MICA/B, UL16BP | Silva-Santos et al. [21] |
| NKG2C | HLA-E | Stankovic et al. [22] |
| NKp30 | B7-H6 | Brandt et al. [23] |
| KIR | HLA-I | Dolstra et al. [24] |
| Transplant Setting | Expanded γδ Subset | Study |
|---|---|---|
| Lung TX | NKG2C+ γδ T cells | Stankovic et al. [22] |
| Liver TX | Vδ1 γδ T cells | Puig-Pey et al. [33], D´Offizi et al. [34] |
| Kidney TX | Vδ2neg, Vγ9-Vδ2+, Vδ1 and Vδ3, CD16+ γδ T cell | Kaminski et al. [35,36], De´chanet et al. [30,31], Lee et al. [37], Couzi et al. [38,39,40,41] |
| HSCT | Vδ2neg, Vδ1 γδ T cell | Prinz et al. [42], Scheper et al. [43], Knight et al. [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaballa, A.; Alagrafi, F.; Uhlin, M.; Stikvoort, A. Revisiting the Role of γδ T Cells in Anti-CMV Immune Response after Transplantation. Viruses 2021, 13, 1031. https://doi.org/10.3390/v13061031
Gaballa A, Alagrafi F, Uhlin M, Stikvoort A. Revisiting the Role of γδ T Cells in Anti-CMV Immune Response after Transplantation. Viruses. 2021; 13(6):1031. https://doi.org/10.3390/v13061031
Chicago/Turabian StyleGaballa, Ahmed, Faisal Alagrafi, Michael Uhlin, and Arwen Stikvoort. 2021. "Revisiting the Role of γδ T Cells in Anti-CMV Immune Response after Transplantation" Viruses 13, no. 6: 1031. https://doi.org/10.3390/v13061031
APA StyleGaballa, A., Alagrafi, F., Uhlin, M., & Stikvoort, A. (2021). Revisiting the Role of γδ T Cells in Anti-CMV Immune Response after Transplantation. Viruses, 13(6), 1031. https://doi.org/10.3390/v13061031







