Global Discrepancies between Numbers of Available SARS-CoV-2 Genomes and Human Development Indexes at Country Scales
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, Y.; McCauley, J. GISAID: Global initiative on sharing all influenza data—from vision to reality. Euro Surveill 2017, 22, 30494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef]
- Levasseur, A.; Delerce, J.; Caputo, A.; Brechard, L.; Colson, P.; Lagier, J.C.; Fournier, P.E.; Raoult, D. Genomic diversity and evolution of coronavirus (SARS-CoV-2) in France from 309 COVID-19-infected patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Colson, P.; Levasseur, A.; Delerce, J.; Chaudet, H.; Bossi, V.; Ben Khedher, M.; Fournier, P.E.; Lagier, J.C.; Raoult, D. Dramatic increase in the SARS-CoV-2 mutation rate and low mortality rate during the second epidemic in summer in Marseille. IHU Pre-Prints 2020. [Google Scholar] [CrossRef]
- Colson, P.; Levasseur, A.; Gautret, P.; Fenollar, F.; Thuan, H.V.; Delerce, J.; Bitam, I.; Saile, R.; Maaloum, M.; Padane, A.; et al. Introduction into the Marseille geographical area of a mild SARS-CoV-2 variant originating from sub-Saharan Africa: An investigational study. Travel Med. Infect. Dis. 2021, 40, 101980. [Google Scholar] [CrossRef]
- Fournier, P.E.; Colson, P.; Levasseur, A.; Gautret, P.; Luciani, L.; Bedotto, M.; Delerce, J.; Brechard, L.; Lagier, J.C.; Chaudet, H.; et al. Genome sequence analysis enabled deciphering the atypical evolution of COVID-19 in Marseille, France. medRxiv 2021. [Google Scholar] [CrossRef]
- Fournier, P.E.; Colson, P.; Levasseur, A.; Devaux, C.; Gautret, P.; Bedotto, M. Emergence and outcome of the SARS-CoV-2 "Marseille-4" variant. Int. J. Infect. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Hodcroft, E.B.; Zuber, M.; Nadeau, S.; Crawford, K.H.D.; Bloom, J.D.; Veesler, D.; Vaughan, T.G.; Comas, I.Ã.; Candelas, F.G.l.; Stadler, T.; et al. Emergence and spread of a SARS-CoV-2 variant through Europe in the summer of 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- Leung, K.; Shum, M.H.; Leung, G.M.; Lam, T.T.; Wu, J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill 2021, 26, 2002106. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Faria, N.R.; Claro, I.M.; Candido, D.; Moyses Franco, L.A.; Andrade, P.S.; Coletti, T.M.; Silva, C.A.M.; Sales, F.C.; Manuli, E.R.; Aguiar, R.S.; et al. Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in Manaus: Preliminary Findings. Virological.org, 2021. Available online: https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586 (accessed on 11 April 2021).
- Ecdc. Risk of Spread of New SARS-CoV-2 Variants of Concern in the EU/EEA—First Update. Ecdc Risk Assessment 21 A.D. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-variants-concern-eueea-first-update#no-link (accessed on 11 April 2021).
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Kemp, S.A.; Collier, D.A.; Datir, R.; Ferreira, I.; Gayed, S.; Jahun, A.; Hosmillo, M.; Rees-Spear, C.; Mlcochova, P.; Lumb, I.U.; et al. Neutralising antibodies in Spike mediated SARS-CoV-2 adaptation. medRxiv 2020. [Google Scholar] [CrossRef]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Cell Host Microbe 2021, 29, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; VanBlargan, L.A.; Bloyet, L.M.; Rothlauf, P.W.; Chen, R.E.; Stumpf, S.; Zhao, H.; Errico, J.M.; Theel, E.S.; Liebeskind, M.J.; et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 2021, 29, 477–488. [Google Scholar] [CrossRef]
- Ho, D.; Wang, P.; Nair, M.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef]
- Hensley, M.K.; Bain, W.G.; Jacobs, J.; Nambulli, S.; Parikh, U.; Cillo, A.; Staines, B.; Heaps, A.; Sobolewski, M.D.; Rennick, L.J.; et al. Intractable COVID-19 and Prolonged SARS-CoV-2 Replication in a CAR-T-cell Therapy Recipient: A Case Study. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Khatamzas, E.; Rehn, A.; Muenchhoff, M.; Hellmuth, J.; Gaitzsch, E.; Weiglein, T.; Georgi, E.; Scherer, C.; Stecher, S.; Weigert, O.; et al. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv 2021. [Google Scholar] [CrossRef]
- Hueso, T.; Pouderoux, C.; Péré, H.; Beaumont, A.L.; Raillon, L.A.; Ader, F.; Chatenoud, L.; Eshagh, D.; Szwebel, T.A.; Martinot, M.; et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020, 136, 2290–2295. [Google Scholar] [CrossRef]
- To, K.K.; Hung, I.F.; Ip, J.D.; Chu, A.W.; Chan, W.M.; Tam, A.R.; Fong, C.H.; Yuan, S.; Tsoi, H.W.; Ng, A.C.; et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Colson, P.; Finaud, M.; Levy, N.; Lagier, J.C.; Raoult, D. Evidence of SARS-CoV-2 re-infection with a different genotype. J. Infect. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Cohen, J.I.; Burbelo, P.D. Reinfection with SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis 2020. online ahead of print. [Google Scholar] [CrossRef]
- Brouqui, P.; Colson, P.; Melenotte, C.; Houhamdi, L.; Bedotto, M.; Devaux, C.; Gautret, P.; Million, M.; Parola, P.; Stoupan, D.; et al. COVID re-infection. Eur. J. Clin. Invest. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Bruel, T.; Grzelak, L.; Guivel-Benhassine, F.; Staropoli, I.; Porrot, F.; Planchais, C.; Buchrieser, J.; Rajah, M.M.; Bishop, E.; et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Yu, J.; Cerutti, G.; Nair, M.S.; Huang, Y.; Kwong, P.D.; Shapiro, L.; Ho, D.D. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Furuse, Y. Genomic sequencing effort for SARS-CoV-2 by country during the pandemic. Int. J. Infect. Dis. 2020, 103, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Dia, N.; Lakh, N.A.; Diagne, M.M.; Mbaye, K.D.; Taieb, F.; Fall, N.M.; Barry, M.A.; Ka, D.; Fall, A.; Diallo, V.M.P.C.; et al. COVID-19 Outbreak, Senegal, 2020. Emerg. Infect. Dis. 2020, 26, 2772–2774. [Google Scholar] [CrossRef] [PubMed]
- Ze, A.N.; Easa Amer, K.; Hafez, M.M.; Hassan, Z.K.; Ahmed, O.S.; Soliman, H.K.; Bahnasy, A.A.; Abdel Hamid, W.; Gad, A.; Ali, M.; et al. Genomic characterization of SARS-CoV-2 in Egypt. J. Adv. Res. 2020. epub ahead of print. [Google Scholar] [CrossRef]
- Happi, C.; Omilabu, S.; Salako, B.L.; Abayomi, A.; Oluniyi, P.E. Virological. 2020. Available online: https://virological.org/t/first-african-sars-cov-2-genome-sequence-from-nigerian-covid-19-case/421 (accessed on 11 April 2021).
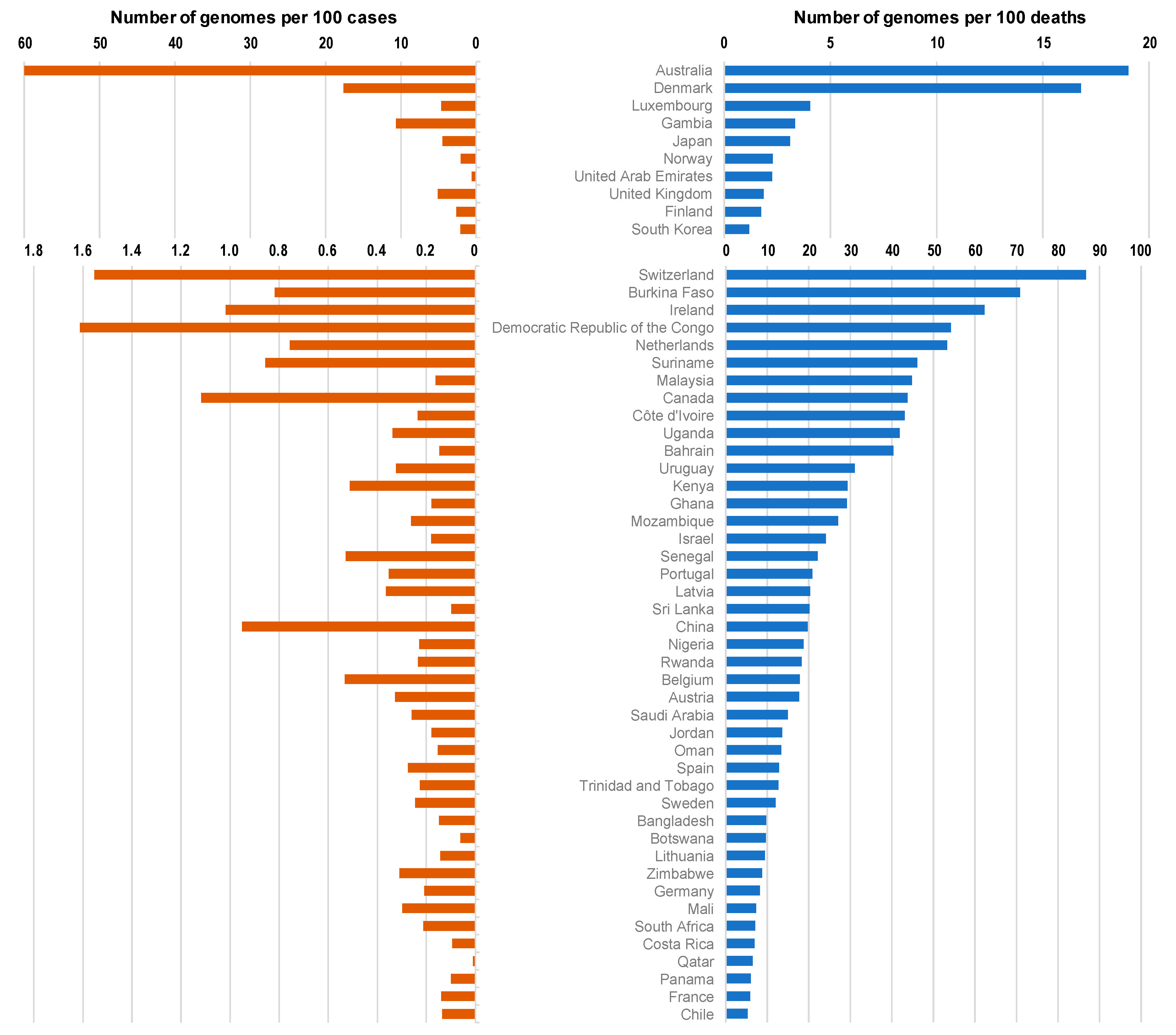
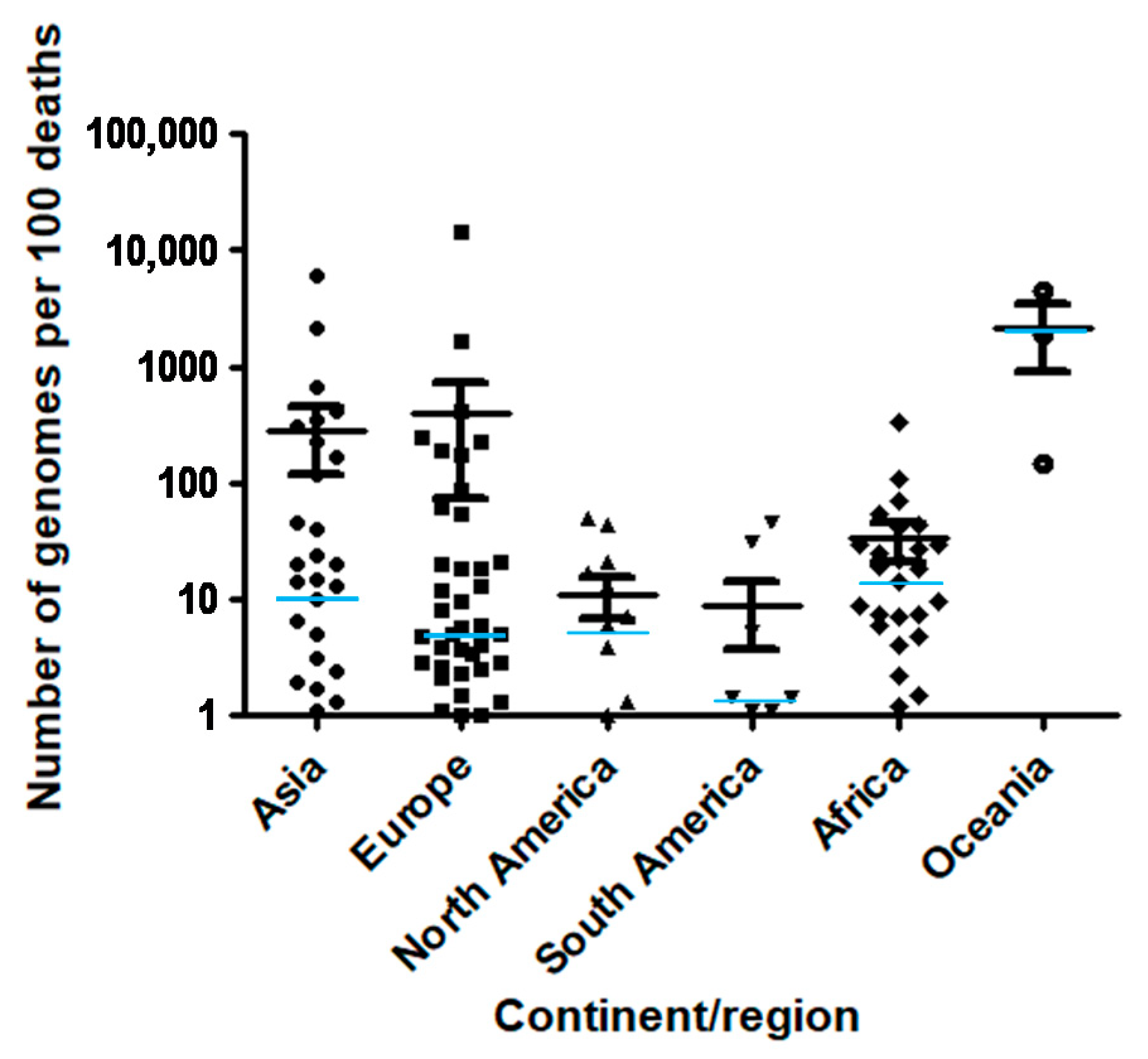
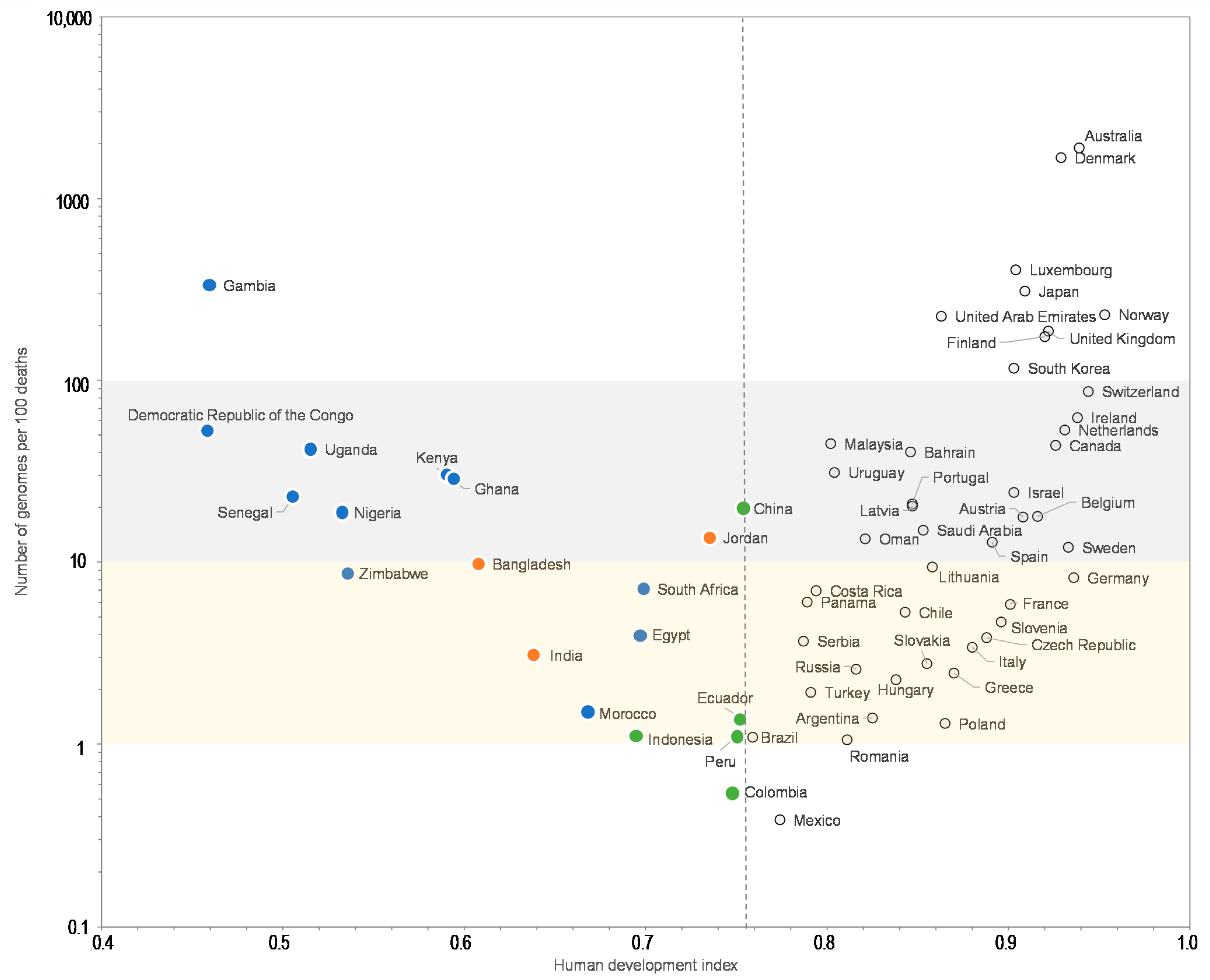

| Country | Continent/Region | Number of Genomes | Number of Genomes per 100 Cases | Number of Genomes per 100 Deaths | Number of Cases | Number of Deaths | Population | GDP per Capita | Human Development Index |
|---|---|---|---|---|---|---|---|---|---|
| Iceland | Europe | 4175 | 69.6 | 14,397 | 6001 | 29 | 341,25 | 46,483 | 0.935 |
| Singapore | Asia | 1731 | 2.9 | 5969 | 59,425 | 29 | 5,850,343 | 85,535 | 0.932 |
| New Zealand | Oceania | 1095 | 47.5 | 4380 | 2305 | 25 | 4,822,233 | 36,086 | 0.917 |
| Taiwan | Asia | 150 | 16.8 | 2143 | 895 | 7 | 23,816,775 | - | - |
| Australia | Oceania | 17,29 | 60.0 | 1902 | 28,799 | 909 | 25,499,881 | 44,649 | 0.939 |
| Denmark | Europe | 34,819 | 17.6 | 1680 | 197,892 | 2072 | 5,792,203 | 46,683 | 0.929 |
| Thailand | Asia | 496 | 3.1 | 653 | 16,221 | 76 | 69,799,978 | 16,278 | 0.755 |
| Vietnam | Asia | 142 | 8.6 | 406 | 1651 | 35 | 97,338,583 | 6172 | 0.694 |
| Luxembourg | Europe | 2325 | 4.6 | 405 | 50,228 | 574 | 625,976 | 94,278 | 0.904 |
| Mongolia | Asia | 7 | 0.4 | 350 | 1,71 | 2 | 3,278,292 | 11,841 | 0.741 |
| Gambia | Africa | 427 | 10.6 | 334 | 4019 | 128 | 2,416,664 | 1562 | 0.460 |
| Japan | Asia | 17,052 | 4.5 | 310 | 380,644 | 5503 | 126,476,458 | 39,002 | 0.909 |
| IHU Méditerranée Infection * | Europe | 1585 | 5.2 | 250 | 30,237 | 633 | - | - | - |
| Norway | Europe | 1278 | 2.1 | 229 | 62,276 | 557 | 5,421,242 | 64,8 | 0.953 |
| United Arab Emirates | Asia | 1845 | 0.6 | 225 | 293,052 | 819 | 9,890,400 | 67,293 | 0.863 |
| United Kingdom | Europe | 192,556 | 5.1 | 186 | 3,754,448 | 103,324 | 67,886,004 | 39,753 | 0.922 |
| Finland | Europe | 1154 | 2.6 | 174 | 44,039 | 664 | 5,540,718 | 40,586 | 0.920 |
| Brunei | Asia | 5 | 2.8 | 167 | 180 | 3 | 437,483 | 71,809 | 0.853 |
| Papua New Guinea | Oceania | 13 | 1.5 | 144 | 851 | 9 | 8947,027 | 3823 | 0.544 |
| South Korea | Asia | 1631 | 2.1 | 117 | 77,395 | 1399 | 51,269,183 | 35,938 | 0.903 |
| Equatorial Guinea | Africa | 95 | 1.7 | 110 | 5492 | 86 | 1,402,985 | 22,605 | 0.591 |
| Switzerland | Europe | 8071 | 1.6 | 87 | 519,404 | 9308 | 8,654,618 | 57,41 | 0.944 |
| Burkina Faso | Africa | 85 | 0.8 | 71 | 10,377 | 120 | 20,903,278 | 1703 | 0.423 |
| Ireland | Europe | 1973 | 1.0 | 62 | 193,645 | 3167 | 4,937,796 | 67,335 | 0.938 |
| Democratic Republic of Congo | Africa | 360 | 1.6 | 54 | 22,322 | 665 | 89,561,404 | 808 | 0.457 |
| Netherlands | Europe | 7422 | 0.8 | 53 | 979,702 | 13,925 | 17,134,873 | 48,473 | 0.931 |
| Saint Vincent and the Grenadines | North America | 1 | 0.1 | 50 | 827 | 2 | 110,947 | 10,727 | 0.723 |
| Surinam | South America | 71 | 0.9 | 46 | 8293 | 154 | 586,634 | 13,767 | 0.720 |
| Malaysia | Asia | 321 | 0.2 | 45 | 198,208 | 717 | 32,365,998 | 26,808 | 0.802 |
| Canada | North America | 8613 | 1.1 | 44 | 770,433 | 19,659 | 37,742,157 | 44,018 | 0.926 |
| Côte d’Ivoire | Africa | 65 | 0.2 | 43 | 27,694 | 151 | 26,378,275 | 3601 | 0.492 |
| Uganda | Africa | 133 | 0.3 | 42 | 39,424 | 318 | 45,741,000 | 1698 | 0.516 |
| Bahrain | Asia | 150 | 0.1 | 40 | 101,503 | 372 | 1,701,583 | 43,291 | 0.846 |
| Uruguay | South America | 129 | 0.3 | 31 | 39,887 | 415 | 3,473,727 | 20,551 | 0.804 |
| Kenya | Africa | 514 | 0.5 | 29 | 100,422 | 1753 | 53,771,300 | 2993 | 0.590 |
| Ghana | Africa | 114 | 0.2 | 29 | 63,883 | 390 | 31,072,945 | 4228 | 0.592 |
| Mozambique | Africa | 94 | 0.3 | 27 | 35,833 | 347 | 31,255,435 | 1136 | 0.437 |
| Benin | Africa | 12 | 0.3 | 25 | 3786 | 48 | 12,123,198 | 2064 | 0.515 |
| Israel | Asia | 1128 | 0.2 | 24 | 628,895 | 4669 | 8,655,541 | 33,132 | 0.903 |
| Senegal | Africa | 136 | 0.5 | 22 | 25,711 | 614 | 16,743,930 | 2471 | 0.505 |
| Portugal | Europe | 2422 | 0.4 | 21 | 685,383 | 11,608 | 10,196,707 | 27,937 | 0.847 |
| USA | North America | 89,814 | 0.3 | 21 | 25,766,681 | 433,196 | 331,002,647 | 54,225 | 0.924 |
| Latvia | Europe | 233 | 0.4 | 20 | 63,992 | 1148 | 1,886,202 | 25,064 | 0.847 |
| Sri Lanka | Asia | 60 | 0.1 | 20 | 61,586 | 297 | 21,413,250 | 11,669 | 0.770 |
| China | Asia | 949 | 1.0 | 20 | 99,746 | 4813 | 1,439,323,774 | 15,309 | 0.752 |
| Nigeria | Africa | 290 | 0.2 | 19 | 127,024 | 1547 | 206,139,587 | 5338 | 0.532 |
| Rwanda | Africa | 34 | 0.2 | 18 | 14,529 | 186 | 12,952,209 | 1854 | 0.524 |
| Belgium | Europe | 3743 | 0.5 | 18 | 702437 | 20,982 | 11,589,616 | 42,659 | 0.916 |
| Austria | Europe | 1344 | 0.3 | 18 | 410,23 | 7607 | 9,006,400 | 45,437 | 0.908 |
| Antigua and Barbuda | North America | 1 | 0.5 | 17 | 215 | 6 | 97,928 | 21,491 | 0.780 |
| Saudi Arabia | Asia | 953 | 0.3 | 15 | 367,276 | 6366 | 34,813,867 | 49,045 | 0.853 |
| Sierra Leone | Africa | 11 | 0.3 | 14 | 3,282 | 77 | 7,976,985 | 1390 | 0.419 |
| Jordan | Asia | 581 | 0.2 | 14 | 324,169 | 4269 | 10,203,140 | 8337 | 0.735 |
| Oman | Asia | 205 | 0.2 | 13 | 133,728 | 1527 | 5,106,622 | 37,961 | 0.821 |
| Spain | Europe | 7431 | 0.3 | 13 | 2,705,001 | 57,806 | 46,754,783 | 34,272 | 0.891 |
| Trinidad and Tobago | North America | 17 | 0.2 | 13 | 7,52 | 134 | 1,399,491 | 28,763 | 0.784 |
| Sweden | Europe | 1388 | 0.2 | 12 | 564,557 | 11,52 | 10,099,270 | 46,949 | 0.933 |
| Bangladesh | Asia | 792 | 0.1 | 9.8 | 533,953 | 8087 | 164,689,383 | 3524 | 0.608 |
| Botswana | Africa | 13 | 0.1 | 9.7 | 21,293 | 134 | 2,351,625 | 15,807 | 0.717 |
| Lithuania | Europe | 258 | 0.1 | 9.4 | 180,16 | 2749 | 2,722,291 | 29,524 | 0.858 |
| Zimbabwe | Africa | 101 | 0.3 | 8.7 | 32,646 | 1160 | 14,862,927 | 1900 | 0.535 |
| Germany | Europe | 4582 | 0.2 | 8.2 | 2,194,562 | 55,883 | 83,783,945 | 45,229 | 0.936 |
| Mali | Africa | 24 | 0.3 | 7.3 | 8056 | 328 | 20,250,834 | 2014 | 0.427 |
| Guinea | Africa | 6 | 0.0 | 7.3 | 14,435 | 82 | 13,132,792 | 1999 | 0.459 |
| South Africa | Africa | 3062 | 0.2 | 7.1 | 1,437,798 | 43,105 | 59,308,690 | 12,295 | 0.699 |
| Costa Rica | North America | 181 | 0.1 | 7.0 | 192,637 | 2599 | 5,094,114 | 15,525 | 0.794 |
| Qatar | Asia | 16 | 0.0 | 6.5 | 150,28 | 248 | 2,881,060 | 116,936 | 0.856 |
| Panama | North America | 314 | 0.1 | 6.0 | 316,808 | 5196 | 4,314,768 | 22,267 | 0.789 |
| Gabon | Africa | 4 | 0.0 | 5.9 | 10,536 | 68 | 2,225,728 | 16,562 | 0.702 |
| France | Europe | 4379 | 0.1 | 5.9 | 3,166,145 | 74,601 | 65,273,512 | 38,606 | 0.901 |
| Liechtenstein | Europe | 3 | 0.1 | 5.8 | 2475 | 52 | 38,137 | - | 0.916 |
| Chile | South America | 966 | 0.1 | 5.3 | 714,143 | 18,174 | 19,116,209 | 22,767 | 0.843 |
| Malta | Europe | 13 | 0.1 | 5.0 | 17,4 | 261 | 441,539 | 36,513 | 0.878 |
| Estonia | Europe | 20 | 0.0 | 4.9 | 42,656 | 406 | 1,326,539 | 29,481 | 0.871 |
| Palestine | Asia | 88 | 0.1 | 4.9 | 157,593 | 1812 | 5,101,416 | 4450 | 0.686 |
| Cameroon | Africa | 22 | 0.1 | 4.8 | 29,617 | 462 | 26,545,864 | 3365 | 0.556 |
| Slovenia | Europe | 162 | 0.1 | 4.7 | 163,235 | 3448 | 2,078,932 | 31,401 | 0.896 |
| Cyprus | Europe | 8 | 0.0 | 4.1 | 30,538 | 197 | 875,899 | 32,415 | 0.869 |
| Egypt | Africa | 366 | 0.2 | 4.0 | 164,282 | 9169 | 102,334,403 | 10,55 | 0.696 |
| Czech Republic | Europe | 614 | 0.1 | 3.9 | 964,66 | 15,944 | 10,708,982 | 32606 | 0.888 |
| Jamaica | North America | 13 | 0.1 | 3.8 | 15,435 | 344 | 2 961,161 | 8194 | 0.732 |
| France minus IHU Méditerranée Infection * | Europe | 2794 | 0.1 | 3.8 | 3,135,908 | 73,968 | - | - | - |
| Serbia | Europe | 146 | 0.0 | 3.7 | 390,637 | 3965 | 6,804,596 | 14,049 | 0.787 |
| Italy | Europe | 2974 | 0.1 | 3.4 | 2,515,507 | 87,381 | 60,461,828 | 35,22 | 0.880 |
| India | Asia | 4778 | 0.0 | 3.1 | 10,720,048 | 154,01 | 1,380,004,385 | 6427 | 0.640 |
| North Macedonia | Europe | 82 | 0.1 | 2.9 | 91,891 | 2831 | 2,083,380 | 13,111 | 0.757 |
| Slovakia | Europe | 122 | 0.1 | 2.8 | 243,427 | 4411 | 5,459,643 | 30,155 | 0.855 |
| Russia | Europe | 1820 | 0.0 | 2.6 | 3,752,548 | 70,533 | 145,934,460 | 24,766 | 0.816 |
| Greece | Europe | 141 | 0.1 | 2.5 | 154,796 | 5742 | 10,423,056 | 24,574 | 0.870 |
| Kuwait | Asia | 23 | 0.0 | 2.4 | 163,45 | 958 | 4,270,563 | 65,531 | 0.803 |
| Hungary | Europe | 278 | 0.1 | 2.3 | 363,45 | 12,291 | 9,660,350 | 26,778 | 0.838 |
| Madagascar | Africa | 6 | 0.0 | 2.2 | 18,743 | 279 | 27,691,019 | 1416 | 0.519 |
| Belarus | Europe | 35 | 0.0 | 2.1 | 242,851 | 1688 | 9,449,321 | 17,168 | 0.808 |
| Turkey | Asia | 493 | 0.0 | 1.9 | 2,457,118 | 25,605 | 84,339,067 | 25,129 | 0.791 |
| Kazakhstan | Asia | 53 | 0.0 | 1.7 | 231,716 | 3040 | 18,776,707 | 24,056 | 0.800 |
| Montenegro | Europe | 12 | 0.0 | 1.5 | 60,288 | 790 | 628,062 | 16,409 | 0.814 |
| Morocco | Africa | 122 | 0.0 | 1.5 | 469,139 | 8224 | 36,910,558 | 7485 | 0.667 |
| Ecuador | South America | 208 | 0.1 | 1.4 | 246 | 14,766 | 17,643,060 | 10,582 | 0.752 |
| Argentina | South America | 662 | 0.0 | 1.4 | 1,905,524 | 47,601 | 45,195,777 | 18,934 | 0.825 |
| Belize | North America | 4 | 0.0 | 1.3 | 11,845 | 298 | 397,621 | 7824 | 0.708 |
| Myanmar | Asia | 41 | 0.0 | 1.3 | 139,152 | 3103 | 54,409,794 | 5592 | 0.578 |
| Poland | Europe | 473 | 0.0 | 1.3 | 1,496,665 | 36,443 | 37,846,605 | 27,216 | 0.865 |
| Tunisia | Africa | 78 | 0.0 | 1.2 | 204,351 | 6508 | 11,818,618 | 10,849 | 0.735 |
| Peru | South America | 441 | 0.0 | 1.1 | 1,113,970 | 40,272 | 32,971,846 | 12,237 | 0.750 |
| Brazil | South America | 2414 | 0.0 | 1.1 | 9,058,687 | 221,547 | 212,559,409 | 14,103 | 0.759 |
| Indonesia | Asia | 313 | 0.0 | 1.1 | 1,037,993 | 29,331 | 273,523,621 | 11,189 | 0.694 |
| Romania | Europe | 191 | 0.0 | 1.1 | 721,513 | 18,105 | 19,237,682 | 23,313 | 0.811 |
| Croatia | Europe | 50 | 0.0 | 1.0 | 230,978 | 4943 | 4,105,268 | 22,67 | 0.831 |
| Andorra | Europe | 1 | 0.0 | 1.0 | 9779 | 100 | 77,265 | - | 0.858 |
| Cuba | North America | 2 | 0.0 | 1.0 | 24,105 | 208 | 11,326,616 | - | 0.777 |
| Lebanon | Asia | 23 | 0.0 | 0.9 | 293,157 | 2621 | 6,825,442 | 13,368 | 0.757 |
| Georgia | Asia | 26 | 0.0 | 0.8 | 256,287 | 3127 | 3,989,175 | 9745 | 0.780 |
| Kosovo | Europe | 12 | 0.0 | 0.8 | 58,988 | 1479 | 1,932,774 | 9796 | - |
| Nepal | Asia | 15 | 0.0 | 0.7 | 270,588 | 2020 | 29,136,808 | 2443 | 0.574 |
| Bosnia and Herzegovina | Europe | 33 | 0.0 | 0.7 | 121,194 | 4659 | 3,280,815 | 11,714 | 0.768 |
| Algeria | Africa | 18 | 0.0 | 0.6 | 106,61 | 2881 | 43,851,043 | 13,914 | 0.754 |
| Guatemala | North America | 32 | 0.0 | 0.6 | 157,595 | 5543 | 17,915,567 | 7424 | 0.650 |
| Colombia | South America | 290 | 0.0 | 0.5 | 2,067,575 | 52,913 | 50,882,884 | 13,255 | 0.747 |
| Pakistan | Asia | 58 | 0.0 | 0.5 | 541,031 | 11,56 | 220,892,331 | 5035 | 0.562 |
| Mexico | North America | 598 | 0.0 | 0.4 | 1,825,519 | 155,145 | 128,932,753 | 17,336 | 0.774 |
| El Salvador | North America | 6 | 0.0 | 0.4 | 53,989 | 1599 | 6,486,201 | 7292 | 0.674 |
| Philippines | Asia | 38 | 0.0 | 0.4 | 519,575 | 10,552 | 109,581,085 | 7599 | 0.699 |
| Dominican Republic | North America | 8 | 0.0 | 0.3 | 208,61 | 2603 | 10,847,904 | 14,601 | 0.736 |
| Ukraine | Europe | 67 | 0.0 | 0.3 | 1,247,674 | 23,469 | 43,733,759 | 7894 | 0.751 |
| Zambia | Africa | 2 | 0.0 | 0.3 | 50,319 | 705 | 18,383,956 | 3689 | 0.588 |
| Bolivia | South America | 27 | 0.0 | 0.3 | 210,726 | 10,226 | 11,673,029 | 6886 | 0.693 |
| Moldova | Europe | 9 | 0.0 | 0.3 | 158,309 | 3413 | 4,033,963 | 5190 | 0.700 |
| Azerbaijan | Asia | 8 | 0.0 | 0.3 | 229,793 | 3113 | 10,139,175 | 15,847 | 0.757 |
| Venezuela | South America | 3 | 0.0 | 0.3 | 125,364 | 1171 | 28,435,943 | 16,745 | 0.761 |
| Iraq | Asia | 31 | 0.0 | 0.2 | 617,202 | 13,024 | 40,222,503 | 15,664 | 0.685 |
| Bulgaria | Europe | 15 | 0.0 | 0.2 | 217,574 | 8973 | 6,948,445 | 18,563 | 0.813 |
| Armenia | Asia | 3 | 0.0 | 0.1 | 166,669 | 3067 | 2,963,234 | 8788 | 0.755 |
| Albania | Europe | 1 | 0.0 | 0.1 | 75,454 | 1350 | 2,877,800 | 11,803 | 0.785 |
| Iran | Asia | 36 | 0.0 | 0.1 | 1,398,841 | 5, 736 | 83,992,953 | 19,083 | 0.798 |
| Saint Kitts and Nevis | North America | 3 | 8.1 | 0.0 | 37 | - | 53,192 | 24,654 | 0.778 |
| Cambodia | Asia | 4 | 0.9 | 0.0 | 463 | - | 16,718,971 | 3645 | 0.582 |
| Hong Kong | Asia | 344 | 0.0 | 0.0 | - | - | 7,496,988 | 56,055 | 0.933 |
| Slovakia | Europe | 122 | 0.1 | 2.8 | 243,427 | 4411 | 5,459,643 | 30,155 | 0.855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colson, P.; Raoult, D. Global Discrepancies between Numbers of Available SARS-CoV-2 Genomes and Human Development Indexes at Country Scales. Viruses 2021, 13, 775. https://doi.org/10.3390/v13050775
Colson P, Raoult D. Global Discrepancies between Numbers of Available SARS-CoV-2 Genomes and Human Development Indexes at Country Scales. Viruses. 2021; 13(5):775. https://doi.org/10.3390/v13050775
Chicago/Turabian StyleColson, Philippe, and Didier Raoult. 2021. "Global Discrepancies between Numbers of Available SARS-CoV-2 Genomes and Human Development Indexes at Country Scales" Viruses 13, no. 5: 775. https://doi.org/10.3390/v13050775






