Loperamide Inhibits Replication of Severe Fever with Thrombocytopenia Syndrome Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Materials
2.2. Virus Infection and Treatment with Compounds
2.3. Viral Titration
2.4. Time-of-Addition Infection Assay
2.5. Counting Fluorescent (SFTSV N-Positive) and DAPI-Stained Cell Numbers
2.6. Calcium Imaging and Analysis
2.7. Statistical Analysis
3. Results
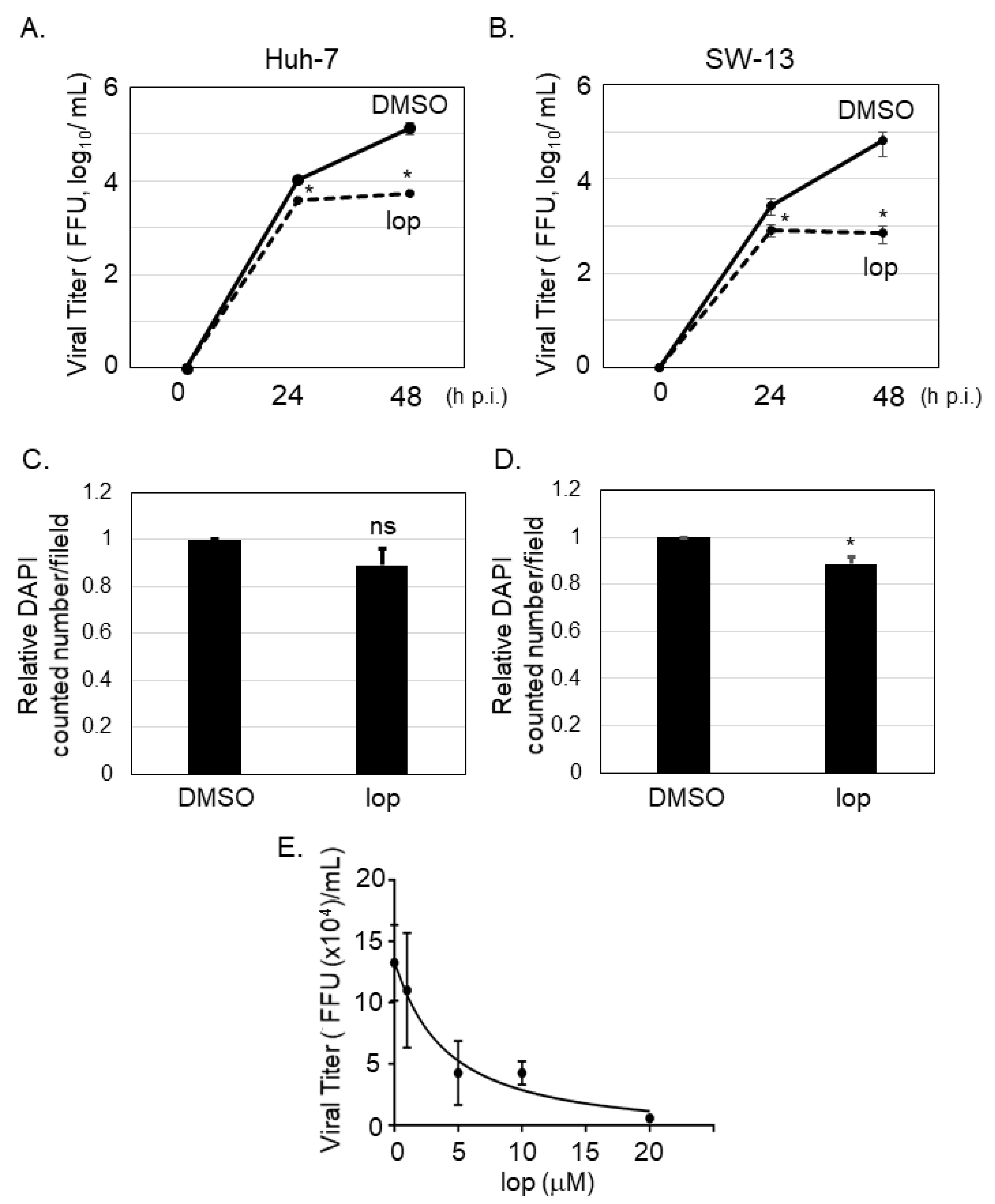
3.1. Loperamide Treatment Inhibited SFTSV Propagation
3.2. Loperamide Inhibited Post-Entry Step, but Not Pre- and during-Entry Stages, of SFTSV Infection
3.3. Nifedipine, but Not Ivabradine, Amantadine, or Naloxone, Inhibited Post-Entry Step of SFTSV Infection
3.4. Calcium Influx Was Inhibited by Loperamide Treatment in Huh-7 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.C.; Yun, Y.; Van An, L.; Kim, S.H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.H.; Lee, K.H. Endemic Severe Fever with Thrombocytopenia Syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029–1031. [Google Scholar] [CrossRef]
- Xu, B.; Liu, L.; Huang, X.; Ma, H.; Zhang, Y.; Du, Y.; Wang, P.; Tang, X.; Wang, H.; Kang, K.; et al. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: Discovery of a new bunyavirus. PLoS Pathog. 2011, 7, e1002369. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.H.; Choe, P.G.; Kim, N.J.; Lee, J.K.; Oh, M.D. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 2013, 19, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of severe Fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef]
- Lin, T.L.; Ou, S.C.; Maeda, K.; Shimoda, H.; Chan, J.P.; Tu, W.C.; Hsu, W.L.; Chou, C.C. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Emerg. Microbes Infect. 2020, 9, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, E.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albarino, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Mourya, D.T.; Yadav, P.D.; Basu, A.; Shete, A.; Patil, D.Y.; Zawar, D.; Majumdar, T.D.; Kokate, P.; Sarkale, P.; Raut, C.G.; et al. Malsoor virus, a novel bat phlebovirus, is closely related to severe fever with thrombocytopenia syndrome virus and heartland virus. J. Virol. 2014, 88, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Duan, X.; Wang, B.; Zhu, L.; Zhang, Y.; Zhang, J.; Wang, J.; Luo, T.; Kou, C.; Liu, D.; et al. A novel tick-borne phlebovirus, closely related to severe fever with thrombocytopenia syndrome virus and Heartland virus, is a potential pathogen. Emerg. Microbes Infect. 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Takayama-Ito, M.; Saijo, M. Antiviral Drugs Against Severe Fever With Thrombocytopenia Syndrome Virus Infection. Front. Microbiol. 2020, 11, 150. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.J.; Gould, R.J.; Snyder, S.H. Loperamide: Blockade of calcium channels as a mechanism for antidiarrheal effects. J Pharm. Exp. 1984, 231, 628–632. [Google Scholar]
- Urata, S.; Uno, Y.; Kurosaki, Y.; Yasuda, J. The cholesterol, fatty acid and triglyceride synthesis pathways regulated by site 1 protease (S1P) are required for efficient replication of severe fever with thrombocytopenia syndrome virus. Biochem. Biophys. Res. Commun. 2018, 503, 631–636. [Google Scholar] [CrossRef]
- Suzuki, T.; Sato, Y.; Sano, K.; Arashiro, T.; Katano, H.; Nakajima, N.; Shimojima, M.; Kataoka, M.; Takahashi, K.; Wada, Y.; et al. Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J. Clin. Investig. 2020, 130, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Shin, Y.; Daly, J.W. Loperamide: A positive modulator for store-operated calcium channels? Proc. Natl. Acad. Sci. USA 1997, 94, 14912–14917. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Vasilyev, D.V.; Shan, Q.J.; Dunlop, J.; Mayer, S.; Bowlby, M.R. Novel pharmacological activity of loperamide and CP-339,818 on human HCN channels characterized with an automated electrophysiology assay. Eur. J. Pharm. 2008, 581, 97–104. [Google Scholar] [CrossRef]
- Novella Romanelli, M.; Sartiani, L.; Masi, A.; Mannaioni, G.; Manetti, D.; Mugelli, A.; Cerbai, E. HCN Channels Modulators: The Need for Selectivity. Curr. Top Med. Chem. 2016, 16, 1764–1791. [Google Scholar] [CrossRef]
- Vasilyev, D.V.; Shan, Q.; Lee, Y.; Mayer, S.C.; Bowlby, M.R.; Strassle, B.W.; Kaftan, E.J.; Rogers, K.E.; Dunlop, J. Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J. Neurophysiol. 2007, 97, 3713–3721. [Google Scholar] [CrossRef]
- Church, J.; Fletcher, E.J.; Abdel-Hamid, K.; MacDonald, J.F. Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurons. Mol. Pharm. 1994, 45, 747–757. [Google Scholar]
- Baker, D.E. Loperamide: A pharmacological review. Rev. Gastroenterol. Disord. 2007, 7 (Suppl. 3), S11–S18. [Google Scholar]
- Hanauer, S.B. The role of loperamide in gastrointestinal disorders. Rev. Gastroenterol. Disord. 2008, 8, 15–20. [Google Scholar]
- DeHaven-Hudkins, D.L.; Burgos, L.C.; Cassel, J.A.; Daubert, J.D.; DeHaven, R.N.; Mansson, E.; Nagasaka, H.; Yu, G.; Yaksh, T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J. Pharm. Exp. 1999, 289, 494–502. [Google Scholar]
- Limapichat, W.; Yu, W.Y.; Branigan, E.; Lester, H.A.; Dougherty, D.A. Key binding interactions for memantine in the NMDA receptor. ACS Chem. Neurosci. 2013, 4, 255–260. [Google Scholar] [CrossRef]
- Guan, Y.; Johanek, L.M.; Hartke, T.V.; Shim, B.; Tao, Y.X.; Ringkamp, M.; Meyer, R.A.; Raja, S.N. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain 2008, 138, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Kosterlitz, H.W.; Leslie, F.M. Comparison of the receptor binding characteristics of opiate agonists interacting with mu- or kappa-receptors. Br. J. Pharm. 1978, 64, 607–614. [Google Scholar] [CrossRef]
- 2017 Annual Review of Diseases Prioritized under the Research and Development Blueprint; WHO Meeting Report; World Health Organization: Geneva, Switzerland, 2017.
- Tani, H.; Fukuma, A.; Fukushi, S.; Taniguchi, S.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Sato, Y.; Suzuki, T.; Nagata, N.; Hasegawa, H.; et al. Efficacy of T-705 (Favipiravir) in the Treatment of Infections with Lethal Severe Fever with Thrombocytopenia Syndrome Virus. mSphere 2016, 1. [Google Scholar] [CrossRef]
- Tani, H.; Komeno, T.; Fukuma, A.; Fukushi, S.; Taniguchi, S.; Shimojima, M.; Uda, A.; Morikawa, S.; Nakajima, N.; Furuta, Y.; et al. Therapeutic effects of favipiravir against severe fever with thrombocytopenia syndrome virus infection in a lethal mouse model: Dose-efficacy studies upon oral administration. PLoS ONE 2018, 13, e0206416. [Google Scholar] [CrossRef]
- Shimojima, M.; Fukushi, S.; Tani, H.; Yoshikawa, T.; Fukuma, A.; Taniguchi, S.; Suda, Y.; Maeda, K.; Takahashi, T.; Morikawa, S.; et al. Effects of ribavirin on severe fever with thrombocytopenia syndrome virus in vitro. Jpn. J. Infect. Dis. 2014, 67, 423–427. [Google Scholar] [CrossRef]
- Shimojima, M.; Fukushi, S.; Tani, H.; Taniguchi, S.; Fukuma, A.; Saijo, M. Combination effects of ribavirin and interferons on severe fever with thrombocytopenia syndrome virus infection. Virol. J. 2015, 12, 181. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Ando, S.; Shimojima, M.; Saijo, M.; Fukasawa, M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J. Infect. Chemother. 2018, 24, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Toyama, M.; Sakakibara, N.; Okamoto, M.; Arima, N.; Saijo, M. Establishment of an antiviral assay system and identification of severe fever with thrombocytopenia syndrome virus inhibitors. Antivir. Chem. Chemother. 2017, 25, 83–89. [Google Scholar] [CrossRef][Green Version]
- Yuan, S.; Chan, J.F.; Ye, Z.W.; Wen, L.; Tsang, T.G.; Cao, J.; Huang, J.; Chan, C.C.; Chik, K.K.; Choi, G.K.; et al. Screening of an FDA-Approved Drug Library with a Two-Tier System Identifies an Entry Inhibitor of Severe Fever with Thrombocytopenia Syndrome Virus. Viruses 2019, 11, 385. [Google Scholar] [CrossRef]
- Smee, D.F.; Jung, K.H.; Westover, J.; Gowen, B.B. 2’-Fluoro-2’-deoxycytidine is a broad-spectrum inhibitor of bunyaviruses in vitro and in phleboviral disease mouse models. Antivir. Res. 2018, 160, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.J.; Mo, Q.; Feng, K.; Min, Y.Q.; Li, M.; Hou, D.; Peng, C.; Zheng, X.; Deng, F.; Hu, Z.; et al. Interferon-gamma-Directed Inhibition of a Novel High-Pathogenic Phlebovirus and Viral Antagonism of the Antiviral Signaling by Targeting STAT1. Front. Immunol. 2019, 10, 1182. [Google Scholar] [CrossRef]
- Ogawa, M.; Shimojima, M.; Saijo, M.; Fukasawa, M. Several catechins and flavonols from green tea inhibit severe fever with thrombocytopenia syndrome virus infection in vitro. J. Infect. Chemother. 2021, 27, 32–39. [Google Scholar] [CrossRef]
- Mendoza, C.A.; Yamaoka, S.; Tsuda, Y.; Matsuno, K.; Weisend, C.M.; Ebihara, H. The NF-kappaB inhibitor, SC75741, is a novel antiviral against emerging tick-borne bandaviruses. Antivir. Res. 2021, 185, 104993. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.K.; Li, S.F.; Zhang, S.F.; Wan, W.W.; Zhang, Y.L.; Xin, Q.L.; Dai, K.; Hu, Y.Y.; Wang, Z.B.; et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019, 29, 739–753. [Google Scholar] [CrossRef]
- Takeda, M.; Pekosz, A.; Shuck, K.; Pinto, L.H.; Lamb, R.A. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J. Virol. 2002, 76, 1391–1399. [Google Scholar] [CrossRef]
- Appleyard, G. Amantadine-resistance as a genetic marker for influenza viruses. J. Gen. Virol. 1977, 36, 249–255. [Google Scholar] [CrossRef]
- Skehel, J.J.; Hay, A.J.; Armstrong, J.A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J. Gen. Virol. 1978, 38, 97–110. [Google Scholar] [CrossRef]
- Dey, D.; Siddiqui, S.I.; Mamidi, P.; Ghosh, S.; Kumar, C.S.; Chattopadhyay, S.; Ghosh, S.; Banerjee, M. The effect of amantadine on an ion channel protein from Chikungunya virus. PLoS Negl. Trop Dis. 2019, 13, e0007548. [Google Scholar] [CrossRef]
- Wang, K.; Xie, S.; Sun, B. Viral proteins function as ion channels. Biochim. Biophys. Acta 2011, 1808, 510–515. [Google Scholar] [CrossRef]
- Koff, W.C.; Elm, J.L., Jr.; Halstead, S.B. Inhibition of dengue virus replication by amantadine hydrochloride. Antimicrob. Agents Chemother. 1980, 18, 125–129. [Google Scholar] [CrossRef]
- Chen, J.; Liang, L.; Li, Y.; Zhang, Y.; Zhang, M.; Yang, T.; Meng, F.; Lai, X.; Li, C.; He, J.; et al. Naloxone regulates the differentiation of neural stem cells via a receptor-independent pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 5917–5930. [Google Scholar] [CrossRef]
- Liang, L.; Chen, J.; Li, Y.; Lai, X.; Sun, H.; Li, C.; Zhang, M.; Yang, T.; Meng, F.; Law, P.Y.; et al. Morphine and Naloxone Facilitate Neural Stem Cells Proliferation via a TET1-Dependent and Receptor-Independent Pathway. Cell Rep. 2020, 30, 3625–3631.e3626. [Google Scholar] [CrossRef]
- Sasaki, A.; Nakashima, Y.; Takasaki, I.; Andoh, T.; Shiraki, K.; Kuraishi, Y. Effects of loperamide on mechanical allodynia induced by herpes simplex virus type-1 in mice. J. Pharm. Sci. 2007, 104, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chertow, D.S.; Uyeki, T.M.; DuPont, H.L. Loperamide therapy for voluminous diarrhea in Ebola virus disease. J. Infect. Dis. 2015, 211, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urata, S.; Yasuda, J.; Iwasaki, M. Loperamide Inhibits Replication of Severe Fever with Thrombocytopenia Syndrome Virus. Viruses 2021, 13, 869. https://doi.org/10.3390/v13050869
Urata S, Yasuda J, Iwasaki M. Loperamide Inhibits Replication of Severe Fever with Thrombocytopenia Syndrome Virus. Viruses. 2021; 13(5):869. https://doi.org/10.3390/v13050869
Chicago/Turabian StyleUrata, Shuzo, Jiro Yasuda, and Masaharu Iwasaki. 2021. "Loperamide Inhibits Replication of Severe Fever with Thrombocytopenia Syndrome Virus" Viruses 13, no. 5: 869. https://doi.org/10.3390/v13050869
APA StyleUrata, S., Yasuda, J., & Iwasaki, M. (2021). Loperamide Inhibits Replication of Severe Fever with Thrombocytopenia Syndrome Virus. Viruses, 13(5), 869. https://doi.org/10.3390/v13050869




