Myeloid-Derived Suppressor Cells Restrain Natural Killer Cell Activity in Acute Coxsackievirus B3-Induced Myocarditis
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Experiments
2.2. In Vivo Depletion of MDSC
2.3. Isolation of Splenic and Cardiac Immune Cells
2.4. Enrichment of Splenic NK and MDSC for In Vitro Experiments
2.5. Flow Cytometry
2.6. RNA Isolation, cDNA Synthesis, and RT-PCR for the Detection of Cytokines and Viral RNA
2.7. Immunohistological Stainings
2.8. Immunofluorescence Staining of Cardiac MDSC
2.9. In Vitro Experiments to Evaluate Regulation of NK Cells by MDSC
2.10. Statistical Analyses
3. Results
3.1. Regulation of Myeloid-Derived Suppressor Cells in the Spleen and Heart of Coxsackievirus B3-Infected A.BY/SnJ Mice
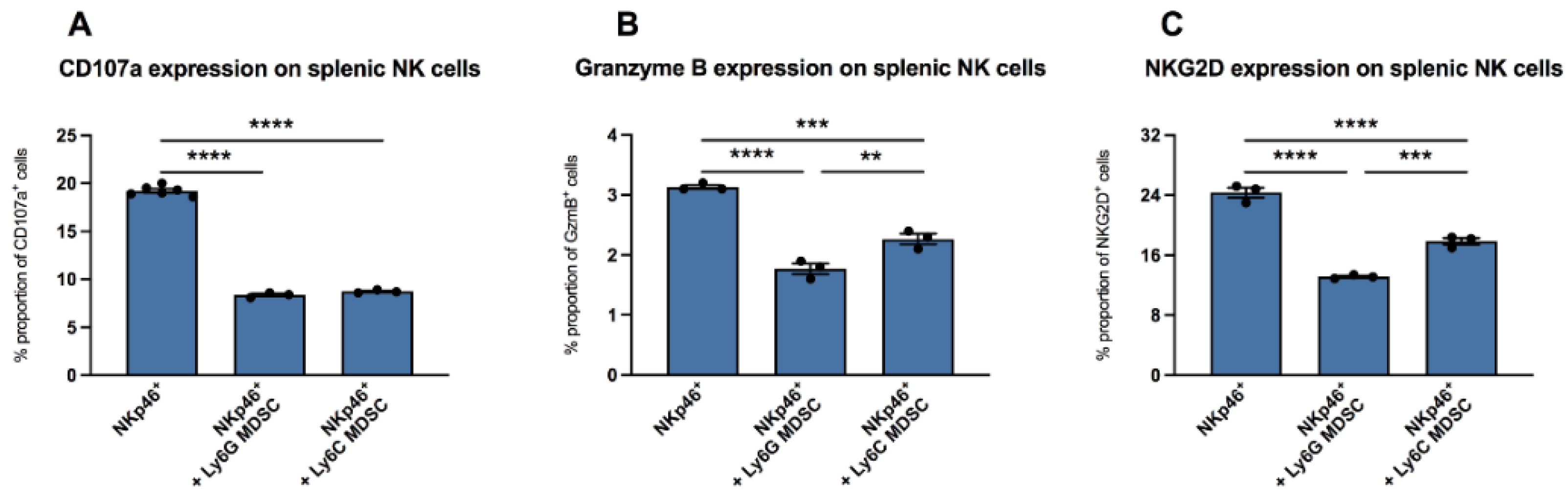
3.2. Impact of MDSC on NK Cells In Vitro
3.3. Impact of MDSC Depletion on Cardiac Virus Load, Damage, and Immune Response in CVB3-Infected A.BY/SnJ Mice
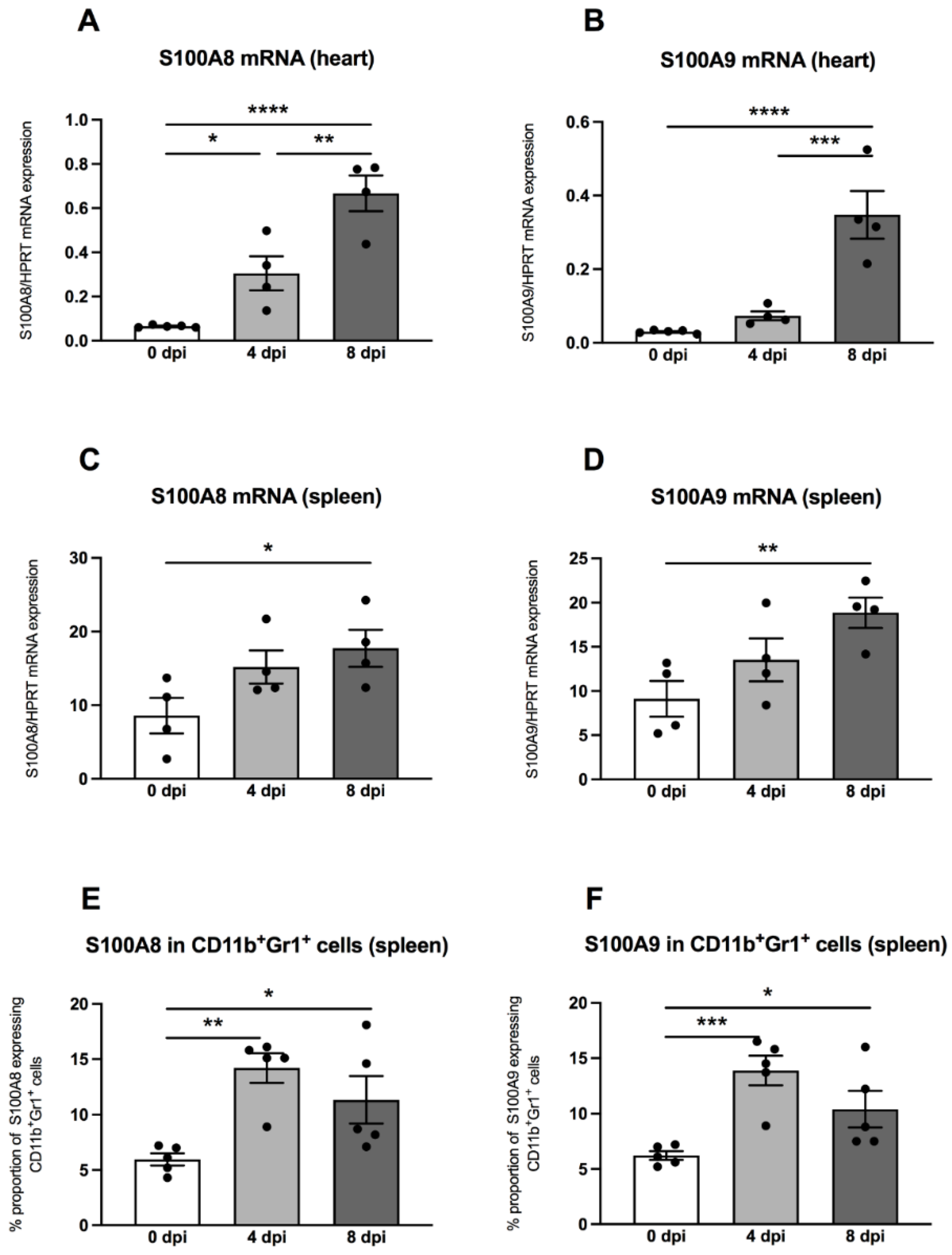
3.4. Regulation of S100A8 and S100A9 in the Heart and Spleen
3.5. Immunohistological Findings of Cardiac S100A8 and S100A9 Expressing Immune Cells
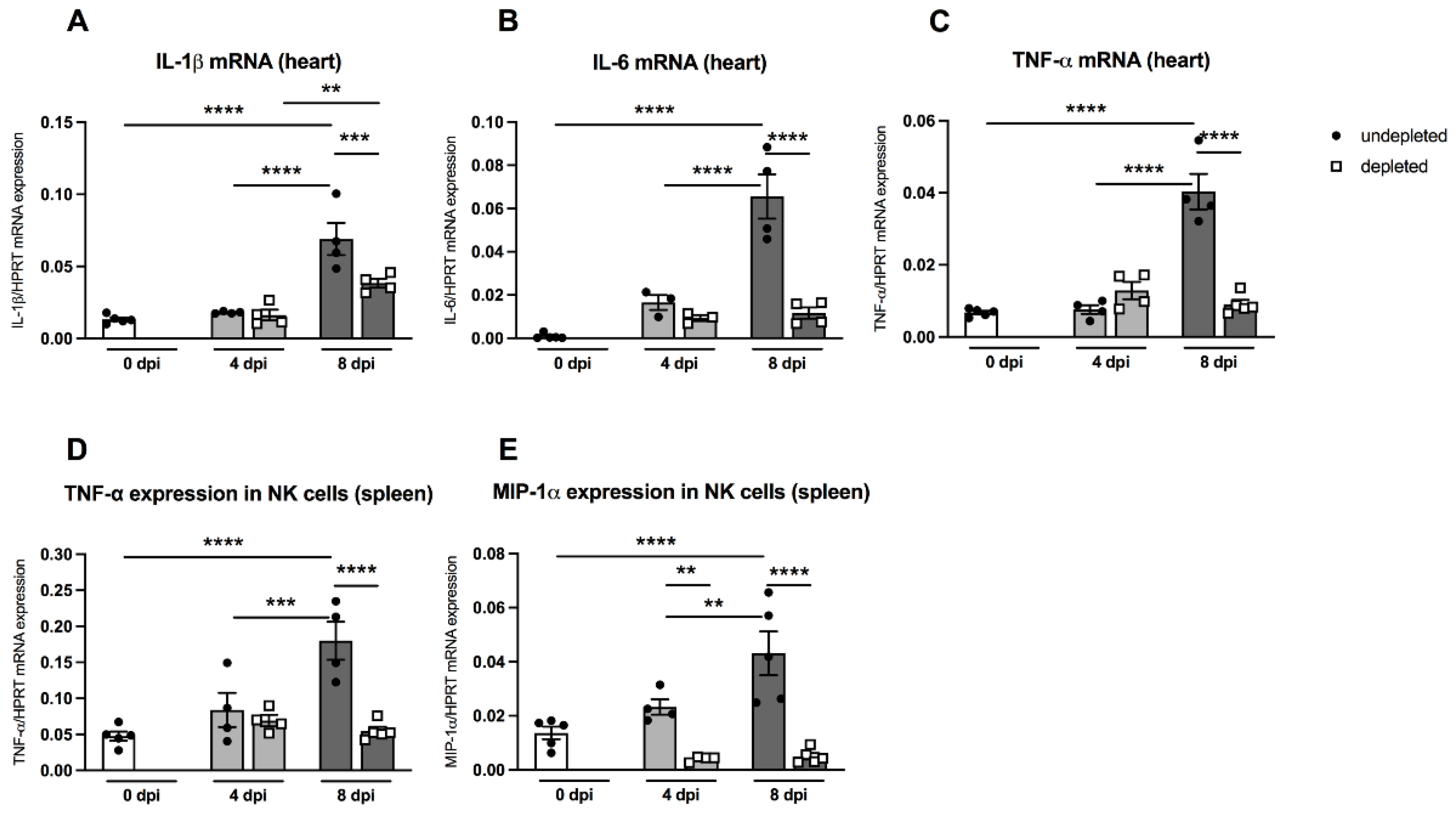
3.6. Impact of MDSC Depletion on Cytokine Levels
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Tschope, C.; Schultheiss, H.P. Lack in treatment options for virus-induced inflammatory cardiomyopathy: Can iPS-derived cardiomyocytes close the gap? Circ. Res. 2014, 115, 540–541. [Google Scholar] [CrossRef]
- Esfandiarei, M.; McManus, B.M. Molecular biology and pathogenesis of viral myocarditis. Annu. Rev. Pathol. 2008, 3, 127–155. [Google Scholar] [CrossRef]
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Klingel, K.; Fabritius, C.; Sauter, M.; Goldner, K.; Stauch, D.; Kandolf, R.; Ettischer, N.; Gahlen, S.; Schonberger, T.; Ebner, S.; et al. The activating receptor NKG2D of natural killer cells promotes resistance against enterovirus-mediated inflammatory cardiomyopathy. J. Pathol. 2014, 234, 164–177. [Google Scholar] [CrossRef]
- Song, X.; Krelin, Y.; Dvorkin, T.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A.; Voronov, E.; Apte, R.N. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J. Immunol. 2005, 175, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.; Huang, X.; Yang, Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J. Immunol. 2012, 189, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.; Narayanan, S.; Hahn, Y.S. Myeloid-derived suppressor cells: The dark knight or the joker in viral infections? Immunol. Rev. 2013, 255, 210–221. [Google Scholar] [CrossRef]
- Lunemann, A.; Lunemann, J.D.; Munz, C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol. Med. 2009, 15, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Vrakas, C.N.; O’Sullivan, R.M.; Evans, S.E.; Ingram, D.A.; Jones, C.B.; Phuong, T.; Kurt, R.A. The Measure of DAMPs and a role for S100A8 in recruiting suppressor cells in breast cancer lung metastasis. Immunol. Investig. 2015, 44, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Zilio, S.; Marigo, I.; Dolcetti, L.; Zanovello, P.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Yue, Y.; Xiong, S. Monocytic myeloid-derived suppressor cells from females, but not males, alleviate CVB3-induced myocarditis by increasing regulatory and CD4(+)IL-10(+) T cells. Sci. Rep. 2016, 6, 22658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; He, L.; Lv, K. Expansion of CD11b(+)Ly-6C(+) myeloid-derived suppressor cells (MDSCs) driven by galectin-9 attenuates CVB3-induced myocarditis. Mol. Immunol. 2017, 83, 62–71. [Google Scholar] [CrossRef]
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T.; et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008, 205, 2235–2249. [Google Scholar] [CrossRef]
- Gruhle, S.; Sauter, M.; Szalay, G.; Ettischer, N.; Kandolf, R.; Klingel, K. The prostacyclin agonist iloprost aggravates fibrosis and enhances viral replication in enteroviral myocarditis by modulation of ERK signaling and increase of iNOS expression. Basic Res. Cardiol. 2012, 107, 287. [Google Scholar] [CrossRef]
- Hohenadl, C.; Klingel, K.; Mertsching, J.; Hofschneider, P.H.; Kandolf, R. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol. Cell. Probes. 1991, 5, 11–20. [Google Scholar] [CrossRef]
- Coombes, J.L.; Han, S.J.; van Rooijen, N.; Raulet, D.H.; Robey, E.A. Infection-induced regulation of natural killer cells by macrophages and collagen at the lymph node subcapsular sinus. Cell Rep. 2012, 2, 124–135. [Google Scholar] [CrossRef]
- Klingel, K.; Kandolf, R. Molecular in situ localization techniques in diagnosis and pathogenicity studies of enteroviral heart disease. Clin. Diagn. Virol. 1996, 5, 157–166. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Klingel, K.; Hohenadl, C.; Canu, A.; Albrecht, M.; Seemann, M.; Mall, G.; Kandolf, R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: Quantitative analysis of virus replication, tissue damage, and inflammation. Proc. Natl. Acad. Sci. USA 1992, 89, 314–318. [Google Scholar] [CrossRef]
- Kandolf, R.; Sauter, M.; Aepinus, C.; Schnorr, J.J.; Selinka, H.C.; Klingel, K. Mechanisms and consequences of enterovirus persistence in cardiac myocytes and cells of the immune system. Virus Res. 1999, 62, 149–158. [Google Scholar] [CrossRef]
- Kraft, L.; Erdenesukh, T.; Sauter, M.; Tschope, C.; Klingel, K. Blocking the IL-1beta signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res. Cardiol. 2019, 114, 11. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Kanterman, J.; Ish-Shalom, E.; Elnekave, M.; Horwitz, E.; Baniyash, M. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 2013, 38, 541–554. [Google Scholar] [CrossRef]
- Roda, J.M.; Parihar, R.; Magro, C.; Nuovo, G.J.; Tridandapani, S.; Carson, W.E., 3rd. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006, 66, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Reefman, E.; Kay, J.G.; Wood, S.M.; Offenhauser, C.; Brown, D.L.; Roy, S.; Stanley, A.C.; Low, P.C.; Manderson, A.P.; Stow, J.L. Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells. J. Immunol. 2010, 184, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Szalay, G.; Sauter, M.; Hald, J.; Weinzierl, A.; Kandolf, R.; Klingel, K. Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am. J. Pathol. 2006, 169, 2085–2093. [Google Scholar] [CrossRef][Green Version]
- Weinzierl, A.O.; Szalay, G.; Wolburg, H.; Sauter, M.; Rammensee, H.G.; Kandolf, R.; Stevanovic, S.; Klingel, K. Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4-/CD8+ dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. J. Virol. 2008, 82, 8149–8160. [Google Scholar] [CrossRef]
- Klingel, K.; McManus, B.M.; Kandolf, R. Enterovirus-infected immune cells of spleen and lymph nodes in the murine model of chronic myocarditis: A role in pathogenesis? Eur. Heart J. 1995, 16 (Suppl. O), 42–45. [Google Scholar] [CrossRef]
- Kawai, C. From myocarditis to cardiomyopathy: Mechanisms of inflammation and cell death: Learning from the past for the future. Circulation 1999, 99, 1091–1100. [Google Scholar] [CrossRef]
- Muller, I.; Vogl, T.; Pappritz, K.; Miteva, K.; Savvatis, K.; Rohde, D.; Most, P.; Lassner, D.; Pieske, B.; Kuhl, U.; et al. Pathogenic Role of the Damage-Associated Molecular Patterns S100A8 and S100A9 in Coxsackievirus B3-Induced Myocarditis. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.; Vogl, T.; Kuhl, U.; Krannich, A.; Banks, A.; Trippel, T.; Noutsias, M.; Maisel, A.S.; van Linthout, S.; Tschope, C. Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Fail. 2020, 7, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 2008, 181, 4666–4675. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, H.; Guerrero, N.A.; Carbajosa, S.; Beschin, A.; De Baetselier, P.; Girones, N.; Fresno, M. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J. Immunol. 2011, 187, 2656–2665. [Google Scholar] [CrossRef]
- Klingel, K.; Schnorr, J.J.; Sauter, M.; Szalay, G.; Kandolf, R. beta2-microglobulin-associated regulation of interferon-gamma and virus-specific immunoglobulin G confer resistance against the development of chronic coxsackievirus myocarditis. Am. J. Pathol. 2003, 162, 1709–1720. [Google Scholar] [CrossRef]
- Shioi, T.; Matsumori, A.; Sasayama, S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation 1996, 94, 2930–2937. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Greene, C. Signal transduction pathways activated by the IL-1 receptor family: Ancient signaling machinery in mammals, insects, and plants. J. Leukoc. Biol. 1998, 63, 650–657. [Google Scholar] [CrossRef]
- Savvatis, K.; Muller, I.; Frohlich, M.; Pappritz, K.; Zietsch, C.; Hamdani, N.; Grote, K.; Schieffer, B.; Klingel, K.; Van Linthout, S.; et al. Interleukin-6 receptor inhibition modulates the immune reaction and restores titin phosphorylation in experimental myocarditis. Basic Res. Cardiol. 2014, 109, 449. [Google Scholar] [CrossRef]
- Skabytska, Y.; Biedermann, T. Cutaneous bacteria induce immunosuppression. Oncotarget 2015, 6, 30441–30442. [Google Scholar] [CrossRef]
- Cook, D.N.; Beck, M.A.; Coffman, T.M.; Kirby, S.L.; Sheridan, J.F.; Pragnell, I.B.; Smithies, O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 1995, 269, 1583–1585. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, I.; Janson, L.; Sauter, M.; Pappritz, K.; Linthout, S.V.; Tschöpe, C.; Klingel, K. Myeloid-Derived Suppressor Cells Restrain Natural Killer Cell Activity in Acute Coxsackievirus B3-Induced Myocarditis. Viruses 2021, 13, 889. https://doi.org/10.3390/v13050889
Müller I, Janson L, Sauter M, Pappritz K, Linthout SV, Tschöpe C, Klingel K. Myeloid-Derived Suppressor Cells Restrain Natural Killer Cell Activity in Acute Coxsackievirus B3-Induced Myocarditis. Viruses. 2021; 13(5):889. https://doi.org/10.3390/v13050889
Chicago/Turabian StyleMüller, Irene, Lisa Janson, Martina Sauter, Kathleen Pappritz, Sophie Van Linthout, Carsten Tschöpe, and Karin Klingel. 2021. "Myeloid-Derived Suppressor Cells Restrain Natural Killer Cell Activity in Acute Coxsackievirus B3-Induced Myocarditis" Viruses 13, no. 5: 889. https://doi.org/10.3390/v13050889
APA StyleMüller, I., Janson, L., Sauter, M., Pappritz, K., Linthout, S. V., Tschöpe, C., & Klingel, K. (2021). Myeloid-Derived Suppressor Cells Restrain Natural Killer Cell Activity in Acute Coxsackievirus B3-Induced Myocarditis. Viruses, 13(5), 889. https://doi.org/10.3390/v13050889






