Experimental Morogoro Virus Infection in Its Natural Host, Mastomys natalensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Monitoring
2.3. Virus Strains
2.4. Inoculation
2.5. Sampling and Analysis
2.6. Statistics and Data Presentation
3. Results
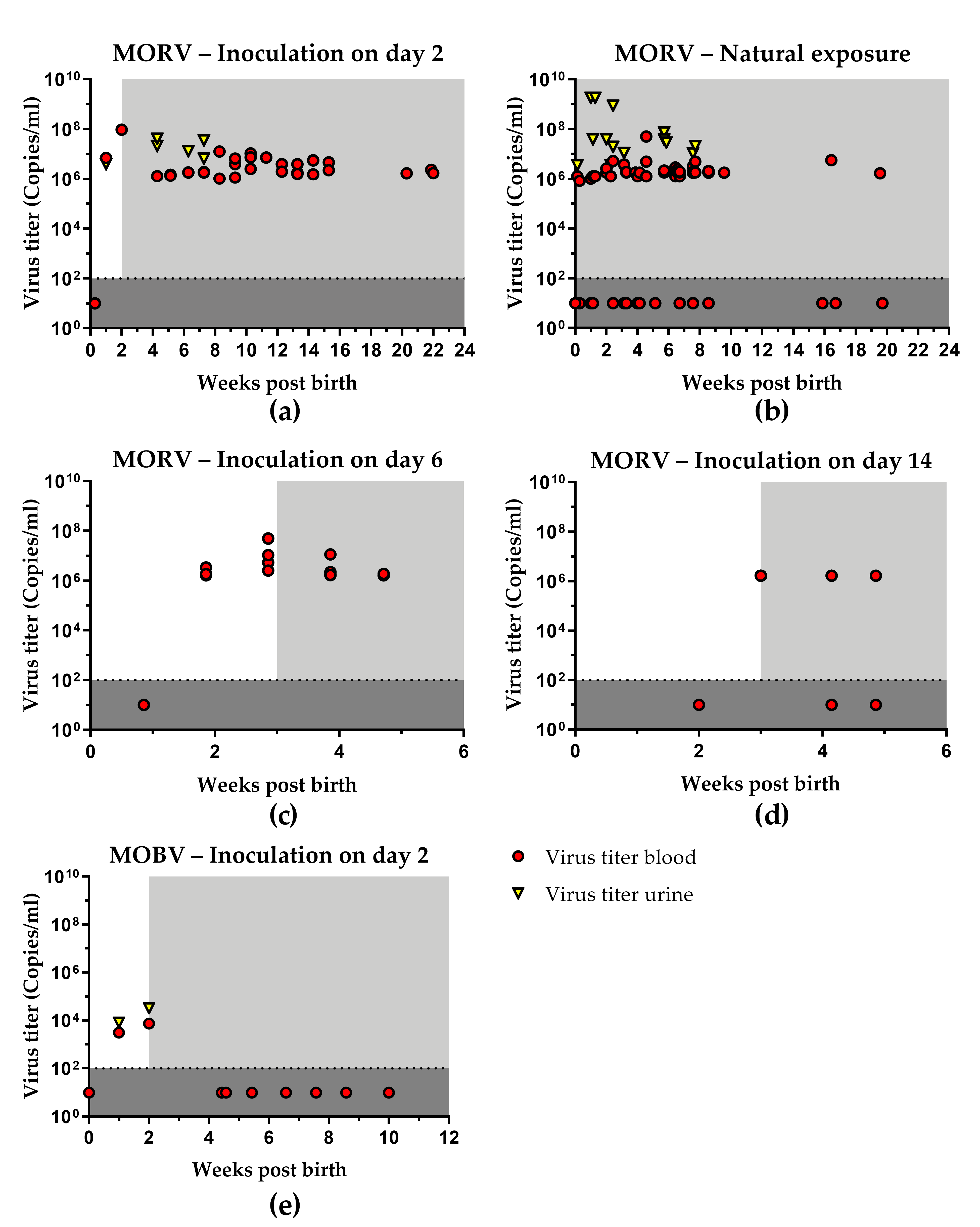
3.1. Infection with MORV
3.1.1. Inoculation of Neonates
3.1.2. Natural Transmission
3.1.3. Age Dependence of Infection
3.2. Infection with MOBV
3.3. Growth and Development of Infected Animals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salazar-Bravo, J.; Ruedas, L.A.; Yates, T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 25–63. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Kuhn, J.H.; Jahrling, P.B.; Bavari, S. Capter 21: Hemorrhagic fever-causing mammarenaviruses. In Medical Aspects of Biological Warfare; Office of The Surgeon General Borden Institute; US Army Medical Department Center and School; Health Readiness Center of Excellence: Fort Sam Houston, TX, USA, 2018. [Google Scholar]
- Fichet-Calvet, E.; Rogers, D.J. Risk Maps of Lassa Fever in West Africa. PLoS Negl. Trop. Dis. 2009, 3, e388. [Google Scholar] [CrossRef]
- McCormick, J.B.; Webb, P.A.; Krebs, J.W.; Johnson, K.M.; Smith, E.S. A Prospective Study of the Epidemiology and Ecology of Lassa Fever. J. Infect. Dis. 1987, 155, 437–444. [Google Scholar] [CrossRef]
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa Virus Isolation from Mastomys natalensis Rodents during an Epidemic in Sierra Leone. Science 1974, 185, 263–265. [Google Scholar] [CrossRef]
- Demby, A.H.; Inapogui, A.; Kargbo, K.; Koninga, J.; Kourouma, K.; Kanu, J.; Coulibaly, M.; Wagoner, K.D.; Ksiazek, T.G.; Peters, C.; et al. Lassa Fever in Guinea: II. Distribution and Prevalence of Lassa Virus Infection in Small Mammals. Vector Borne Zoonotic Dis. 2001, 1, 283–297. [Google Scholar] [CrossRef]
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulemou, K.; Sylla, O.; Kourouma, F.; Dore, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Hoch, S.P.; Tomori, O.; Nasidi, A.; Perez-Oronoz, G.I.; Fakile, Y.; Hutwagner, L.; McCormick, J.B. Review of cases of nosocomial Lassa fever in Nigeria: The high price of poor medical practice. BMJ 1995, 311, 857–859. [Google Scholar] [CrossRef]
- Iacono, G.L.; Cunningham, A.A.; Fichet-Calvet, E.; Garry, R.F.; Grant, D.S.; Khan, S.H.; Leach, M.; Moses, L.M.; Schieffelin, J.S.; Shaffer, J.G.; et al. Using Modelling to Disentangle the Relative Contributions of Zoonotic and Anthroponotic Transmission: The Case of Lassa Fever. PLoS Negl. Trop. Dis. 2015, 9, e3398. [Google Scholar] [CrossRef]
- Kafetzopoulou, L.E.; Pullan, S.T.; Lemey, P.; Suchard, M.A.; Ehichioya, D.U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J.; Oestereich, L.; Wozniak, D.M.; et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science 2019, 363, 74–77. [Google Scholar] [CrossRef]
- Siddle, K.J.; Eromon, P.; Barnes, K.G.; Mehta, S.; Oguzie, J.U.; Odia, I.; Schaffner, S.F.; Winnicki, S.M.; Shah, R.R.; Qu, J.; et al. Genomic Analysis of Lassa Virus during an Increase in Cases in Nigeria in 2018. N. Engl. J. Med. 2018, 379, 1745–1753. [Google Scholar] [CrossRef]
- Olayemi, A.; Cadar, D.; Magassouba, N.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New Hosts of The Lassa Virus. Sci. Rep. 2016, 6, 25280. [Google Scholar] [CrossRef]
- Yadouleton, A.; Agolinou, A.; Kourouma, F.; Saizonou, R.; Pahlmann, M.; Bedié, S.K.; Bankolé, H.; Becker-Ziaja, B.; Gbaguidi, F.; Thielebein, A.; et al. Lassa Virus in Pygmy Mice, Benin, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1977–1979. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Becker-Ziaja, B.; Koivogui, L.; Günther, S. Lassa Serology in Natural Populations of Rodents and Horizontal Transmission. Vector Borne Zoonotic Dis. 2014, 14, 665–674. [Google Scholar] [CrossRef]
- Mylne, A.Q.N.; Pigott, D.M.; Longbottom, J.; Shearer, F.; Duda, K.A.; Messina, J.P.; Weiss, D.J.; Moyes, C.L.; Golding, N.; Hay, S.I. Mapping the zoonotic niche of Lassa fever in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 483–492. [Google Scholar] [CrossRef]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- WHO. Lassa Fever Research and Development (R&D) Roadmap; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ishii, A.; Thomas, Y.; Moonga, L.; Nakamura, I.; Ohnuma, A.; Hang’Ombe, B.; Takada, A.; Mweene, A.; Sawa, H. Novel Arenavirus, Zambia. Emerg. Infect. Dis. 2011, 17, 1921–1924. [Google Scholar] [CrossRef]
- Gryseels, S.; Rieger, T.; Oestereich, L.; Cuypers, B.; Borremans, B.; Makundi, R.; Leirs, H.; Günther, S.; De Bellocq, J.G. Gairo virus, a novel arenavirus of the widespread Mastomys natalensis: Genetically divergent, but ecologically similar to Lassa and Morogoro viruses. Virology 2015, 476, 249–256. [Google Scholar] [CrossRef]
- Wulff, H.; McIntosh, B.M.; Hamner, D.B.; Johnson, K.M. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull. World Health Organ. 1977, 55, 441–444. [Google Scholar]
- Günther, S.; Hoofd, G.; Charrel, R.; Röser, C.; Becker-Ziaja, B.; Lloyd, G.; Sabuni, C.; Verhagen, R.; Van Der Groen, G.; Kennis, J.; et al. Mopeia Virus–related Arenavirus in Natal Multimammate Mice, Morogoro, Tanzania. Emerg. Infect. Dis. 2009, 15, 2008–2012. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; McCormick, J.B.; Saluzzo, J.F.; Herve, J.P.; Georges, A.J.; Johnson, K.M. An arenavirus isolated from wild-caught rodents (Pramys species) in the Central African Republic. Intervirology 1983, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, S.; Baird, S.J.E.; Borremans, B.; Makundi, R.; Leirs, H.; De Bellocq, J.G. When Viruses Don’t Go Viral: The Importance of Host Phylogeographic Structure in the Spatial Spread of Arenaviruses. PLoS Pathog. 2017, 13, e1006073. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Lecompte, E.; Koivogui, L.; Soropogui, B.; Doré, A.; Kourouma, F.; Sylla, O.; Daffis, S.; Koulémou, K.; Ter Meulen, J. Fluctuation of Abundance and Lassa Virus Prevalence in Mastomys natalensis in Guinea, West Africa. Vector Borne Zoonotic Dis. 2007, 7, 119–128. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Lecompte, E.; Koivogui, L.; Daffis, S.; Meulen, J.T. Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector Borne Zoonotic Dis. 2008, 8, 41–48. [Google Scholar] [CrossRef]
- Walker, D.H.; Wulff, H.; Lange, J.V.; Murphy, F.A. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull. World Health Organ. 1975, 52, 523–534. [Google Scholar]
- Borremans, B.; Vossen, R.; Becker-Ziaja, B.; Gryseels, S.; Hughes, N.; Van Gestel, M.; Van Houtte, N.; Günther, S.; Leirs, H. Shedding dynamics of Morogoro virus, an African arenavirus closely related to Lassa virus, in its natural reservoir host Mastomys natalensis. Sci. Rep. 2015, 5, 10445. [Google Scholar] [CrossRef] [PubMed]
- Rieger, T.; Merkler, D.; Günther, S. Infection of Type I Interferon Receptor-Deficient Mice with Various Old World Arenaviruses: A Model for Studying Virulence and Host Species Barriers. PLoS ONE 2013, 8, e72290. [Google Scholar] [CrossRef] [PubMed]
- Nikisins, S.; Rieger, T.; Patel, P.; Müller, R.; Günther, S.; Niedrig, M. International External Quality Assessment Study for Molecular Detection of Lassa Virus. PLoS Negl. Trop. Dis. 2015, 9, e0003793. [Google Scholar] [CrossRef]
- Gunther, S. Imported Lassa Fever in Germany: Molecular Characterization of a New Lassa Virus Strain. Emerg. Infect. Dis. 2000, 6, 466–476. [Google Scholar] [CrossRef]
- Hufert, F.T.; Schmitz, H. Epitope mapping of the Lassa virus nucleoprotein using monoclonal anti-nucleocapsid antibodies. Arch. Virol. 1989, 106, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Vitullo, A.D.; Hodara, V.L.; Merani, M.S. Effect of Persistent Infection with Junin Virus on Growth and Reproduction of its Natural Reservoir, Calomys musculinus. Am. J. Trop. Med. Hyg. 1987, 37, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Fulhorst, C.F.; Ksiazek, T.G.; Peters, C.J.; Tesh, R.B. Experimental Infection of the Cane MouseZygodontomys brevicauda(Family Muridae) with Guanarito Virus (Arenaviridae), the Etiologic Agent of Venezuelan Hemorrhagic Fever. J. Infect. Dis. 1999, 180, 966–969. [Google Scholar] [CrossRef]
- Mariën, J.; Borremans, B.; Verhaeren, C.; Kirkpatrick, L.; Gryseels, S.; De Bellocq, J.G.; Günther, S.; Sabuni, C.A.; Massawe, A.W.; Reijniers, J.; et al. Density dependence and persistence of Morogoro arenavirus transmission in a fluctuating population of its reservoir host. J. Anim. Ecol. 2019, 89, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Bonwitt, J.; Sáez, A.M.; Lamin, J.; Ansumana, R.; Dawson, M.; Buanie, J.; Lamin, J.; Sondufu, D.; Borchert, M.; Sahr, F.; et al. At Home with Mastomys and Rattus: Human-Rodent Interactions and Potential for Primary Transmission of Lassa Virus in Domestic Spaces. Am. J. Trop. Med. Hyg. 2017, 96, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.L.; Boisen, M.L.; Nelson, D.K.S.; Bush, D.J.; Cross, R.W.; Koval, A.P.; Hoffmann, A.R.; Beddingfield, B.J.; Hastie, K.M.; Rowland, M.M.; et al. Antibodies from Sierra Leonean and Nigerian Lassa fever survivors cross-react with recombinant proteins representing Lassa viruses of divergent lineages. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, P.B.; Frame, J.D.; Rhoderick, J.B.; Monson, M.H. Endemic lassa fever in liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 380–384. [Google Scholar] [CrossRef]
- Baize, S.; Marianneau, P.; Loth, P.; Reynard, S.; Journeaux, A.; Chevallier, M.; Tordo, N.; Deubel, V.; Contamin, H. Early and Strong Immune Responses Are Associated with Control of Viral Replication and Recovery in Lassa Virus-Infected Cynomolgus Monkeys. J. Virol. 2009, 83, 5890–5903. [Google Scholar] [CrossRef]
- Baillet, N.; Reynard, S.; Perthame, E.; Hortion, J.; Journeaux, A.; Mateo, M.; Carnec, X.; Schaeffer, J.; Picard, C.; Barrot, L.; et al. Systemic viral spreading and defective host responses are associated with fatal Lassa fever in macaques. Commun. Biol. 2021, 4, 1–19. [Google Scholar] [CrossRef]
- Frame, J.D.; Verbrugge, G.P.; Gill, R.; Pinneo, L. The use of Lassa fever convalescent plasma in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 319–324. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Peters, C.J. Passive antibody therapy of Lassa fever in cynomolgus monkeys: Importance of neutralizing antibody and Lassa virus strain. Infect. Immun. 1984, 44, 528–533. [Google Scholar] [CrossRef]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef]
- Oldstone, M.B.A.; Dixon, F.J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. J. Exp. Med. 1969, 129, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Traub, E. Epidemiology of lymphocytic choriomeningitis in a mouse stock observed for four years. J. Exp. Med. 1939, 69, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.; Aoki, T.; Dixon, F.J. The antibody response of mice to murine leukemia virus in spontaneous infection: Absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc. Natl. Acad. Sci. USA 1972, 69, 134–138. [Google Scholar] [PubMed]
- Hotchin, J.; Weigand, H. Studies of lymphocytic choriomeningitis in mice. I. The relationship between age at inoculation and outcome of infection. J. Immunol. 1961, 86, 392–400. [Google Scholar] [PubMed]
- Zajac, A.J.; Blattman, J.N.; Murali-Krishna, K.; Sourdive, D.J.; Suresh, M.; Altman, J.D.; Ahmed, R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998, 188, 2205–2213. [Google Scholar] [CrossRef]
- Mariën, J.; Borremans, B.; Gryseels, S.; Soropogui, B.; De Bruyn, L.; Bongo, G.N.; Becker-Ziaja, B.; De Bellocq, J.G.; Guenther, S.; Magassouba, N.; et al. No measurable adverse effects of Lassa, Morogoro and Gairo arenaviruses on their rodent reservoir host in natural conditions. Parasites Vectors 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Ter Meulen, J.; Badusche, M.; Kuhnt, K.; Doetze, A.; Satoguina, J.; Marti, T.; Loeliger, C.; Koulemou, K.; Koivogui, L.; Schmitz, H.; et al. Characterization of human CD4(+) T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J. Virol. 2000, 74, 2186–2192. [Google Scholar] [CrossRef]
- Port, J.R.; Wozniak, D.M.; Oestereich, L.; Pallasch, E.; Becker-Ziaja, B.; Müller, J.; Rottstegge, M.; Olal, C.; Gómez-Medina, S.; Oyakhliome, J.; et al. Severe human Lassa fever is characterized by non-specific T-cell activation and lymphocyte homing to inflamed tissues. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
| Primer/Probe 1 | Sequence (5′ → 3′) |
|---|---|
| Nikisins F2 MORV | AAT CAA TTT GTG AAT GTG CCA |
| Nikisins R MORV | GCT CAG GTT TCA TAT AGT TTA GAC CA |
| Nikisins TM MORV | /56-FAM/AAG TGG GGC/ZEN/CCA ATG ATG TCC CCA TT/3′ IB®FQ/ |
| Nikisins F2 MOBV | AAC CAA CTT ATG GAT ATG CCA |
| Nikisins R MOBV | TGG GCC TTC TAT CTT ATA GCC TGG ACC A |
| Nikisins TM MOBV | /56-FAM/AAT GGG GGC/ZEN/CTA TGA TGA CCC CCT T/3′ IB®FQ/ |
| Blood | Urine | Organs | |||
|---|---|---|---|---|---|
| Group | Sampling Period (Weeks Post-Birth) | PCR Positive Samples/Tested Samples 1 per Sampling Period | Ab Positive Samples/Tested Samples 1 per Sampling Period | PCR Positive Samples/Tested Samples 1 per Sampling Period | Virus Positive Animals/Tested Animals 2 per Sampling Period |
| MORV inoculation day 2 (n = 5) | 1 | 1/1 | 0/1 | 1/1 | 1/1 |
| 2 | 1/1 | 1/1 | n.t. | 1/1 | |
| 4–6 | 9/9 | 9/9 | 3/3 | n.t. | |
| 7–9 | 8/8 | 7/8 | 1/1 | n.t. | |
| 10–12 | 8/8 | 8/8 | n.t. | n.t. | |
| 13–15 | 8/8 | 8/8 | n.t. | n.t. | |
| 20–22 | 3/3 | 3/3 | n.t. | 3/3 | |
| MORV inoculation day 6 (n = 15) | 2 | 4/4 | 0/4 | n.t. | 4/4 |
| 3 | 4/4 | 4/4 | n.t. | 4/4 | |
| 4 | 4/4 | 4/4 | n.t. | 3/4 | |
| 5 | 3/3 | 3/3 | n.t. | 2/3 | |
| MORV inoculation day 14 (n = 10) | 3 | 4/4 | 1/4 | n.t. | 4/4 |
| 4 | 2/3 | 3/3 | n.t. | 0/3 | |
| 5 | 2/3 | 3/3 | n.t. | n.t. | |
| MORV inoculation day 27 (n = 10) | 5 | 0/2 | 1/2 | n.t. | 0/2 |
| 6 | 0/2 | 1/2 | n.t. | n.t. | |
| 7 | 0/2 | 2/2 | n.t. | n.t. | |
| 8 | 0/2 | 2/2 | n.t. | 0/2 | |
| 9 | 1/2 | 2/2 | n.t. | n.t. | |
| MORV exposure to persistently infected inviduals from birth (n = 37) | 0–1 | 6/10 | 8/10 | 4/9 | 5/12 |
| 2–3 | 10/14 | 14/14 | 6/8 | 6/8 | |
| 4–7 | 26/32 | 31/32 | 2/4 | 0/4 | |
| 8–10 | 14/20 | 20/20 | 2/3 | 4/5 | |
| 16–20 | 2/6 | 6/6 | n.t. | 2/5 | |
| MORV exposure to persistently infected inviduals from day 25 (n = 9) | 4–5 | 7/9 | 2/9 | n.t. | n.t. |
| 6–7 | 5/18 | 18/18 | 0/3 | n.t. | |
| 8 | 0/9 | 9/9 | 0/7 | 0/3 | |
| MORV exposure of adults to infected individuals (n = 14) | 0–1 | 0/10 | 3/10 | n.t. | n.t. |
| 2–4 | 3/15 | 9/15 | n.t. | 0/1 | |
| 5–9 | 1/8 | 8/8 | n.t. | 0/2 | |
| 11–13 | 0/2 | 2/2 | n.t. | 0/2 | |
| MOBV inoculation day 2 (n = 4) | 1 | 1/1 | 0/1 | 1/1 | 0/1 |
| 2 | 1/1 | 1/1 | 1/1 | 1/1 | |
| 4–7 | 0/6 | 6/6 | 0/4 | n.t. | |
| 8–10 | 0/6 | 6/6 | 0/2 | 0/2 | |
| MOBV exposure to infected individuals from birth (n = 12) | 1–4 | 0/12 | 0/8 | n.t. | 0/4 |
| 5–7 | 0/8 | 0/8 | n.t. | n.t. | |
| MOBV exposure of adults to infected individuals (n = 2) | 1–2 | 0/3 | 0/3 | n.t. | n.t. |
| 5–10 | 0/3 | 0/3 | n.t. | n.t. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, C.; Wurr, S.; Pallasch, E.; Bockholt, S.; Rieger, T.; Günther, S.; Oestereich, L. Experimental Morogoro Virus Infection in Its Natural Host, Mastomys natalensis. Viruses 2021, 13, 851. https://doi.org/10.3390/v13050851
Hoffmann C, Wurr S, Pallasch E, Bockholt S, Rieger T, Günther S, Oestereich L. Experimental Morogoro Virus Infection in Its Natural Host, Mastomys natalensis. Viruses. 2021; 13(5):851. https://doi.org/10.3390/v13050851
Chicago/Turabian StyleHoffmann, Chris, Stephanie Wurr, Elisa Pallasch, Sabrina Bockholt, Toni Rieger, Stephan Günther, and Lisa Oestereich. 2021. "Experimental Morogoro Virus Infection in Its Natural Host, Mastomys natalensis" Viruses 13, no. 5: 851. https://doi.org/10.3390/v13050851
APA StyleHoffmann, C., Wurr, S., Pallasch, E., Bockholt, S., Rieger, T., Günther, S., & Oestereich, L. (2021). Experimental Morogoro Virus Infection in Its Natural Host, Mastomys natalensis. Viruses, 13(5), 851. https://doi.org/10.3390/v13050851






