Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children
Abstract
1. Introduction
2. Methods
2.1. Subjects and Sample Collection
2.2. Determination of Antibody Reactivity Profiles by MeDALL Allergen- and PreDicta RV-Chip
2.3. Statistical Analysis
3. Results
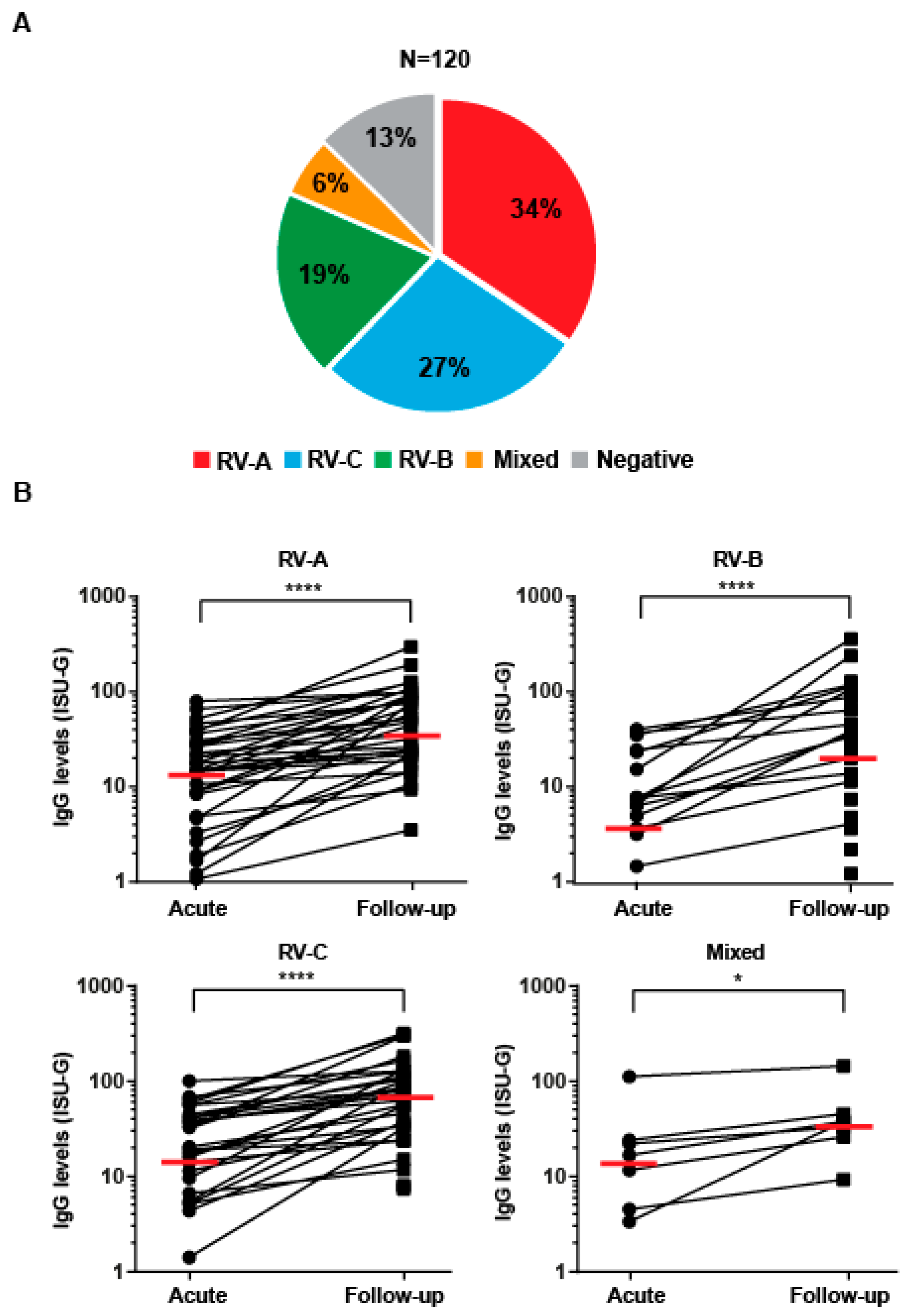
3.1. The Majority of Pre-School Children Presenting with Acute Wheezing Attacks Show RV Species-Specific IgG Increases
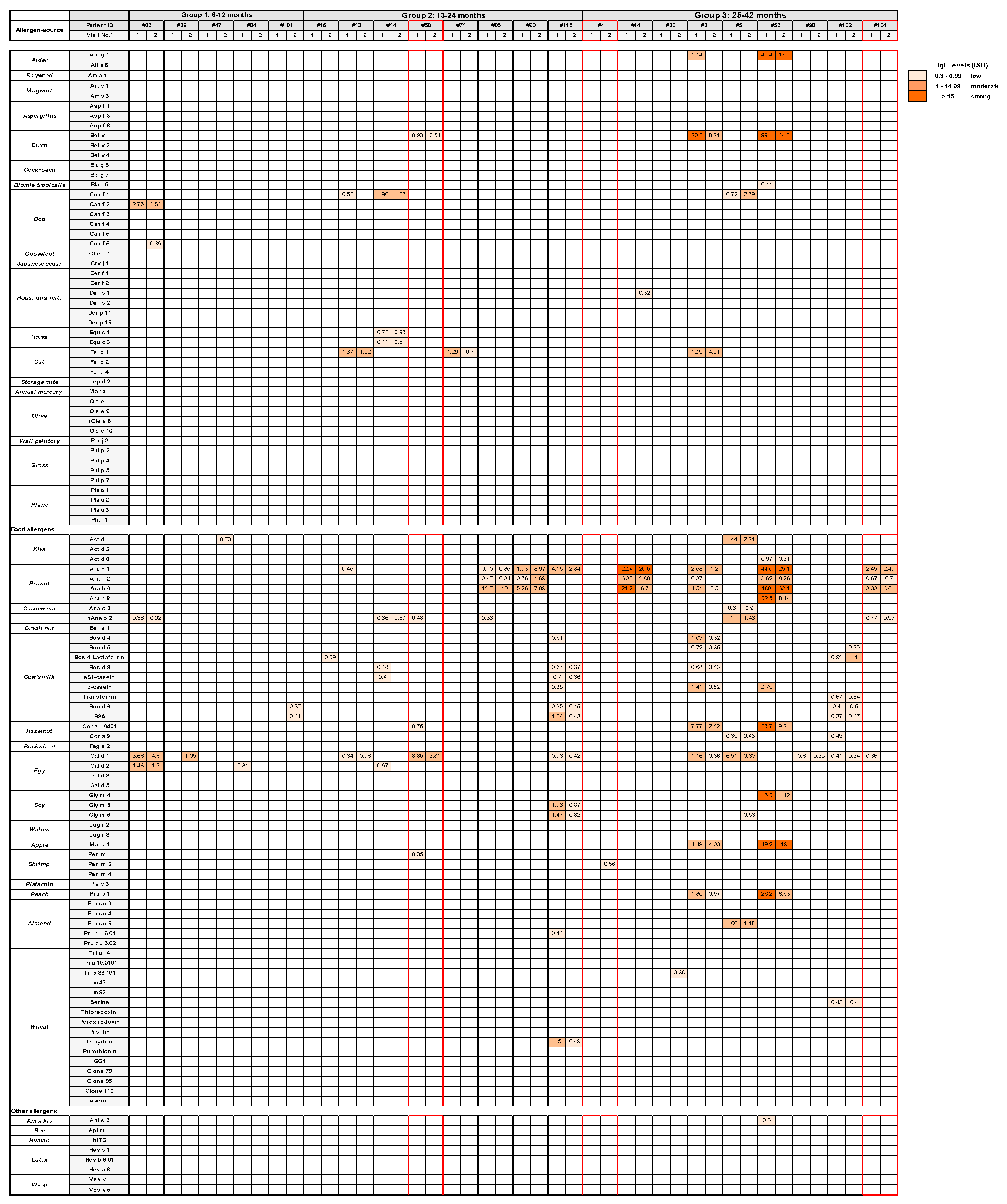
3.2. Role of Allergen-Specific IgE sensitizations and allergen Exposure as Possible Trigger Factors for Acute Wheezing in Certain Pre-School Children
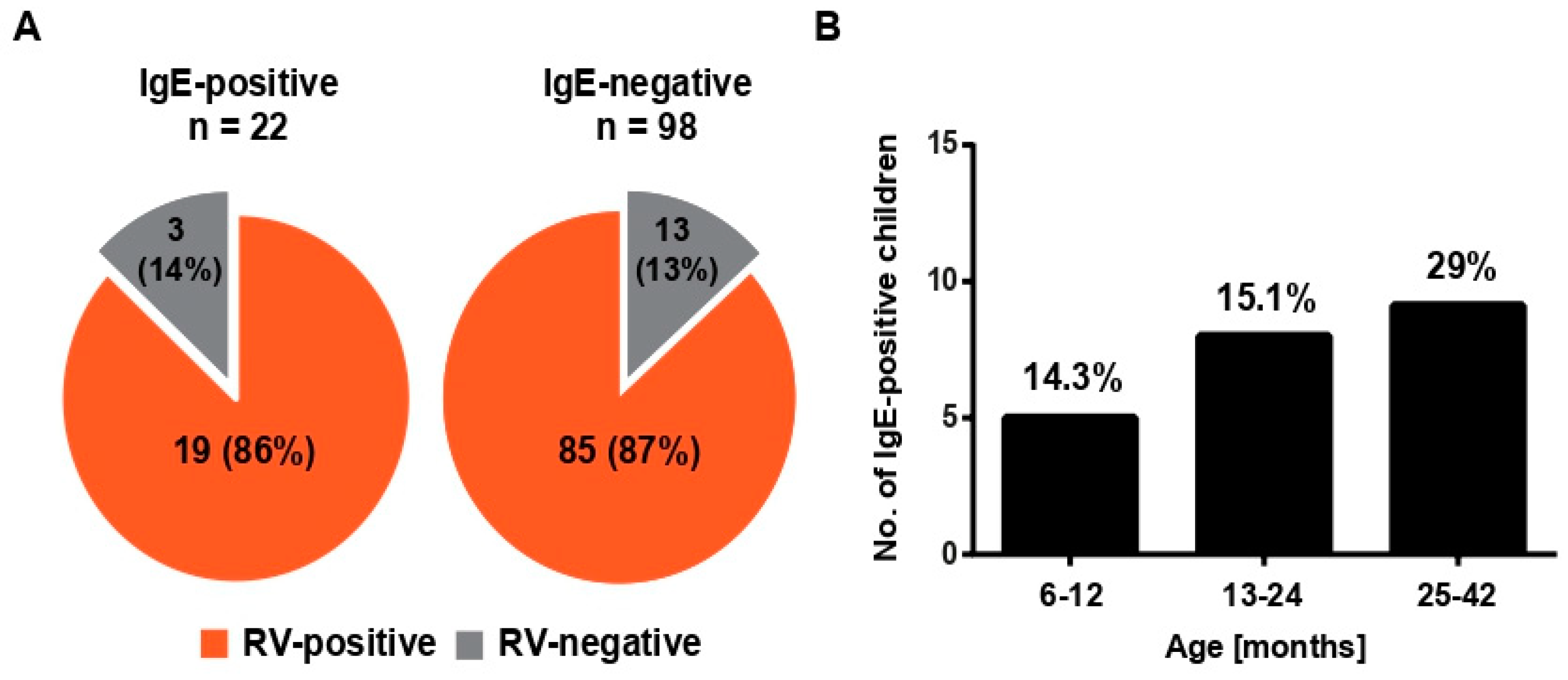
3.3. RV-Induced Wheeze Attacks Occur at the Same Frequencies in IgE-Sensitized, as Well as in Nonsensitized, Pre-School Children
3.4. Evidence for Allergen Exposure as Trigger Factor for Early Childhood Wheezing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heymann, P.W.; Carper, H.T.; Murphy, D.D.; Platts-Mills, T.A.; Patrie, J.; McLaughlin, A.P.; Erwin, E.A.; Shaker, M.S.; Hellems, M.; Peerzada, J.; et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004, 114, 239–247. [Google Scholar] [CrossRef]
- Green, R.M.; Custovic, A.; Sanderson, G.; Hunter, J.; Johnston, S.L.; Woodcock, A. Synergism between allergens and viruses and risk of hospital admission with asthma: Case-control study. BMJ 2002, 324, 763. [Google Scholar] [CrossRef]
- Rakes, G.P.; Arruda, E.; Ingram, J.M.; Hoover, G.E.; Zambrano, J.C.; Hayden, F.G.; Platts-Mills, T.A.; Heymann, P.W. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999, 159, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, J.C.; Carper, H.T.; Rakes, G.P.; Patrie, J.; Murphy, D.D.; Platts-Mills, T.A.; Hayden, F.G.; Gwaltney, J.M., Jr.; Hatley, T.K.; Owens, A.M. Experimental rhinovirus challenges in adults with mild asthma: Response to infection in relation to IgE. J. Allergy Clin. Immunol. 2003, 111, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, A.; Khaitov, M.; Jackson, D.J.; Traub, S.; Trujillo-Torralbo, M.B.; Kudlay, D.A.; Dvornikov, A.S.; Del-Rosario, A.; Valenta, R.; Stanciu, L.A.; et al. M1-like macrophages are potent producers of anti-viral interferons and M1-associated marker-positive lung macrophages are decreased during rhinovirus-induced asthma exacerbations. EBioMedicine 2020, 54, 102734. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Kuusipalo, H.; Vuorinen, T.; Söderlund-Venermo, M.; Allander, T.; Waris, M.; Hartiala, J.; Ruuskanen, O. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr. Allergy Immunol. 2010, 21, 1008–1014. [Google Scholar] [CrossRef]
- Haahtela, T.; Burbach, G.J.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bousquet, P.J.; Bresciani, M.; Bruno, A.; et al. Clinical relevance is associated with allergen-specific wheal size in skin prick testing. Clin. Exp. Allergy 2014, 44, 407–416. [Google Scholar] [CrossRef]
- Psarras, S.; Papadopoulos, N.G.; Johnston, S.L. Pathogenesis of respiratory syncytial virus bronchiolitis-related wheezing. Paediatr. Respir. Rev. 2004, 5 (Suppl. A), S179–S184. [Google Scholar] [CrossRef]
- Stenberg-Hammar, K.; Hedlin, G.; Söderhäll, C. Rhinovirus and preschool wheeze. Pediatr. Allergy Immunol. 2017, 28, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.; Asarnoj, A.; Hamsten, C.; Wickman, M.; van Hage, M. Windows of opportunity for tolerance induction for allergy by studying the evolution of allergic sensitization in birth cohorts. Semin. Immunol. 2017, 30, 61–66. [Google Scholar] [CrossRef]
- Soto-Quiros, M.; Avila, L.; Platts-Mills, T.A.; Hunt, J.F.; Erdman, D.D.; Carper, H.; Murphy, D.D.; Odio, S.; James, H.R.; Patrie, J.T.; et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J. Allergy Clin. Immunol. 2012, 129, 1499–1505. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.A.; Avila, P.C.; Heymann, P.W.; Miller, E.K.; Papadopoulos, N.G.; Peebles, R.S., Jr.; Gern, J.E. Infections and Asthma Committee, Environmental and Occupational Respiratory Diseases Interest Section, American Academy of Allergy, Asthma & Immunology. Viral respiratory tract infections and asthma: The course ahead. J. Allergy Clin. Immunol. 2010, 125, 1212–1217. [Google Scholar] [PubMed]
- Gangl, K.; Waltl, E.E.; Vetr, H.; Cabauatan, C.R.; Niespodziana, K.; Valenta, R.; Niederberger, V. Infection with Rhinovirus Facilitates Allergen Penetration Across a Respiratory Epithelial Cell Layer. Int. Arch. Allergy Immunol. 2015, 166, 291–296. [Google Scholar] [CrossRef]
- Waltl, E.E.; Selb, R.; Eckl-Dorna, J.; Mueller, C.A.; Cabauatan, C.R.; Eiwegger, T.; Resch-Marat, Y.; Niespodziana, K.; Vrtala, S.; Valenta, R.; et al. Betamethasone prevents human rhinovirus- and cigarette smoke- induced loss of respiratory epithelial barrier function. Sci. Rep. 2018, 8, 9688. [Google Scholar] [CrossRef]
- Bossios, A.; Gourgiotis, D.; Skevaki, C.L.; Saxoni-Papageorgiou, P.; Lötvall, J.; Psarras, S.; Karpathios, T.; Constandopoulos, A.G.; Johnston, S.L.; Papadopoulos, N.G. Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin. Exp. Allergy 2008, 38, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Hiller, R.; Laffer, S.; Harwanegg, C.; Huber, M.; Schmidt, W.M.; Twardosz, A.; Barletta, B.; Becker, W.M.; Blaser, K.; Breiteneder, H.; et al. Microarrayed allergen molecules: Diagnostic gatekeepers for allergy treatment. FASEB J. 2002, 16, 414–416. [Google Scholar] [CrossRef]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef]
- Huang, H.J.; Campana, R.; Akinfenwa, O.; Curin, M.; Sarzsinszky, E.; Karsonova, A.; Riabova, K.; Karaulov, A.; Niespodziana, K.; Elisyutina, O.; et al. Microrray-based allergy diagnosis: Quo Vadis? Front Immunol. 2021, 11, 594978. [Google Scholar] [CrossRef]
- Niederberger, V.; Ring, J.; Rakoski, J.; Jager, S.; Spitzauer, S.; Valent, P.; Horak, F.; Kundi, M.; Valenta, R. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int. Arch. Allergy Immunol. 2007, 142, 133–144. [Google Scholar] [CrossRef]
- Niespodziana, K.; Napora, K.; Cabauatan, C.; Focke-Tejkl, M.; Keller, W.; Niederberger, V.; Tsolia, M.; Christodoulou, I.; Papadopoulos, N.G.; Valenta, R. Misdirected antibody responses against an N-terminal epitope on human rhinovirus VP1 as explanation for recurrent RV infections. FASEB J. 2012, 26, 1001–1008. [Google Scholar] [CrossRef]
- Niespodziana, K.; Cabauatan, C.R.; Jackson, D.J.; Gallerano, D.; Trujillo-Torralbo, B.; Del Rosario, A.; Mallia, P.; Valenta, R.; Johnston, S.L. Rhinovirus-induced VP1-specific Antibodies are Group-specific and Associated with Severity of Respiratory Symptoms. EBioMedicine 2014, 2, 64–70. [Google Scholar] [CrossRef]
- Stenberg-Hammar, K.; Niespodziana, K.; Söderhäll, C.; James, A.; Cabauatan, C.R.; Konradsen, J.R.; Melén, E.; van Hage, M.; Valenta, R.; Hedlin, G. Rhinovirus-specific antibody responses in preschool children with acute wheeze reflect severity of respiratory symptoms. Allergy 2016, 71, 1728–1735. [Google Scholar] [CrossRef]
- Niespodziana, K.; Stenberg-Hammar, K.; Megremis, S.; Cabauatan, C.R.; Napora-Wijata, K.; Vacal, P.C.; Gallerano, D.; Lupinek, C.; Ebner, D.; Schlederer, T.; et al. PreDicta chip-based high resolution diagnosis of rhinovirus-induced wheeze. Nat. Commun. 2018, 9, 2382. [Google Scholar] [CrossRef] [PubMed]
- Megremis, S.; Niespodziana, K.; Cabauatan, C.; Xepapadaki, P.; Kowalski, M.L.; Jartti, T.; Bachert, C.; Finotto, S.; West, P.; Stamataki, S.; et al. Rhinovirus Species-Specific Antibodies Differentially Reflect Clinical Outcomes in Health and Asthma. Am. J. Respir. Crit. Care Med. 2018, 198, 1490–1499. [Google Scholar] [CrossRef]
- Niespodziana, K.; Borochova, K.; Pazderova, P.; Schlederer, T.; Astafyeva, N.; Baranovskaya, T.; Barbouche, M.R.; Beltyukov, E.; Berger, A.; Borzova, E.; et al. Toward personalization of asthma treatment according to trigger factors. J. Allergy. Clin. Immunol. 2020, 145, 1529–1534. [Google Scholar] [CrossRef]
- Hammar, K.S.; Hedlin, G.; Konradsen, J.R.; Nordlund, B.; Kull, I.; Giske, C.G.; Pedroletti, C.; Söderhäll, C.; Melén, E. Subnormal levels of vitamin D are associated with acute wheeze in young children. Acta Paediatr. 2014, 103, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509.e9. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Kostoudi, S.; Tzeli, K.; Kitsioulis, N.; Papadopoulos, N.G. A pilot study to investigate the influence of upper respiratory infections on IgE reactivity to food allergens. Pediatr. Allergy Immunol. 2019, 30, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Egger, C.; Horak, F.; Vrtala, S.; Valenta, R.; Niederberger, V. Nasal application of rBet v 1 or non-IgE-reactive T-cell epitope-containing rBet v 1 fragments has different effects on systemic allergen-specific antibody responses. J. Allergy Clin. Immunol. 2010, 126, 1312–1315.e4. [Google Scholar] [CrossRef]
- Eckl-Dorna, J.; Fröschl, R.; Lupinek, C.; Kiss, R.; Gattinger, P.; Marth, K.; Campana, R.; Mittermann, I.; Blatt, K.; Valent, P.; et al. Intranasal administration of allergen increases specific IgE whereas intranasal omalizumab does not increase serum IgE levels-A pilot study. Allergy 2018, 73, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Egger, C.; Lupinek, C.; Ristl, R.; Lemell, P.; Horak, F.; Zieglmayer, P.; Spitzauer, S.; Valenta, R.; Niederberger, V. Effects of nasal corticosteroids on boosts of systemic allergen-specific IgE production induced by nasal allergen exposure. PLoS ONE 2015, 23, e0114991. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, H.; Manabe, R.; Furukawa, H.; Kuwahara, N.; Kimura, T.; Jinno, M.; Matsukara, S.; Tanaka, A.; Uchida, Y.; Fujiwara, A.; et al. Influence of Omalizumab on Allergen-Specific IgE in Patients with Adult Asthma. Int. Arch. Allergy Immunol. 2015, 168, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Skrindo, I.; Lupinek, C.; Valenta, R.; Hovland, V.; Pahr, S.; Baar, A.; Carlsen, K.H.; Mowinckel, P.; Wickman, M.; Melen, E.; et al. The use of the MeDALL-chip to assess IgE sensitization: A new diagnostic tool for allergic disease? Pediatr. Allergy Immunol. 2015, 26, 239–246. [Google Scholar] [CrossRef]
- Lupinek, C.; Hochwallner, H.; Johansson, C.; Mie, A.; Rigler, E.; Scheynius, A.; Alm, J.; Valenta, R. Maternal allergen-specific IgG might protect the child against allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Hesla, H.M.; Stenius, F.; Järnbert-Pettersson, H.; Alm, J. Allergy-related disease in relation to early life exposures-the ALADDIN birth cohort. J. Allergy Clin. Immunol. 2017, 139, 686–688. [Google Scholar] [CrossRef]
- Wickman, M.; Lupinek, C.; Andersson, N.; Belgrave, D.; Asarnoj, A.; Benet, M.; Pinart, M.; Wieser, S.; Garcia-Aymerich, J.; Baar, A.; et al. Detection of IgE Reactivity to a Handful of Allergen Molecules in Early Childhood Predicts Respiratory Allergy in Adolescence. EBioMedicine 2017, 26, 91–99. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Manios, Y.; Liarigkovinos, T.; Grammatikaki, E.; Douladiris, N.; Kortsalioudaki, C.; Papadopoulos, N.G. Association of passive exposure of pregnant women to environmental tobacco smoke with asthma symptoms in children. Pediatr. Allergy Immunol. 2009, 20, 423–429. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Bachert, C.; Finotto, S.; Jartti, T.; Konstantinou, G.N.; Kiefer, A.; Kowalski, M.; Lewandowska-Polak, A.; Lukkarinen, H.; Roumpedaki, E.; et al. Contribution of repeated infections in asthma persistence from preschool to school age: Design and characteristics of the PreDicta cohort. Pediatr. Allergy Immunol. 2018, 29, 383–393. [Google Scholar] [CrossRef]
- Pavia, A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect Dis. 2011, 52, 284–289. [Google Scholar] [CrossRef]
- Advani, S.; Sengupta, A.; Forman, M.; Valsamakis, A.; Milstone, A.M. Detecting respiratory viruses in asymptomatic children. Pediatr. Infect. Dis. J. 2021, 31, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Van der Zalm, M.M.; van Ewijk, B.E.; Wilbrink, B.; Uiterwaal, C.S.; Wolfs, T.F.; van der Ent, C.K. Respiratory pathogens in children with and without respiratory symptoms. J. Pediatr. 2009, 154, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; Hayden, F.G.; Hendley, J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J. Med. Virol. 2006, 78, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Samoliński, B.; Fronczak, A.; Kuna, P.; Akdis, C.A.; Anto, J.M.; Bialoszewski, A.Z.; Burney, P.G.; Bush, A.; Czupryniak, A.; Dahl, R.; et al. Council on the European Union. Prevention and control of childhood asthma and allergy in the EU from the public health point of view: Polish Presidency of the European Union. Allergy 2012, 67, 726–731. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Value |
|---|---|
| Age in months, median (min-max) | 18 (6–42) |
| 6–12 months, n = 35 (median) | 10 |
| 13–24 months, n = 53 (median) | 17 |
| 25–42 months, n = 32 (median) | 32 |
| Male, n (%) | 76 (63) |
| 6–12 months, n (%) | 35 (29) |
| 13–24 months, n (%) | 53 (44) |
| 25–42 months, n (%) | 31 (26) |
| Ever wheeze before, n (%) | 92 (77) |
| Allergic sensitization 1, n (%) | 22 (18) |
| 6–12 months, n (%) | 5 (4.2) |
| 13–24 months, n (%) | 8 (6.6) |
| 25–42 months, n (%) | 9 (7.5) |
| Weeks until follow-up, median (min-max) | 11 (6–32) |
| 6–12 months, median (min-max) | 11 (7–27) |
| 13–24 months, median (min-max) | 11 (7–24) |
| 25–42 months, median (min-max) | 12.5 (9–30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niespodziana, K.; Stenberg-Hammar, K.; Papadopoulos, N.G.; Focke-Tejkl, M.; Errhalt, P.; Konradsen, J.R.; Söderhäll, C.; van Hage, M.; Hedlin, G.; Valenta, R. Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children. Viruses 2021, 13, 915. https://doi.org/10.3390/v13050915
Niespodziana K, Stenberg-Hammar K, Papadopoulos NG, Focke-Tejkl M, Errhalt P, Konradsen JR, Söderhäll C, van Hage M, Hedlin G, Valenta R. Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children. Viruses. 2021; 13(5):915. https://doi.org/10.3390/v13050915
Chicago/Turabian StyleNiespodziana, Katarzyna, Katarina Stenberg-Hammar, Nikolaos G. Papadopoulos, Margarete Focke-Tejkl, Peter Errhalt, Jon R. Konradsen, Cilla Söderhäll, Marianne van Hage, Gunilla Hedlin, and Rudolf Valenta. 2021. "Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children" Viruses 13, no. 5: 915. https://doi.org/10.3390/v13050915
APA StyleNiespodziana, K., Stenberg-Hammar, K., Papadopoulos, N. G., Focke-Tejkl, M., Errhalt, P., Konradsen, J. R., Söderhäll, C., van Hage, M., Hedlin, G., & Valenta, R. (2021). Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children. Viruses, 13(5), 915. https://doi.org/10.3390/v13050915







