Abstract
Hepatitis B virus (HBV) chronic infection causes progressive liver damage, although about 20% of patients develop extrahepatic manifestations such as cryoglobulinemic vasculitis (CV). Clinical manifestations range from mild to moderate (purpura, asthenia, arthralgia) to severe (leg ulcers, peripheral neuropathy, glomerulonephritis, non-Hodgkin lymphoma). A comprehensive review of therapeutic options for HBV-related CV is lacking. Nucleos(t)ide analogues (NA) suppress HBV replication in 90–100% of cases and induce clinical response in most patients with mild-to-moderate CV. Plasma exchange can be performed in patients with severe CV and should be considered in severe or life-threatening cases combined with high doses of corticosteroids and antiviral treatment. A cautious use of rituximab can be considered only in association with NA treatment in refractory cases. A review of the literature and an analysis of data collected by six centers of the Italian Group for the Study of Cryoglobulinemia on 18 HBV-CV nucleotide/nucleoside analogues (NAs)-treated patients were carried out.
1. Introduction
Hepatitis B virus (HBV) infection is still a major global health problem with about 350 million chronically infected subjects worldwide. HBV infection can cause acute or fulminant hepatitis as well as chronic hepatitis evolving into cirrhosis and hepatocellular carcinoma, and it is responsible for 887,000 deaths every year [1]. About 20% of HBV patients may develop extrahepatic manifestations, such as polyarteritis nodosa and glomerulonephritis, dermatitis, arthralgia, arthritis, aplastic anemia and cryoglobulinemic vasculitis (CV) [2].
In the past, CV was termed “essential” due to its unknown etiology. After discovering hepatitis C virus (HCV) in 1989, it became clear that most CV cases were HCV positive [3,4].
CV can be described as an immune complex-mediated systemic vasculitis involving medium/small-size vessels. It is characterized by the presence, in the serum, of immunoglobulins able to precipitate when temperature goes below 37 °C [5]. According to Brouet and colleagues [6], cryoglobulinemias are classified into three types: I, II, and III [7]. In type I, the cryoglobulins are formed by monoclonal immunoglobulins, IgM or IgG only, and it is associated with lymphoproliferative disorders (multiple myeloma, Waldenstrom’s disease, or non-Hodgkin’s lymphoma, NHL). In types II and III, called mixed cryoglobulinemia (MC), the cryoglobulins are immunocomplexes composed by the antigen and monoclonal IgMs or polyclonal IgGs. The IgMs are usually endowed with rheumatoid factor (RF) activity against polyclonal IgGs. MC is strongly associated with HCV infection (80–90%) [8], but a fraction of cases is HCV-negative (10–20%), being secondary to other viral infections (HBV and HIV are the most common), or to systemic autoimmune diseases (primary Sjögren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis), or finally to chronic lymphoproliferative disorders [9,10,11,12,13,14,15,16,17,18,19,20]. MC can occur in 0.5 to 5.5% of HBV patients [21,22,23,24,25]. The potential role of HBV, as MC etiologic agent, was firstly suggested by Levo and colleagues [26] more than 40 years ago. Monti and colleagues [24] retrospectively analyzed a cohort of 717 subjects with essential cryoglobulinemia followed by the Italian Group for the Study of Cryoglobulinemia (GISC). HBsAg data were available only for 400 patients, and the authors reported a 5.5% prevalence of HBsAg positivity. Subsequently, Ferri and colleagues [23] evaluated 231 patients with MC, observing a 1.8% prevalence of HBsAg. In a recent study by Mazzaro and colleagues [27], the prevalence of HBsAg positivity in a group of 246 patients with MC was 4.5%. Furthermore, no correlation was found between MC and different HBV genotypes [2]. So far, no studies have evaluated the incidence of CV in HBV subjects. Our review focuses on clinical manifestations and treatments for HBV-related CV.
1.1. Main Clinical Manifestations of HBV-Associated CV
Since few clinical and epidemiological studies have suggested the casual relationship between HBV and MC (Table 1) [28,29,30], large population studies regarding HBV-related MC are lacking in the literature.

Table 1.
Summary of the clinical-serological and virological characteristics reported by the main studies on HBV-related CV.
About 50% of HBV-MC patients show chronic hepatitis, while cirrhosis is present in 30% of cases.
The disease features vary: 45% to 100% of cases show mild–moderate clinical symptoms (palpable leg purpura, asthenia, and arthralgia, commonly called a Meltzer and Franklin triad [31]). The articular involvement is usually characterized by bilateral and symmetric joint pain, non-deforming, and mainly involve knees and hands. Skin ulcers may occur in 10–30% of cases. Sicca syndrome and Raynaud’s phenomenon have been reported in a few patients. Neurologic manifestations range from distal sensory polyneuropathy to sensory–motor polyneuropathy in 20–60% of cases. Peripheral neuropathy presents with leg pain and symmetric burning paresthesia. Motor deficit is irregular and mainly affects the lower limbs, appearing either a few months after sensory symptoms or simultaneously. Severe clinical symptoms such as glomerulonephritis, progressive peripheral neuropathy, gastrointestinal vasculitis, and NHL may occur in a few cases [22,28,29,30].
Similar to HCV-related CV, the most frequent kidney manifestation is type I membrano-proliferative glomerulonephritis (MPGN). A very common aspect of HBV-MPGN is nephrotic-range proteinuria and microscopic hematuria, often with evidence of renal insufficiency. In a recent study on 12 patients affected by HBV-MPGN [30], proteinuria was present with a nephrotic range in all of them, and 9 (75%) patients had impaired renal function. Microscopic hematuria was found in all patients, and gross hematuria in three.
The histological picture found in MPGN has revealed diffuse endocapillary proliferation, thickening, and double-contour appearance of the glomerular basement membrane. The glomeruli were infiltrated by many monocytes and polymorph nuclear cells. The capillary lumen showed PAS-positive hyaline thrombi. The distinctive histological features are markedly hypercellular and endoluminal thrombi due to the massive precipitation of cryoglobulins. Immune complexes comprising HBV antigens were also detected in some cases [30]. Overall, kidney involvement emerged as an unfavorable prognostic factor [32,33].
1.2. Therapeutic Management of HBV-Related CV
HBV-associated CV is considered a rare disease and, consequently, few data are available regarding the clinical management, because large cohort studies are lacking. Furthermore, the implementation of universal HBV vaccination programs is successfully decreasing HBV infection prevalence worldwide [34], thus making HBV-associated CV progressively less frequent.
Guidelines for treatment of HBV-related CV have not been published yet, but, similarly to HCV-related CV, the treatment is based on the following four targeting approaches: (1) antiviral therapy; (2) B-cell depleting therapy; (3) immunosuppressive drugs; and (4) anti-inflammatory drugs.
3. Discussion
CV occurs in a smaller fraction of HBV patients, and for this reason only case reports or studies on a limited number of subjects are available in the literature. Nevertheless, the clinical manifestations are similar to those induced by HCV, varying from mild–moderate symptoms to severe and life-threatening manifestations (Table 4). CV pathogenesis in HBV patients remains largely unresolved even if it has been suggested that HBV antigens (as well as HCV) stimulate, through CD81, the B-cell clones producing IgM-RF [62]. Therefore, HBV-related CV could represent an evolution from polyclonal B-cell population to oligoclonal B-cell expansion in the setting of a long-term antigen stimulation, and it is considered a transitional phase between autoimmunity and neoplasm [64,65].

Table 4.
Clinical features and pathologic counterpart in cryoglobulinemic vasculitis.
No specific treatment guidelines are currently available for patients with HBV-related CV and the best treatment has yet to be established. As demonstrated in HCV-related CV, direct antiviral agents are effective in a significant proportion of patients and usually introduced as the first-line treatment [66]. The literature reviews herein described, together with the original results we reported in a long-term follow-up of the largest series of patients treated with NAs, suggest that HBV replication is the triggering factor, and antiviral therapy may be the first-line treatment. The suppression of HBV replication is the goal to obtain CV remission, to prevent organ complications, and the possible evolution to lymphoproliferative disorders [67], which is a common neoplastic evolution in cryoglobulinemias, with possible underlying similar hyperactivated pathways and risk factors [68].
4. Conclusions
NAs therapy should be preferred for the therapeutic management of HBV-related CV patients, in subjects with mild-to-moderate manifestations. The administration of short courses of low doses of corticosteroids may be considered in mild-to-moderate HBV-related CV (Figure 1), while long-term use of corticosteroids is discouraged. For severe cases, therapeutic measures applied in severe HCV-related or non-infectious CV may be borrowed with some differences (63). In severe cases, plasma exchange with high-dose corticosteroids should be combined with NAs. Rituximab should be considered as a subsequent option for non-responders to the antiviral therapy, or relapsing patients. To prevent dangerous HBV flare, rituximab administration always requires the association with NAs therapy (Figure 1). In life-threatening CV (i.e., rapid progressive glomerulonephritis, gastrointestinal vasculitis, acute hyper-viscosity syndrome) rituximab and/or plasma exchange with high-dose corticosteroids should be combined with NAs. Nonetheless, further investigations in a larger population on the combination of NAs with second-line therapy options will be valuable to set up the most appropriate strategy for the therapeutic management of HBV-related CV.
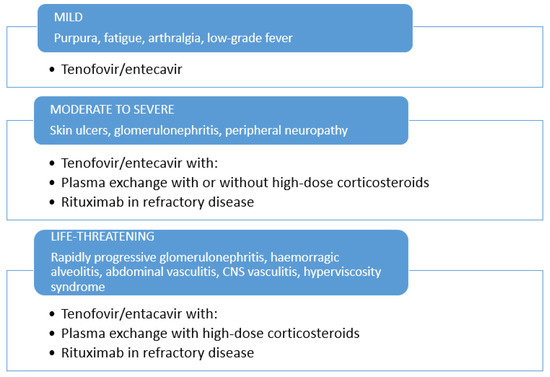
Figure 1.
Therapeutic management of HBV-related cryoglobulinemic vasculitis.
Author Contributions
Conceptualization and methodology, C.M., L.Q; software, CM.; validation, C.M., L.D.M. and L.Q.; formal analysis, C.M., L.D.M., L.Q.; investigation, C.M., L.Q., L.G., M.V., F.S., D.F., P.A., A.L.Z., V.G., G.M., M.G.; data curation, C.M.; writing—original draft preparation, C.M., L.Q., G.M., M.G.; writing—review and editing, C.M., L.Q., L.D.M., L.G., M.V., F.S., D.F., P.A., A.L.Z., V.G., G.M., M.G.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Patients were treated according to the international guidelines and protocols, and no formal approval by Institutional Review Board was required. Pseudonymization and de-identification procedures were applied for data management.
Informed Consent Statement
Patient consent was waived due to the retrospective observational nature of the study.
Data Availability Statement
All data available are published. Further details can be directly asked from the Corresponding Author.
Acknowledgments
The authors wish to thank Luigina Mei for editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gamen, D.; Prince, A.M. Hepatitis B virus infection –natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar]
- Cacoub, P.; Saadoun, D.; Bourliere, M.; Khiri, H.; Martineau, A.; Benhamou, Y.; Varastet, M.; Pol, S.; Thibault, V.; Rotily, M.; et al. Hepatitis B virus genotypes and extrahepatic manifestations. J. Hepatol. 2005, 43, 764–770. [Google Scholar] [CrossRef]
- Cacoub, P.; Hausfater, P.; Musset, L.; Piette, J.C. Mixed cryoglobulinemia in hepatitis C patients. GERMIVIC. Ann. Med. Intern. 2000, 151, 20–29. [Google Scholar]
- Agnello, V.; Chung, R.T.; Kaplan, L.M. A role for hepatitis C virus infection in type II cryoglobulinemia? N. Engl. J. Med. 1992, 327, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Kolopp-Sarda, M.N.; Miossec, P. Cryoglobulins: An update on detection, mechanisms and clinical contribution. Autoimmun. Rev. 2018, 17, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Brouet, J.C.; Cauvel, J.P.; Danon, F.; Klein, M.; Seligmann, M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am. J. Med. 1974, 57, 775–788. [Google Scholar] [CrossRef]
- Gorevic, P.D.; Frangione, B. Mixed cryoglobulinemia cross-reactive idiotypes: Implications for the relationship of MC to rheumatic and lymphoproliferative diseases. Semin. Hematol. 1991, 28, 79–94. [Google Scholar] [PubMed]
- Zignego, A.L.; Ramos-Casals, M.; Ferri, C.; Saadoun, D.; Arcaini, L.; Roccatello, D.; Antonelli, A.; Desbois, A.C.; Comarmond, C.; Gragnani, L.; et al. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun. Rev. 2017, 16, 523–541. [Google Scholar] [CrossRef]
- Pasqual, F.; Perrin, L.; Giostra, E.; Schifferli, J.A. Hepatitis Cvirus in patients with cryoglobulinemia type II. Infect. Dis. 1990, 162, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Monti, G.; Monteverde, A.; et al. Hepatitis C virus and mixed cryoglobulinemia. Lancet 1992, 339, 989. [Google Scholar] [CrossRef]
- Ferri, C.; Greco, F.; Longobardo, G.; et al. Association between hepatitis C and mixed cryoglobulinemia. Clin. Exp. Rheumatol. 1992, 9, 621–624. [Google Scholar]
- Ferri, C.; Zignego, A.L.; Giuggioli, D.; Sebastiani, M.; Cazzato, M.; Antonelli, A.; La Civita, L.; Fadda, P.; Longombardo, G.; Pileri, S. HCV and cryoglobulinemic vasculitis. Cleve Clin. J. Med. 2002, 69 (Suppl. S2), SII20–SII23. [Google Scholar] [CrossRef]
- Ferri, C.; Sebastiani, M.; Giuggioli, D.; Colaci, M.; Fallahi, P.; Piluso, A.; Antonelli, A.; Zignego, A.L. Hepatitis C virus syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World J. Hepatol. 2015, 7, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Oreni, L.; Saccardo, F.; Castelnovo, L.; Filippini, D.; Marson, P.; Mascia, M.T.; Mazzaro, C.; Origgi, L.; Ossi, E.; et al. HCV-unrelated cryoglobulinaemic vasculitis: The results of a prospective observational study by the Italian Group for the Study of Cryoglobulinaemias (GISC). Clin. Exp. Rheumatol. 2017, 35 (Suppl. S103), 67–76. [Google Scholar] [PubMed]
- Roccatello, D.; Saadoun, D.; Ramos-Casals, M.; Tzioufas, A.G.; Fervenza, F.C.; Cacoub, P.; Zignego, A.L.; Ferri, C. Cryoglobulinaemia. Nat. Rev. Dis. Primers. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitis. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Quartuccio, L.; Baldini, C.; Priori, R.; Bartoloni, E.; Carubbi, F.; Alunno, A.; Gandolfo, S.; Colafrancesco, S.; Giacomelli, R.; Gerli, R.; et al. Cryoglobulinemia in Sjögren Syndrome: A Disease Subset that Links Higher Systemic Disease Activity, Autoimmunity, and Local B Cell Proliferation in Mucosa-associated Lymphoid Tissue. J. Rheumatol. 2017, 44, 1179–1183. [Google Scholar] [CrossRef]
- De Vita, S.; Quartuccio, L.; Salvin, S.; Corazza, L.; Zabotti, A.; Fabris, M. Cryoglobulinaemia related to Sjogren’s syndrome or HCV infection: Differences based on the pattern of bone marrow involvement, lymphoma evolution and laboratory tests after parotidectomy. Rheumatology 2012, 51, 627–633. [Google Scholar] [CrossRef]
- Retamozo, S.; Gheitasi, H.; Quartuccio, L.; Kostov, B.; Corazza, L.; Bové, A.; Sisó-Almirall, A.; Gandía, M.; Ramos-Casals, M.; De Vita, S.; et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary Sjögren syndrome: Analysis of 515 patients. Rheumatology 2016, 55, 1443–1451. [Google Scholar] [CrossRef][Green Version]
- Quartuccio, L.; Isola, M.; Corazza, L.; Maset, M.; Monti, G.; Gabrielli, A.; Tzioufas, A.G.; Ferri, C.; Ferraccioli, G.; Ramos-Casals, M.; et al. Performance of the preliminary classification criteria for cryoglobulinaemic vasculitis and clinical manifestations in hepatitis C virus-unrelated cryoglobulinaemic vasculitis. Clin. Exp. Rheumatol. 2012, 30 (Suppl. S70), S48–S52. [Google Scholar]
- Trejo, O.; Ramos-Casals, M.; García-Carrasco, M.; Yagüe, J.; Jiménez, S.; de la Red, G.; Cervera, R.; Font, J.; Ingelmo, M.; et al. Cryoglobulinemia: Study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine 2001, 80, 252–262. [Google Scholar] [CrossRef]
- Terrier, B.; Marie, I.; Lacraz, A.; Belenotti, P.; Bonnet, F.; Chiche, L.; Graffin, B.; Hot, A.; Kahn, J.E.; Michel, C.; et al. Non HCV-related infectious cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey and systematic review of the literature. J. Autoimmun. 2015, 65, 74–81. [Google Scholar] [CrossRef]
- Ferri, C.; Sebastiani, M.; Giuggioli, D.; Cazzato, M.; Longombardo, G.; Antonelli, A.; Puccini, R.; Michelassi, C.; Zignego, A.L. Mixed cryoglobulinemia: Demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rhem. 2004, 33, 355–374. [Google Scholar] [CrossRef]
- Monti, G.; Galli, M.; Invernizzi, F.; Pioltelli, P.; Saccardo, F.; Monteverde, A.; Pietrogrande, M.; Renoldi, P.; Bombardieri, S.; Bordin, G.; et al. Cryoglobulinemias: A multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISCF. Italian Group for the Study of Cryoglobulinaemias. QJM 1995, 88, 115–126. [Google Scholar]
- Cacoub, P.; Terrier, B. Hepatitis B-related autoimmune manifestations. Rheum. Dis. Clin. North Am. 2009, 35, 125–137. [Google Scholar] [CrossRef]
- Levo, Y.; Gorevic, P.D.; Kassab, H.J.; Tobias, H.; Franklin, E.C. Liver involvement in the syndrome of mixed cryoglobulinemia. Ann. Intern. Med. 1977, 87, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Dal Maso, L.; Mauro, E.; Gattei, V.; Ghersetti, M.; Bulian, P.; Moratelli, G.; Grassi, G.; Zorat, F.; Pozzato, G. Survival and prognostic factors in mixed cryoglobulinemia: Data from 246 cases. Diseases 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Boglione, L.; D’Avolio, A.; Cariti, G.; di Perri, G. Telbivudine in treatment of hepatitis B–associated cryoglobulinemia. J. Clin. Virol. 2013, 56, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Dal Maso, L.; Urraro, T.; Mauro, E.; Castelnovo, L.; Casarin, P.; Monti, G.; Gattei, V.; Zignego, A.L.; Pozzato, G. Hepatitis, B virus relate cryoglobulinemic vasculitis: A multicentre open label study from the Gruppo Italiano di Studio delle Crioglobulinemie (GISC). Dig. Liver Dis. 2016, 48, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Xu, S.T.; Chen, H.P.; Zhang, M.C.; Xu, F.; Cheng, S.Q.; Liu, Z.H. Clinical and morphologic spectrum of renal involvement in patients with HBV-associated cryoglobulinaemia. Nephrology 2017, 22, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.; Franklin, E.C. Cryoglobulinemia--a study of twenty-nine patients. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am. J. Med. 1966, 40, 828–836. [Google Scholar] [CrossRef]
- Cacoub, P.; Fabiani, F.L.; Musset, L.; Perrin, M.; Frangeul, L.; Leger, J.M.; Huraux, J.M.; Piette, J.C.; Godeau, P. Mixed cryoglobulinemia and hepatitis C virus. Am. J. Med. 1994, 96, 124–132. [Google Scholar] [CrossRef]
- Roccatello, D.; Fornasieri, A.; Giachino, O.; Rossi, D.; Beltrame, A.; Banfi, G.; Confalonieri, R.; Tarantino, A.; Pasquali, S.; Amoroso, A.; et al. Multicenter study on hepatitis C virus related cryoglobulinemic glomerulonephritis. Am. J. Kidney Dis. 2007, 4, 69–82. [Google Scholar] [CrossRef]
- Nelson, N.P.; Easterbrook, P.J.; McMahon, B.J. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin. Liver Dis. 2016, 20, 607–628. [Google Scholar] [CrossRef]
- Cakir, N.; Nuri Pamuk, O.; Umit, H.; Midlli, K. Successful treatment with Adefovir of one patient whose CV relapsed under lamivudine therapy and who was diagnosed to have HBV virologic breakthrough with YMDD mutations. Intern. Med. 2006, 45, 1213–1215. [Google Scholar] [PubMed]
- Kawakami, T.; Oaka, S.; Mizoguchi Soma, Y. Remission of hepatitis B virus-related cryoglobulinemic vasculitis with entecavir. Ann. Intern. Med. 2008, 149, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Makanishi, T.; Ishii, M.; Tamori, A.; Kawada, N. Entecavir to treat hepatitis B–associated cryoglobulinemic vasculitis. Ann. Intern. Med. 2008, 149, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Conca, P.; Riccio, A.; Tarantino, G. Successful lamivudine monotherapy in an elderly patient suffering from HBV-related decompensated cirrhosis associated with widespread leukocytoclastic vasculitis. Int. J. Immunolopathol. Pharmacol. 2009, 22, 531–535. [Google Scholar] [CrossRef]
- D’Amico, E.; Pace-Palitti, V.; di Lembo, E.; Palazzi, C. Successful treatment of hepatitis B virus infection and related cryoglobulinemic purpura, with nucleoside/nucleotide analogues. Clin. Exp. Rheumatol. 2013, 31, 155. [Google Scholar] [PubMed]
- Viganò, M.; Martin, P.; Cappelletti, M.; Fabrizi, F. HBV-associated cryoglobulinemic vasculitis: Remission after antiviral therapy with Entecavir. Kidney Blood Press. Res. 2014, 39, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Akimoto, T.; Okuda, K.; Sugase, T.; Takeshima, E.; Numata, A.; Morishita, Y.; Iwazu, Y.; Yoshizawa, H.; Komada, T.; et al. Purpura with ulcerative skin lesions and mixed cryoglobulinemia in a quiescent hepatitis B virus carrier. Intern. Med. 2014, 53, 115–118. [Google Scholar] [CrossRef][Green Version]
- Visentini, M.; Pascolini, S.; Mitrevski, M.; Marrapodi, R.; Del Padre, M.; Todi, L.; Camponeschi, A.; Axiotis, E.; Carlesimo, M.; De Santis, A.; et al. Hepatitis B virus caused mixed cryoglobulinemia by driving clonal expansion of innate B-cells producing a VH1-69-encoded antibody. Clin. Exp. Rheum. 2016, 34, S28–S32. [Google Scholar]
- Visentini, M.; Cagliuso, M.; Conti, V.; Carbonari, M.; Casato, M.; Fiorilli, M. The V(H)1-69-expressing marginal zone B cells expanded in HCV-associated mixed cryoglobulinemia display proliferative anergy irrespective of CD21(low) phenotype. Blood 2011, 118, 3440–3441. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Löhr, H.; Goergen, B.; Weber, W.; Gödderz, W.; Meyer zum Büschenfelde, K.H.; Gerken, G.; et al. Mixed cryoglobulinemia type II in chronic hepatitis B associated with HBe-minus HBV mutant: Cellular immune reactions and response to interferon treatment. J. Med. Virol. 1994, 44, 330–335. [Google Scholar] [CrossRef] [PubMed]
- La Civita, L.; Zignego, A.L.; Lombardini, F.; Monti, M.; Longombardo, G.; Pasero, G.; Ferri, C. Exacerbation of peripheral neuropathy during alpha-interferon therapy in a patient with mixed cryoglobulinemia and hepatitis B virus infection. J. Rheumatol. 1996, 23, 1641–1643. [Google Scholar]
- Zaja, F.; de Vita, S.; Mazzaro, C.; Sacco, S.; Damiani, D.; De Marchi, G.; Michelutti, A.; Baccarani, M.; Fanin, R.; Ferraccioli, G. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood 2003, 101, 3827–3834. [Google Scholar] [CrossRef]
- Roccatello, D.; Baldovino, S.; Rossi, D.; Mansouri, M.; Naretto, C.; Gennaro, M.; Cavallo, R.; Alpa, M.; Costanzo, P.; Giachino, O.; et al. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol. Dial. Transplant. 2004, 19, 3054–3061. [Google Scholar] [CrossRef][Green Version]
- Quartuccio, L.; Soardo, G.; Romano, G.; Zaja, F.; Scott, C.A.; De Marchi, G.; Fabris, M.; Ferraccioli, G.; De Vita, S.; et al. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: Efficacy and safety in the absence of steroids. Rheumatology 2006, 45, 842–846. [Google Scholar] [CrossRef][Green Version]
- Rossi, D.; Sciascia, S.; Fenoglio, R.; Ferro, M.; Baldovino, S.; Kamgaing, J.; Ventrella, F.; Kalikatzaros, H.; Viziello, L.; Solfietti, L.; et al. Cryoglobulinemic glomerulonephritis: Clinical presentation and histological features, diagnostic piftalls, and controversies in the management. State of the art and the experience on a large monocentric cohort treated with B cell depletion therapy. Minerva Med. 2020, 112, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, C.; Visentini, M.; Gragnani, L.; Fraticelli, P.; Tavoni, A.; Filippini, D.; Saccardo, F.; Lauletta, G.; Colantuono, S.; Atzeni, F.; et al. Safety and effectiveness of biosimilar of Rituximab CT-P10 in the treatment of cryoglobulinemic vasculitis: The MARBLe study (Mixed cryoglobulinemiA Rituximab BiosimiLar). Intern. Emerg. Med. 2020, 16, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, F.; Combarnous, F.; Macgregor, B.; Coppere, B.; Mausservey, C.; Ninet, J.; Hot, A.; et al. Safety and efficacy of rituximab treatment for vasculitis in hepatitis B virus-associated type II cryoglobulinemia: A case report. J. Med. Case Rep. 2012, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Hanbali, A.; Khaled, Y. Incidence of hepatitis B reactivation following rituximab therapy. Am. J. Hematol. 2009, 84, 195. [Google Scholar] [CrossRef]
- Monti, G.; Saccardo, F. Emergency in cryoglobulinemic syndrome: What to do? Dig. Liver Dis. 2007, 39 (Suppl. S1), S112–S115. [Google Scholar] [CrossRef]
- Auzerie, V.; Chiali, A.; Bussel, A.; Brouet, J.C.; Fermand, J.P.; Dubertret, L.; Senet, P.; et al. Leg ulcers associated with cryoglobulinemia: Clinical study of 15 patients and response to treatment. Arch. Dermatol. 2003, 139, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Moriconi, L.; Gremignai, G.; Migliorini, P.; Paleologo, G.; Fosella, P.V.; Bombardieri, S.; et al. Treatment of the renal involvement in mixed cryoglobulinemia with prolonged plasma exchange. Nephron 1986, 43, 246–253. [Google Scholar] [CrossRef]
- Stefanutti, C.; Vivenzio, A.; Di Giacomo, S.; Labbadia, G.; Mazza, F.; D’Alessandri, G.; Ferraro, P.M.; Masala, C.; et al. Immunoadsorption apheresis and immunosoppressive drug therapy in the treatment of complicated HCV-related cryoglobulinemic. J. Clin. Apher. 2009, 24, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Marson, P.; Monti, G.; Montani, F.; Riva, A.; Mascia, M.T.; Castelnovo, L.; Filippini, D.; Capuzzo, E.; Moretto, M.; D’Alessandri, G.; et al. Apheresis treatment of cryoglobulinemic vasculitis: A multicentre cohort study of 159 patients. Tranf. Apher. Sci. 2018, 57, 639–645. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Stone, J.H.; Cid, M.C.; Bosch, X. The cryoglobulinaemias. Lancet 2012, 379, 348–360. [Google Scholar] [CrossRef]
- Galli, M.; Monti, G.; Marson, P.; Pietrogrande, M.; Candela, M.; Castelnovo, L.; Faggioli, P.; Novati, P.; Zani, R.; et al. Recommendation for managing the manifestations of severe and life-threatening mixed cryoglobulinemia syndrome. Autoimmun. Rev. 2019, 18, 778–785. [Google Scholar] [CrossRef]
- Scwartz, J.; Padmanabhan, A.; Aqui, N.; Balogun, R.A.; Connelly-Smith, L.; Delaney, M.; Dunbar, N.M.; Witt, V.; Wu, Y.; Shaz, B.H.; et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J. Clin. Apher. 2016, 31, 149–162. [Google Scholar] [CrossRef]
- Pietrogrande, M.; De Vita, S.; Zignego, A.L.; Pioltelli, P.; Sansonno, D.; Sollima, S.; Atzeni, F.; Saccardo, F.; Quartuccio, L.; Bruno, S.; et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected 215 patients. Autoimmun. Rev. 2011, 10, 444–454. [Google Scholar] [CrossRef]
- Scarpato, S.; Atzeni, F.; Sarzi Puttini, P.; Brucato, A.; Quartuccio, L.; Pietrogrande, M.; Monti, G.; Galli, M.; Italian Group for Study of Cryoglobulinaemia (GISC). Pain management in cryoglobulinemia syndrome. Best Pract. Res. Clin. Rheumatol. 2015, 29, 77–89. [Google Scholar] [CrossRef]
- Tedeschi, A.; Baratè, C.; Minola, E.; Morra, E. Cryoglobulinemia. Blood Rev. 2007, 21, 183–200. [Google Scholar] [CrossRef]
- Pozzato, G.; Mazzaro, C.; Gattei, V. Hepatitis C Virus-Associated Non-Hodgkin Lymphomas: Biology, Epidemiology, and Treatment. Clin. Liver Dis. 2017, 21, 499–515. [Google Scholar] [CrossRef]
- Mazzaro, C.; Dal Maso, L.; Mauro, E.; Visentini, M.; Tonizzo, M.; Gattei, V.; Andreone, P.; Pozzato, G. Hepatitis C virus- related cryoglobulinemic vasculitis: A review of the role of the new direct antiviral agents (DAAs) therapy. Autoimmun. Rev. 2020, 19, 102589. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Dal Maso, L.; Visentini, M.; Ermacora, A.; Tonizzo, M.; Gattei, V.; Andreone, P. Recent news in the treatment of hepatitis B virus-related cryoglobulinemic vasculitis. Minerva Med. 2020, 111, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Mavragani, C.P.; Nezos, A.; Zintzaras, E.; Quartuccio, L.; De Vita, S.; Koutsilieris, M.; Tzioufas, A.G.; Moutsopoulos, H.M.; Voulgarelis, M. A BAFF receptor His159Tyr mutation in Sjögren’s syndrome-related lymphoproliferation. Arthritis Rheumatol. 2015, 67, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).