Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Virus Production
2.3. Infections and Immunizations
2.4. Serum IgG Endpoint Titer
2.5. DENV Genome Quantification in Serum
2.6. Statistical Analysis
3. Results
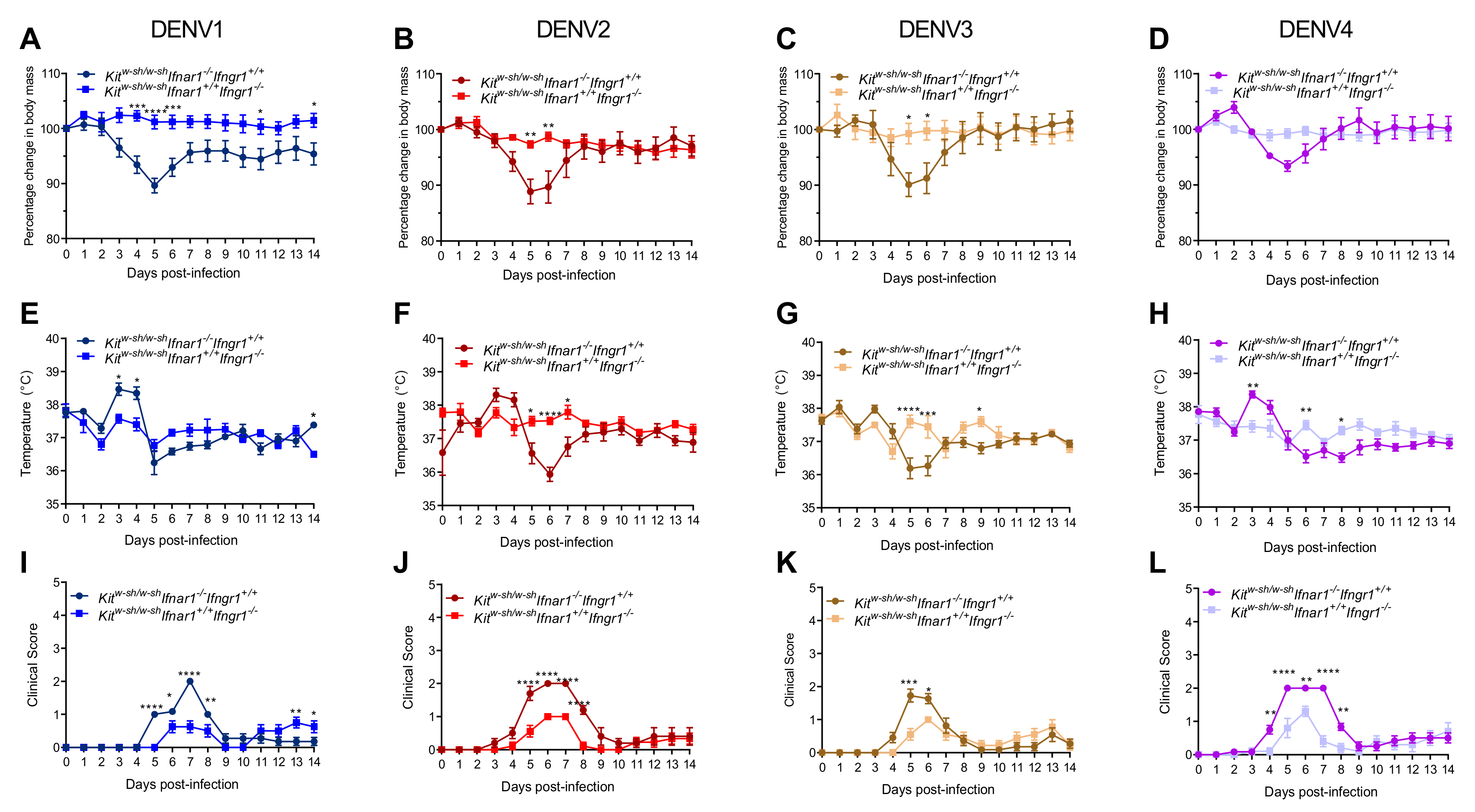
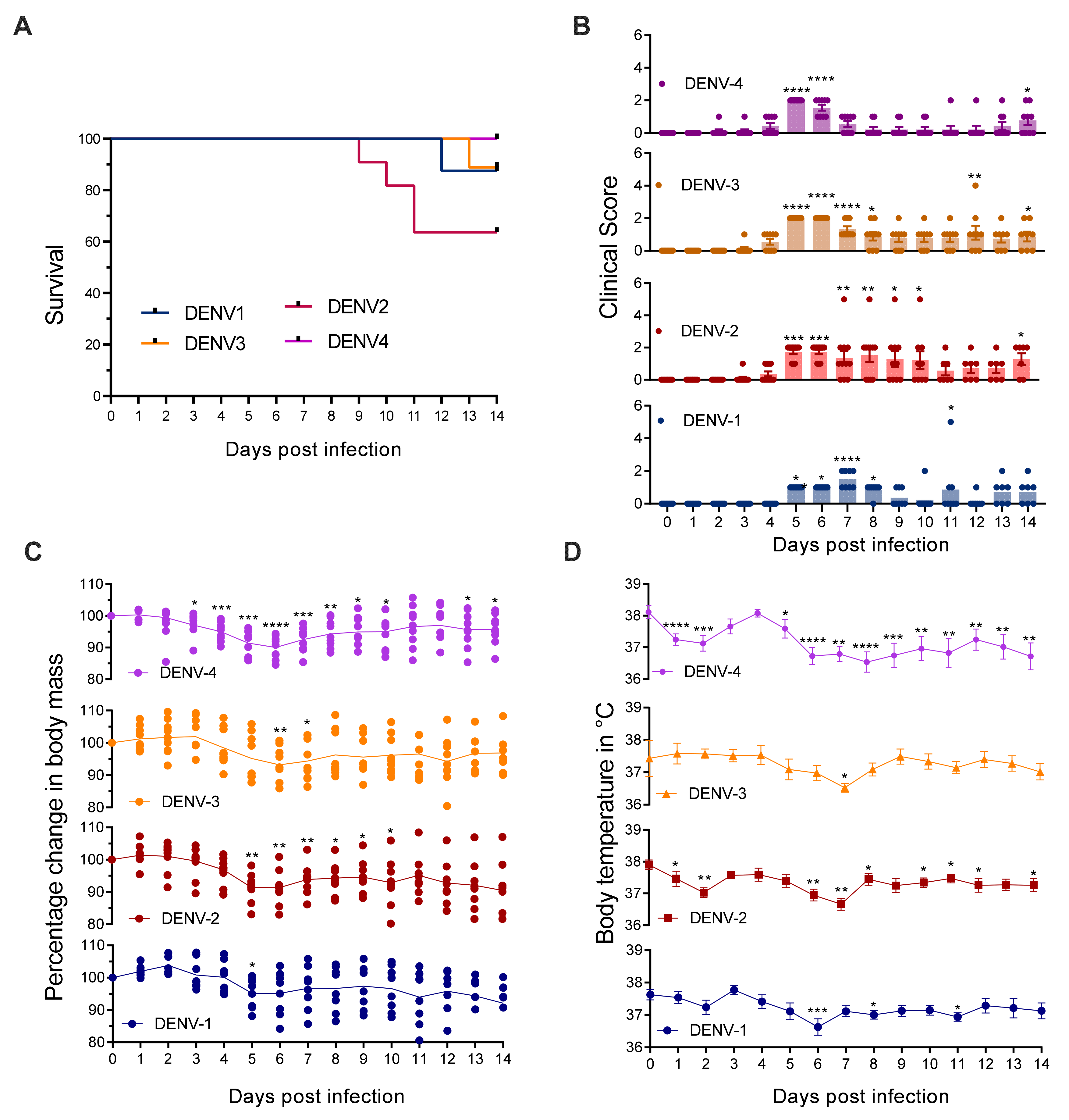
3.1. Mice Lacking MCs in Addition to Type-I and Type-II Interferon Receptors Are Susceptible to DENV Infection
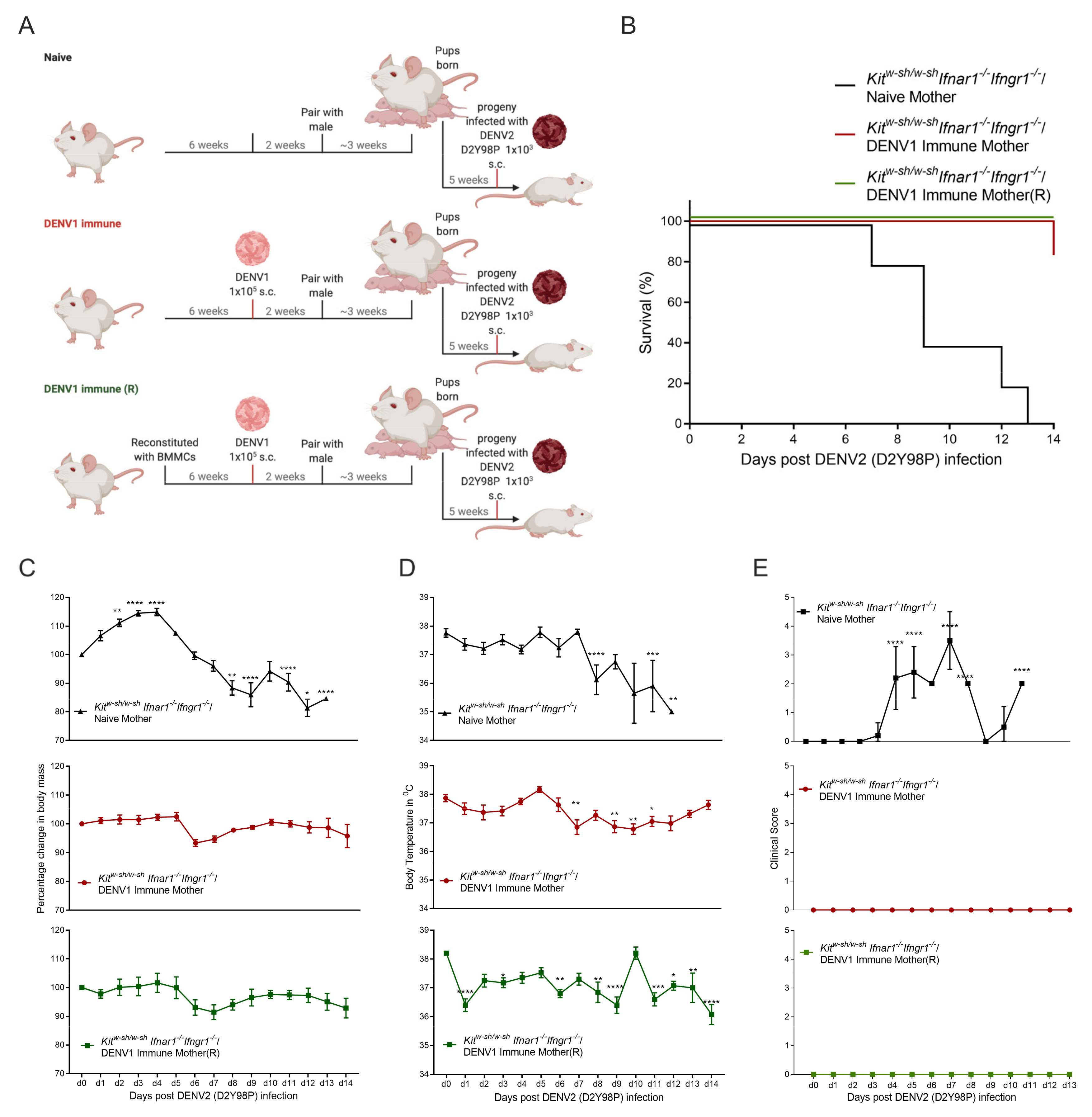
3.2. Progeny of DENV1 Immune KitW-sh/W-sh Ifnar1−/− Ifngr1−/− Mothers Are Protected from Subsequent Heterologous DENV2 Infection
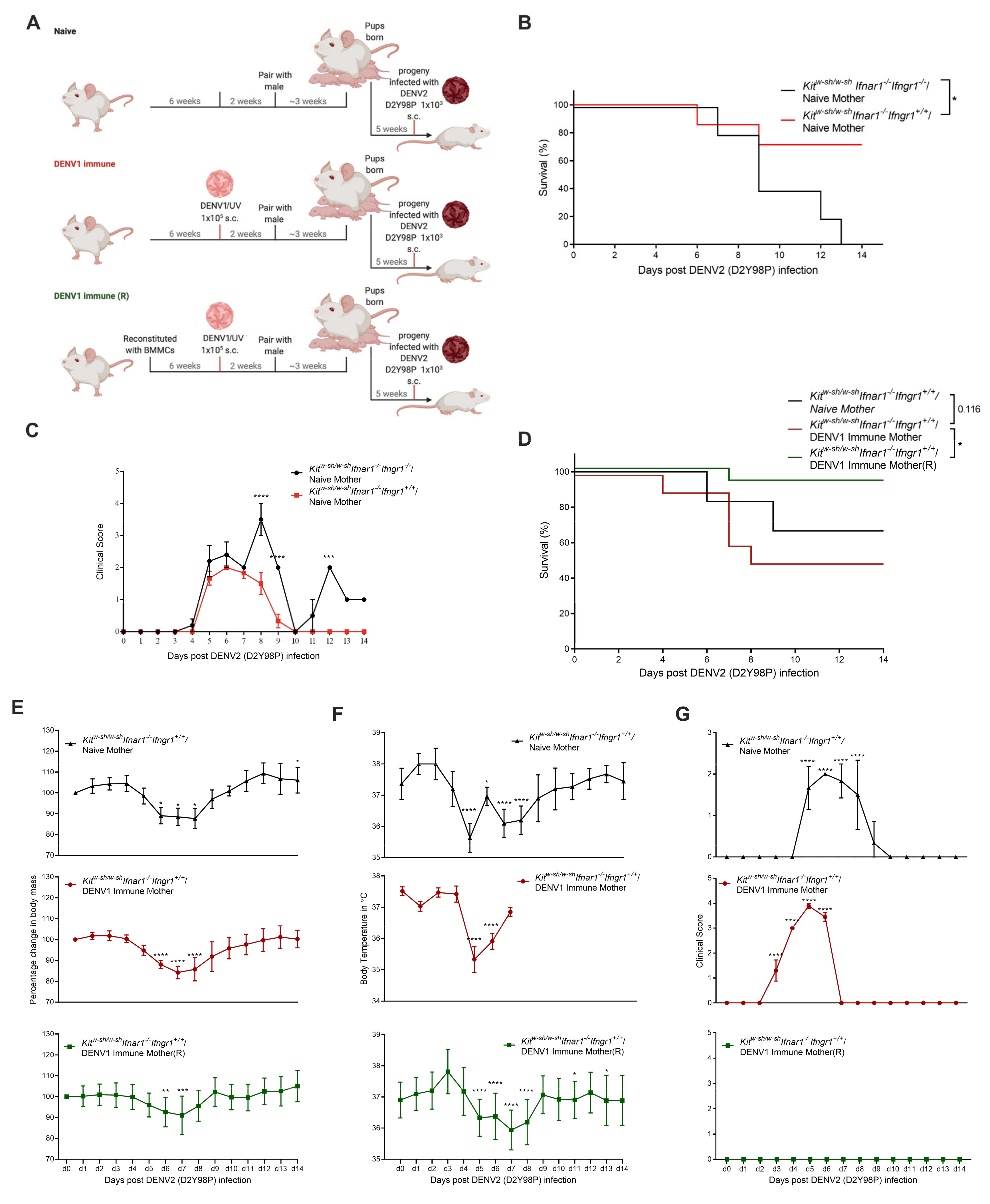
3.3. Increased Disease Severity of Heterologous DENV2 Infection in Progeny of DENV1-Immune KitW-sh/W-sh Ifnar1−/− Ifngr1+/+ Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nat. Cell Biol. 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Koshy, J.M.; Joseph, D.M.; John, M.; Mani, A.; Malhotra, N.; Abraham, G.M.; Pandian, J. Spectrum of neurological manifestations in dengue virus infection in Northwest India. Trop. Dr. 2012, 42, 191–194. [Google Scholar] [CrossRef]
- Garg, R.K.; Malhotra, H.S.; Verma, R.; Sharma, P.; Singh, M.K. Etiological spectrum of hypokalemic paralysis: A retrospective analysis of 29 patients. Ann. Indian Acad. Neurol. 2013, 16, 365. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Neurological manifestations of dengue viral infection. Res. Rep. Trop. Med. 2014, 5, 95–104. [Google Scholar] [CrossRef] [PubMed]
- John, A.L.S.; Rathore, A.P.S.; Yap, H.; Ng, M.-L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef] [PubMed]
- Mantri, C.K.; John, A.L.S. Immune synapses between mast cells and gammadelta T cells limit viral infection. J. Clin. Investig. 2019, 129, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; John, A.L.S. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- John, A.L.S. Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology. PLoS Pathog. 2013, 9, e1003783. [Google Scholar] [CrossRef]
- Syenina, A.; Jagaraj, C.J.; Ab Aman, S.; Sridharan, A.; John, A.L.S. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcgamma receptors. Elife 2015, 4, e05291. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Roehrig, J.T. New Mouse Model for Dengue Virus Vaccine Testing. J. Virol. 1999, 73, 783–786. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef]
- Watanabe, S.; Chan, K.W.K.; Wang, J.; Rivino, L.; Lok, S.-M.; Vasudevan, S.G. Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. J. Virol. 2015, 89, 5847–5861. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L. Subversion of Interferon by Dengue Virus. In Dengue Virus; Rothman, A.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 35–44. [Google Scholar]
- Ngono, A.E.; Shresta, S. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front. Immunol. 2019, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Uno, N.; Ross, T.M. Dengue virus and the host innate immune response. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type I Interferons Potently Enhance Humoral Immunity and Can Promote Isotype Switching by Stimulating Dendritic Cells In Vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Noma, T.; Kou, K.; Yoshizawa, I.; Yata, J. Regulation of human IgG subclass production by cytokines: Human IgG subclass production enhanced differentially by interleukin-6. Immunology 1995, 84, 278–284. [Google Scholar]
- Finkelman, F.D.; Katona, I.M.; Mosmann, T.R.; Coffman, R.L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J. Immunol. 1988, 140, 1022–1027. [Google Scholar]
- Palacio, N.; Dangi, T.; Chung, Y.R.; Wang, Y.; Loredo-Varela, J.L.; Zhang, Z.; Penaloza-MacMaster, P. Early type I IFN blockade improves the efficacy of viral vaccines. J. Exp. Med. 2020, 217, e20191220. [Google Scholar] [CrossRef] [PubMed]
- John, A.L.S.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.; Farouk, F.S.; John, A.L.S. Risk factors and biomarkers of severe dengue. Curr. Opin. Virol. 2020, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-T.; Chuang, Y.-J.; Lin, Y.-S.; Chang, C.-P.; Wan, S.-W.; Lin, S.-H.; Chen, C.-L.; Lin, C.-F. Antibody-Dependent Enhancement Infection Facilitates Dengue Virus-Regulated Signaling of IL-10 Production in Monocytes. PLoS Negl. Trop. Dis. 2014, 8, e3320. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Lim, J.Y.; Beltramello, M.; Quinn, M.; Chen, H.; Liu, S.-N.; Martinez-Sobrido, L.; Diamond, M.S.; Schlesinger, J.J.; De Silva, A.; et al. Human antibodies against dengue enhance dengue viral infectivity without suppressing type I interferon secretion in primary human monocytes. Virology 2011, 410, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.N.B.; Hieu, N.T.; Anders, K.L.; Wolbers, M.; Lien, L.B.; Hieu, L.T.M.; Hien, T.T.; Hung, N.T.; Farrar, J.; Whitehead, S.; et al. Dengue Virus Infections and Maternal Antibody Decay in a Prospective Birth Cohort Study of Vietnamese Infants. J. Infect. Dis. 2009, 200, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.W.; Zhang, S.L.; Tan, H.C.; Yan, B.; Gomez, J.M.M.; Tan, W.Y.; Lam, J.H.; Tan, G.K.X.; Ooi, E.E.; Alonso, S. First Experimental In Vivo Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies. PLoS Pathog. 2014, 10, e1004031. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M.M.; Ong, L.C.; Lam, J.H.; Aman, S.A.B.; Libau, E.A.; Lee, P.X.; John, A.L.S.; Alonso, S. Maternal Antibody-Mediated Disease Enhancement in Type I Interferon-Deficient Mice Leads to Lethal Disease Associated with Liver Damage. PLoS Negl. Trop. Dis. 2016, 10, e0004536. [Google Scholar] [CrossRef]
- Lee, P.X.; Ong, L.C.; Libau, E.A.; Alonso, S. Relative Contribution of Dengue IgG Antibodies Acquired during Gestation or Breastfeeding in Mediating Dengue Disease Enhancement and Protection in Type I Interferon Receptor-Deficient Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004805. [Google Scholar] [CrossRef]
- Kuczera, D.; Assolini, J.P.; Tomiotto-Pellissier, F.; Pavanelli, W.R.; Silveira, G.F. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J. Interf. Cytokine Res. 2018, 38, 69–80. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; John, A.L.S. Maternal immunity and antibodies to dengue virus promote infection and Zika virus–induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef] [PubMed]
- Low, J.G.H.; Ooi, E.-E.; Tolfvenstam, T.; Leo, Y.-S.; Hibberd, M.L.; Ng, L.-C.; Lai, Y.-L.; Yap, G.S.L.; Li, C.S.C.; Vasudevan, S.G.; et al. Early Dengue infection and outcome study (EDEN)-study design and preliminary findings. Ann. Acad. Med. Singap. 2006, 35, 783–789. [Google Scholar]
- Schulze, I.T.; Schlesinger, R. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology 1963, 19, 40–48. [Google Scholar] [CrossRef]
- Morrison, J.; Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Nishida, A.; John, A.L.S. Transcriptional Profiling Confirms the Therapeutic Effects of Mast Cell Stabilization in a Dengue Disease Model. J. Virol. 2017, 91, e0061717. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.; Mantri, C.K.; Aman, S.A.; Syenina, A.; Ooi, J.; Jagaraj, C.J.; Goh, C.C.; Tissera, H.; Wilder-Smith, A.; Ng, L.G.; et al. Dengue virus–elicited tryptase induces endothelial permeability and shock. J. Clin. Investig. 2019, 129, 4180–4193. [Google Scholar] [CrossRef] [PubMed]
- Merluzzi, S.; Frossi, B.; Gri, G.; Parusso, S.; Tripodo, C.; Pucillo, C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 2010, 115, 2810–2817. [Google Scholar] [CrossRef]
- John, A.L.S.; Abraham, S.N. Innate Immunity and Its Regulation by Mast Cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- John, A.L.S.; Chan, C.Y.; Staats, H.F.; Leong, K.W.; Abraham, S.N. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 2012, 11, 250–257. [Google Scholar] [CrossRef]
- McLachlan, J.B.; Shelburne, C.P.; Hart, J.P.; Pizzo, S.V.; Goyal, R.; Brooking-Dixon, R.; Staats, H.F.; Abraham, S.N. Mast cell activators: A new class of highly effective vaccine adjuvants. Nat. Med. 2008, 14, 536–541. [Google Scholar] [CrossRef] [PubMed]
- John, A.L.S.; Choi, H.W.; Walker, Q.D.; Blough, B.; Kuhn, C.M.; Abraham, S.N.; Staats, H.F. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. NPJ Vaccines 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Lee, C.Y.; Carissimo, G.; Chen, Z.; Lum, F.; Abu Bakar, F.; Rajarethinam, R.; Teo, T.; Torres-Ruesta, A.; Renia, L.; Ng, L.F. Type I interferon shapes the quantity and quality of the anti-Zika virus antibody response. Clin. Transl. Immunol. 2020, 9, e1126. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, V.V.; Milligan, G.N.; Bourne, N.; Barrett, A.D. Mouse models of dengue virus infection for vaccine testing. Vaccine 2015, 33, 7051–7060. [Google Scholar] [CrossRef]
- Vargas-Sánchez, Á.; Chiquete, E.; Gutiérrez-Plascencia, P.; Castañeda-Moreno, V.; Alfaro-Castellanos, D.; Paredes-Casillas, P.; Ruiz-Sandovala, J.L. Cerebellar Hemorrhage in a Patient during the Convalescent Phase of Dengue Fever. J. Stroke 2014, 16, 202–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantri, C.K.; Soundarajan, G.; Saron, W.A.A.; Rathore, A.P.S.; Alonso, S.; St. John, A.L. Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue. Viruses 2021, 13, 900. https://doi.org/10.3390/v13050900
Mantri CK, Soundarajan G, Saron WAA, Rathore APS, Alonso S, St. John AL. Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue. Viruses. 2021; 13(5):900. https://doi.org/10.3390/v13050900
Chicago/Turabian StyleMantri, Chinmay Kumar, Gayathri Soundarajan, Wilfried A. A. Saron, Abhay P. S. Rathore, Sylvie Alonso, and Ashley L. St. John. 2021. "Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue" Viruses 13, no. 5: 900. https://doi.org/10.3390/v13050900
APA StyleMantri, C. K., Soundarajan, G., Saron, W. A. A., Rathore, A. P. S., Alonso, S., & St. John, A. L. (2021). Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue. Viruses, 13(5), 900. https://doi.org/10.3390/v13050900





