Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus
2.2. Chemical Compounds
2.3. DENV Infection and MEL Treatment
2.4. Cell Viability Assay
2.5. Focus-Forming Unit (FFU) Assay
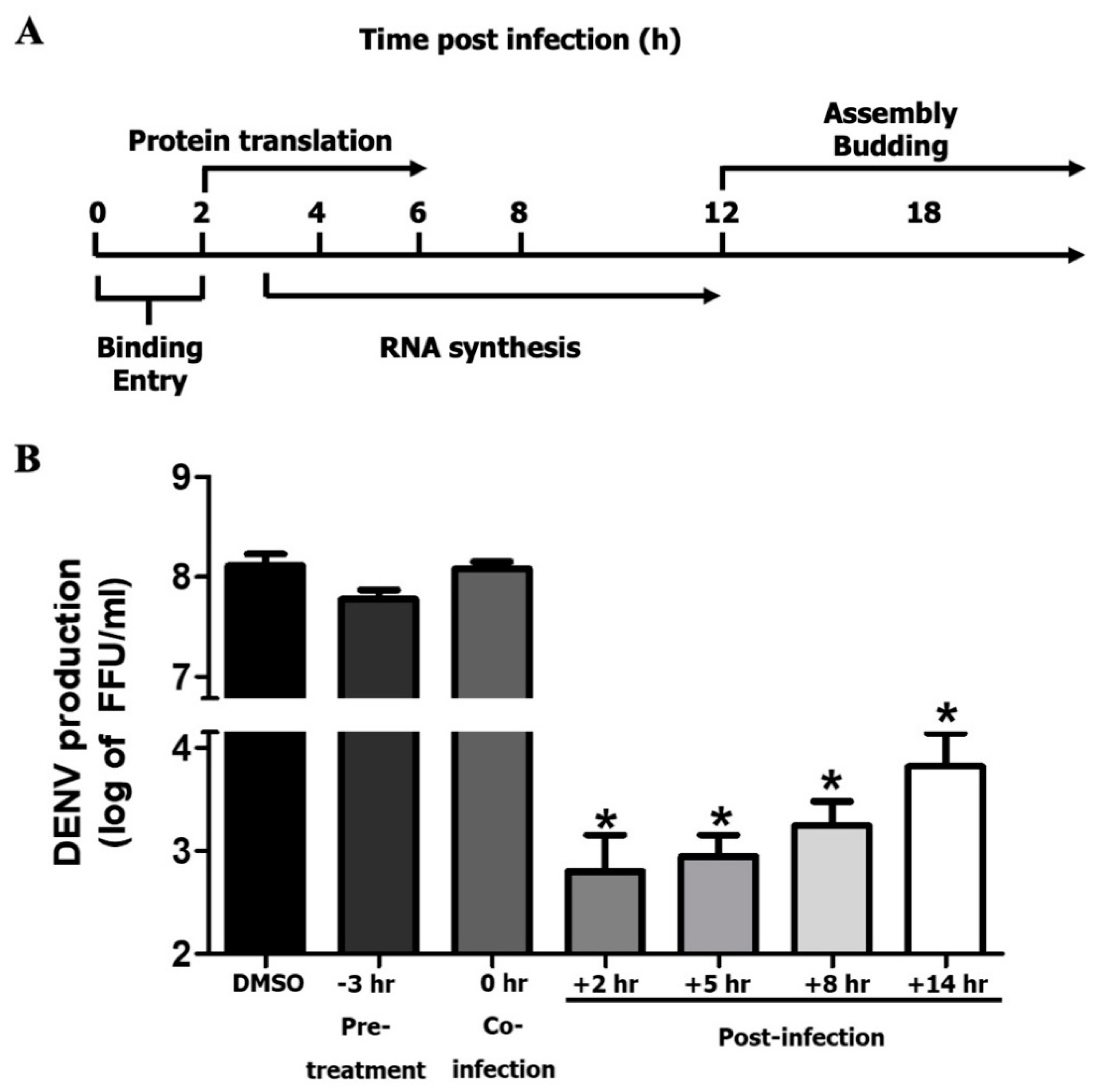
2.6. Time-of-Addition Assay
2.7. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) for mRNA Expression
2.8. Immunofluorescence Assay (IFA) for Detection of Viral Protein
2.9. Western Blot Analysis for SIRT1 Protein Expression
2.10. Statistical Analysis
3. Results
3.1. MEL Inhibits DENV Production
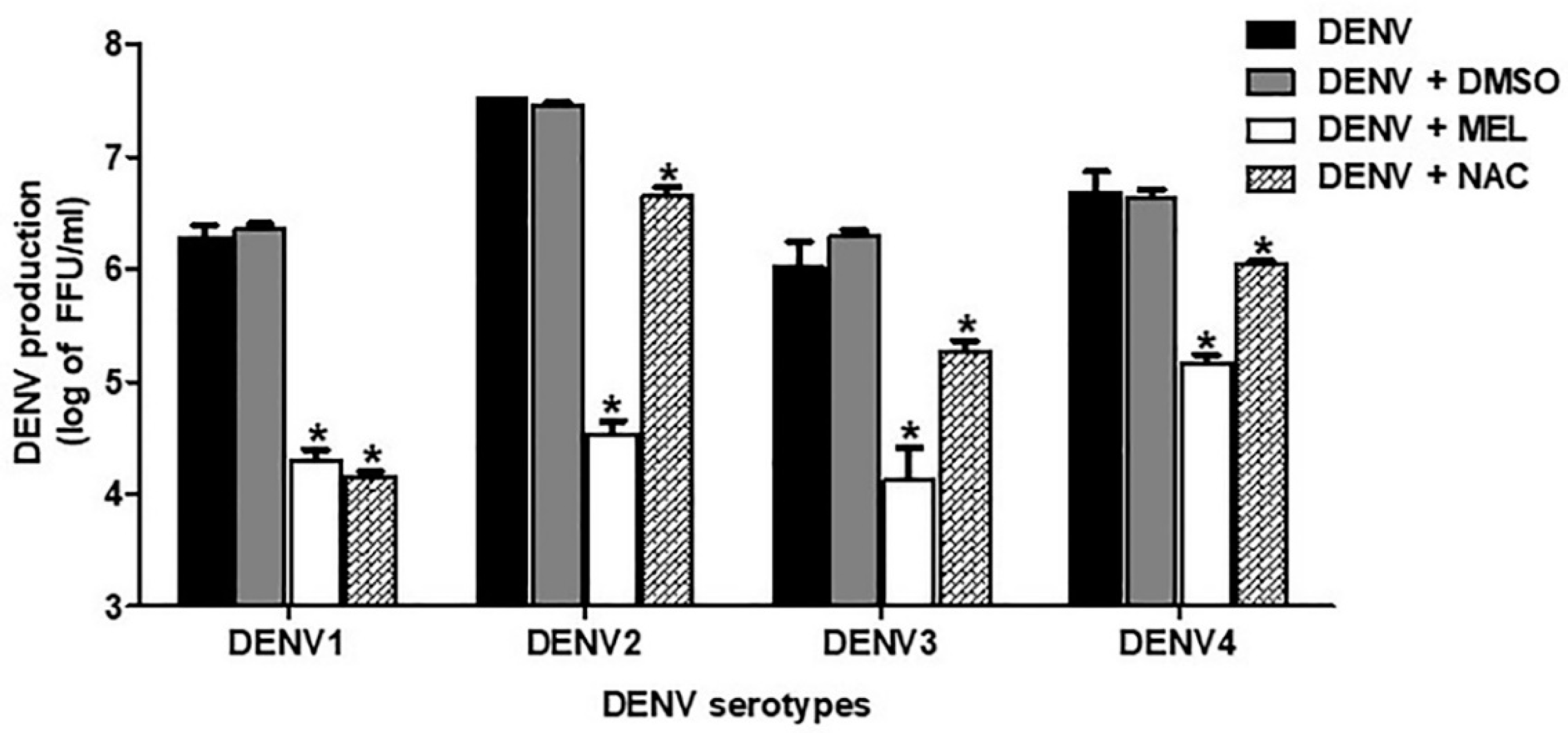
3.2. MEL Exhibits Anti-DENV Activity in All Serotypes
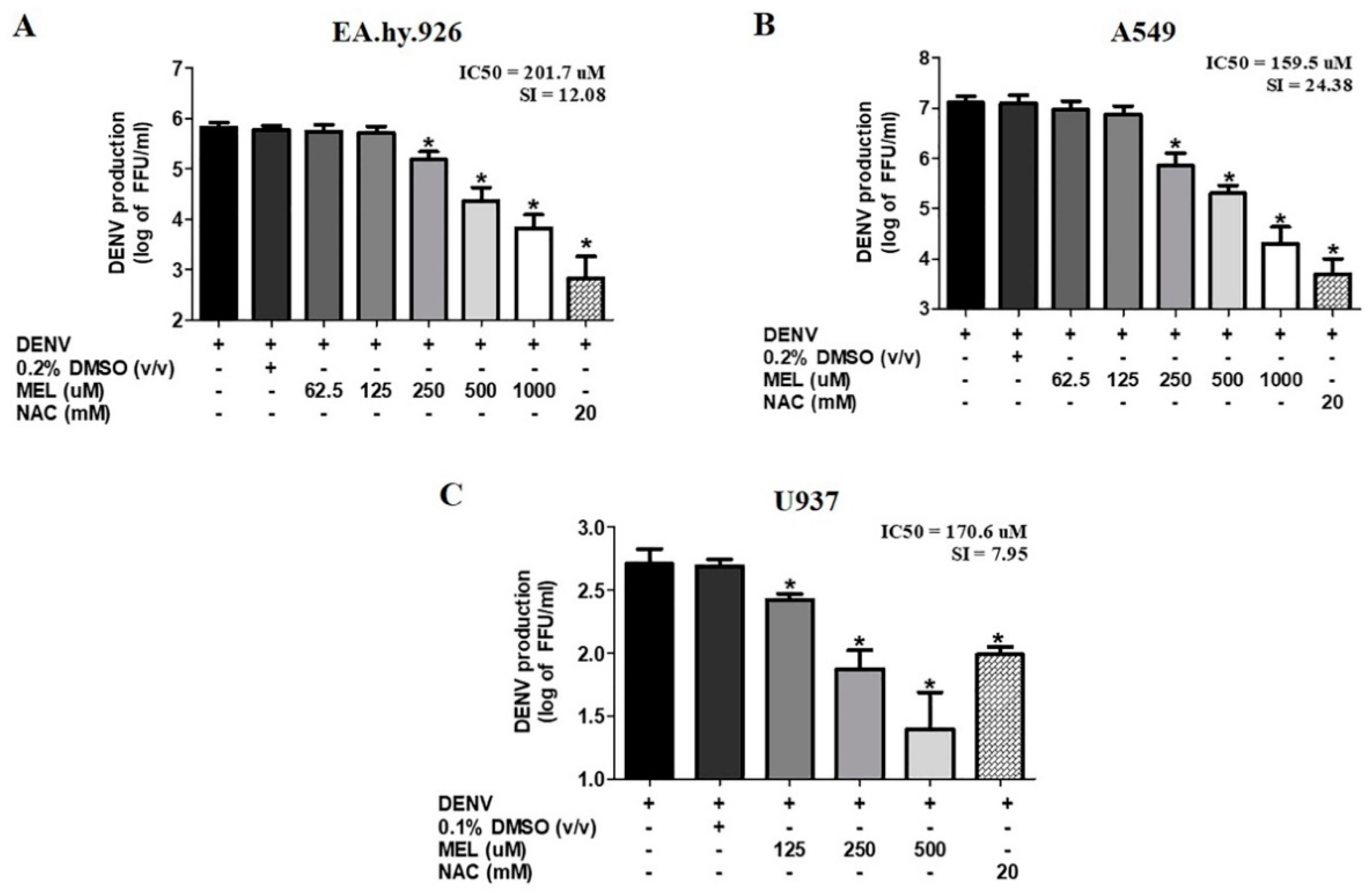
3.3. MEL Exhibits Anti-DENV Activity in Different Cell Lines
3.4. MEL Acts on Post-Entry Step of DENV Infection
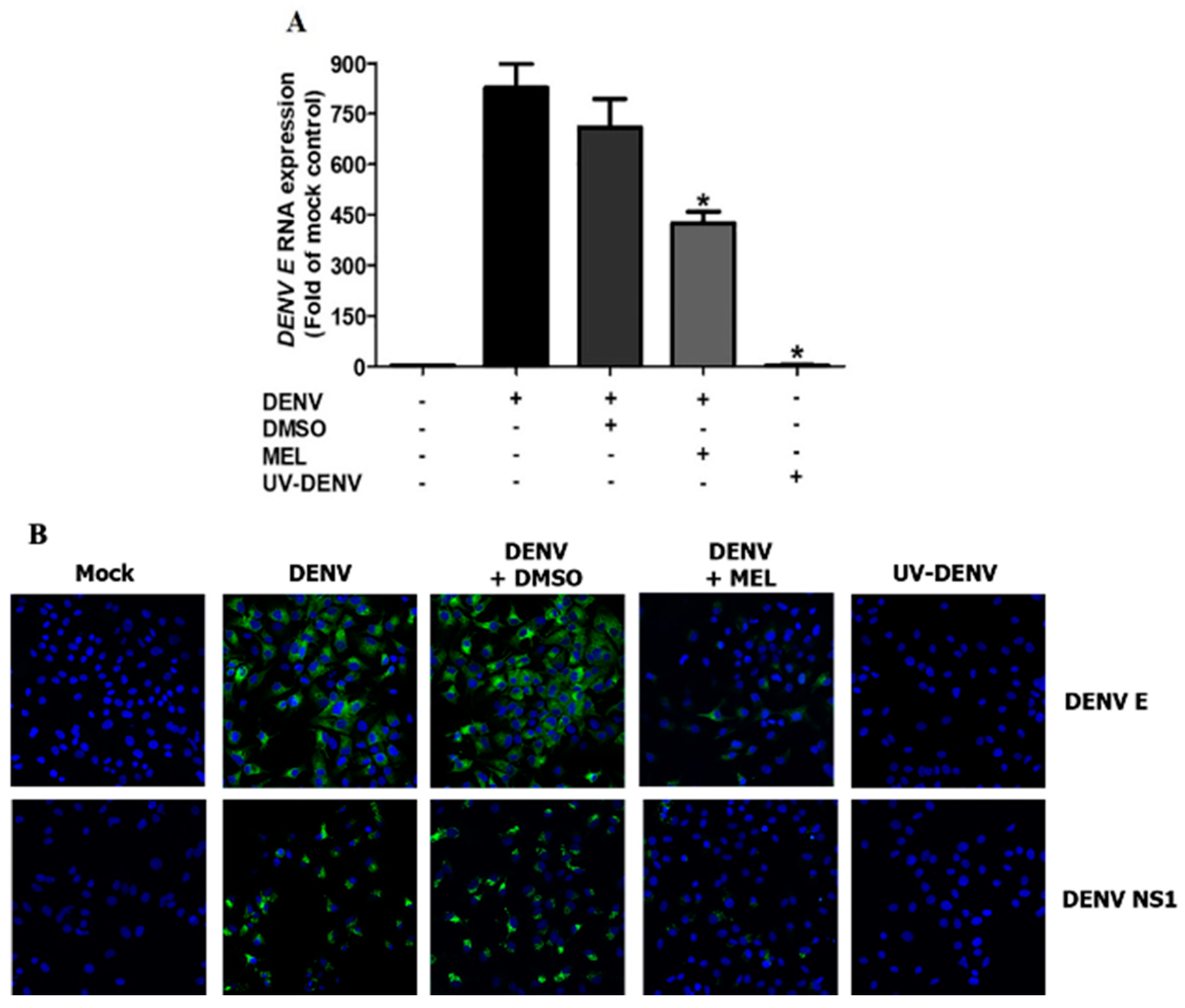
3.5. MEL Interferes with Viral RNA and Protein Expression
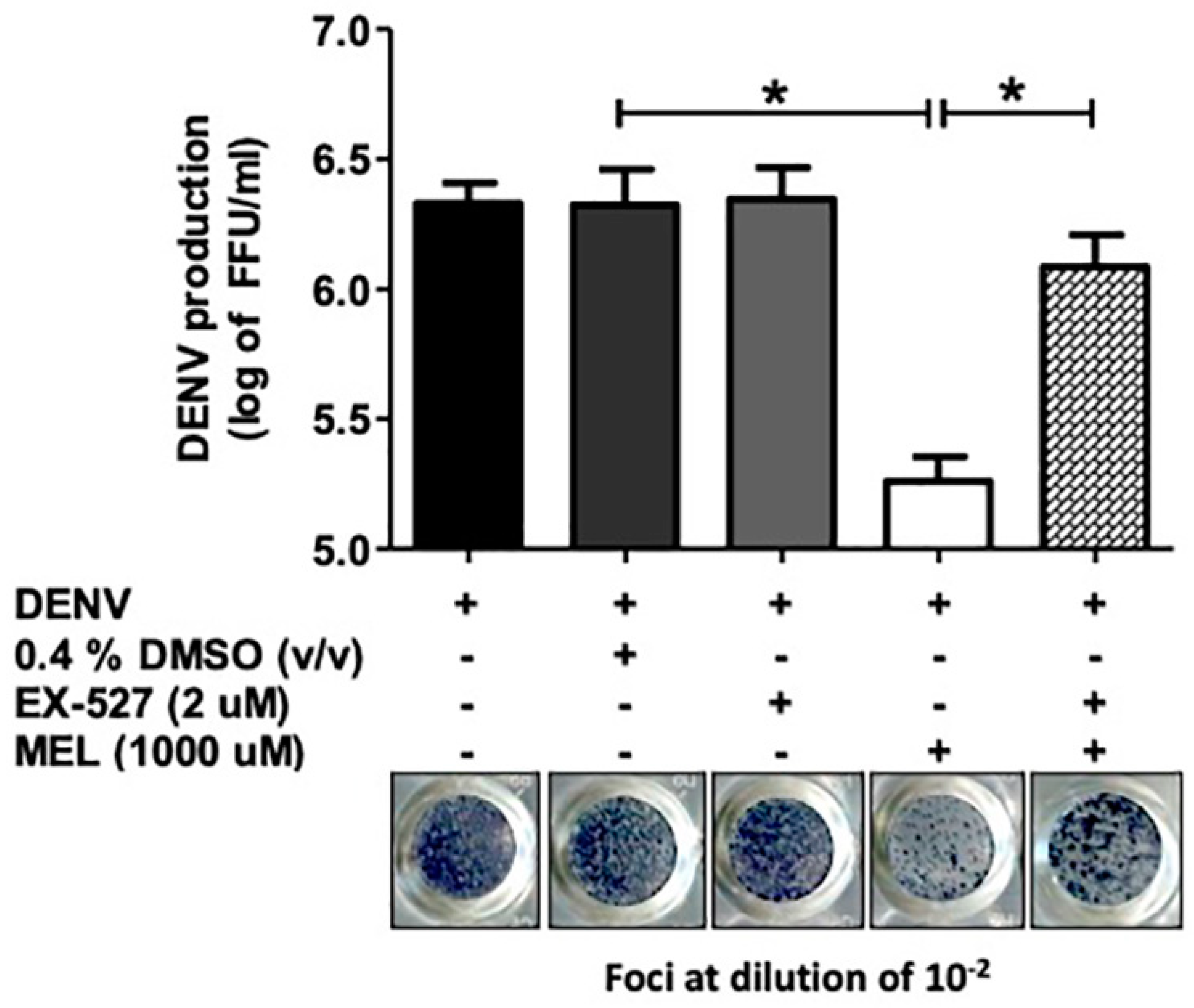
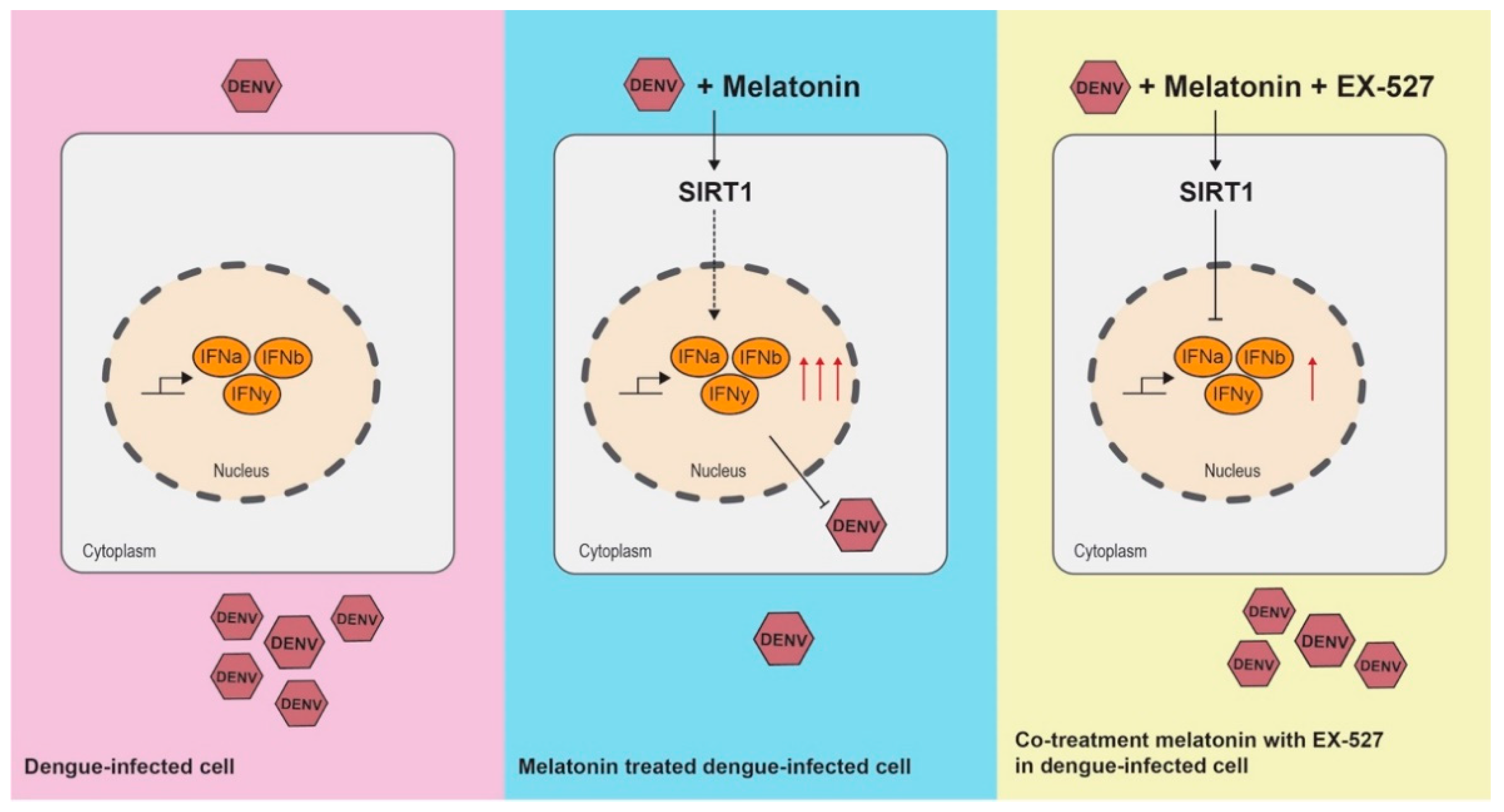
3.6. Inhibition of the SIRT1 Reverses the Anti-DENV Effect of MEL
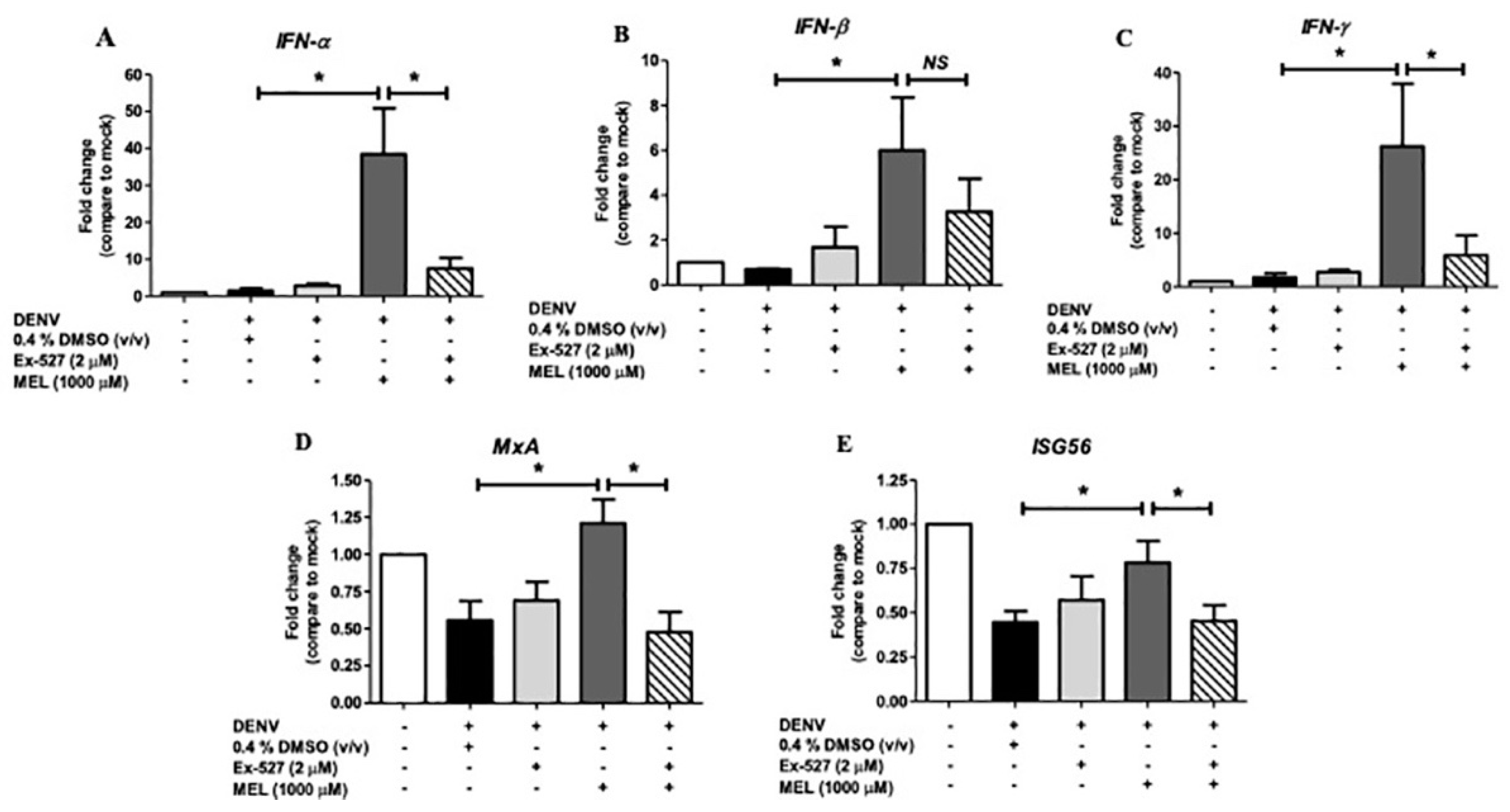
3.7. MEL Enhances Antiviral IFN Response via the SIRT1 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Kuczera, D.; Assolini, J.P.; Tomiotto-Pellissier, F.; Pavanelli, W.R.; Silveira, G.F. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J. Interferon Cytokine Res. 2018, 38, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.G.; Chen, Y.L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.Y.; Shi, P.Y. Strategies for development of Dengue virus inhibitors. Antivir. Res. 2010, 85, 450–462. [Google Scholar] [CrossRef]
- Kaptein, S.J.; Neyts, J. Towards antiviral therapies for treating dengue virus infections. Curr. Opin. Pharmacol. 2016, 30, 1–7. [Google Scholar] [CrossRef]
- Cajochen, C.; Krauchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef]
- Crespo, I.; Miguel, B.S.; Laliena, A.; Alvarez, M.; Culebras, J.M.; Gonzalez-Gallego, J.; Tunon, M.J. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal Res. 2010, 49, 193–200. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Ansari Dezfouli, M.; Zahmatkesh, M.; Farahmandfar, M.; Khodagholi, F. Melatonin protective effect against amyloid beta-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal Sirtuin-1 signaling pathway. Physiol. Behav. 2019, 204, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franco, C.; Stacchiotti, A.; Rodella, L.F.; Rezzani, R. Sirtuin1 Role in the Melatonin Protective Effects Against Obesity-Related Heart Injury. Front. Physiol. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.; Chang, C.P.; Cheng, Y.L.; Wu, Y.W.; Anderson, R.; Wan, S.W.; Chen, C.L.; Ho, T.S.; AbuBakar, S.; Lin, Y.S. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci. Rep. 2017, 7, 42998. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Gentry, M.K.; McCown, J.M.; Brandt, W.E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 1982, 31, 830–836. [Google Scholar] [CrossRef]
- Puttikhunt, C.; Kasinrerk, W.; Srisa-ad, S.; Duangchinda, T.; Silakate, W.; Moonsom, S.; Sittisombut, N.; Malasit, P. Production of anti-dengue NS1 monoclonal antibodies by DNA immunization. J. Virol. Methods 2003, 109, 55–61. [Google Scholar] [CrossRef]
- Manoj, E.M.; Ranasinghe, G.; Ragunathan, M.K. Successful use of N-acetyl cysteine and activated recombinant factor VII in fulminant hepatic failure and massive bleeding secondary to dengue hemorrhagic fever. J. Emerg. Trauma Shock 2014, 7, 313–315. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Panaampon, J.; Suttitheptumrong, A.; Chuncharunee, A.; Bootkunha, J.; Yenchitsomanus, P.T.; Limjindaporn, T. Drug repurposing of N-acetyl cysteine as antiviral against dengue virus infection. Antivir. Res. 2019, 166, 42–55. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Montazeri, M.; Pagheh, A.S.; Sharif, M.; Sarvi, S.; Hosseinzadeh, A.; Reiter, R.J.; Hadighi, R.; Joghataei, M.T.; Ghaznavi, H.; et al. The potential use of melatonin to treat protozoan parasitic infections: A review. Biomed. Pharmacother. 2018, 97, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Javad-Moosavi, S.A.; Reiter, R.J.; Hemati, K.; Ghaznavi, H.; Mehrzadi, S. Idiopathic pulmonary fibrosis (IPF) signaling pathways and protective roles of melatonin. Life Sci. 2018, 201, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Kamrava, S.K.; Moore, B.C.J.; Reiter, R.J.; Ghaznavi, H.; Kamali, M.; Mehrzadi, S. Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr. Drug Targets 2019, 20, 1112–1128. [Google Scholar] [CrossRef]
- Juybari, K.B.; Hosseinzadeh, A.; Ghaznavi, H.; Kamali, M.; Sedaghat, A.; Mehrzadi, S.; Naseripour, M. Melatonin As a Modulator of Degenerative and Regenerative Signaling Pathways in Injured Retinal Ganglion Cells. Curr. Pharm. Des. 2019, 25, 3057–3073. [Google Scholar] [CrossRef]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Harpsoe, N.G.; Fuglsang, H.; Rosenberg, J.; Gogenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Barchas, J.; DaCosta, F.; Spector, S. Acute pharmacology of melatonin. Nature 1967, 214, 919–920. [Google Scholar] [CrossRef]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci 1999, 50, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.R.; Herbinger, K.H.; Froschl, G.; Malta Romano, C.; de Souza Areias Cabidelle, A.; Cerutti Junior, C. Serotype influences on dengue severity: A cross-sectional study on 485 confirmed dengue cases in Vitoria, Brazil. BMC Infect. Dis. 2016, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Paemanee, A.; Hitakarun, A.; Roytrakul, S.; Smith, D.R. Screening of melatonin, alpha-tocopherol, folic acid, acetyl-L-carnitine and resveratrol for anti-dengue 2 virus activity. BMC Res. Notes 2018, 11, 307. [Google Scholar] [CrossRef]
- Tu, Y.; Song, E.; Wang, Z.; Ji, N.; Zhu, L.; Wang, K.; Sun, H.; Zhang, Y.; Zhu, Q.; Liu, X.; et al. Melatonin attenuates oxidative stress and inflammation of Muller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed. Pharmacother. 2021, 137, 111274. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef] [PubMed]
- Napper, A.D.; Hixon, J.; McDonagh, T.; Keavey, K.; Pons, J.F.; Barker, J.; Yau, W.T.; Amouzegh, P.; Flegg, A.; Hamelin, E.; et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J. Med. Chem. 2005, 48, 8045–8054. [Google Scholar] [CrossRef] [PubMed]
- Hackett, B.A.; Dittmar, M.; Segrist, E.; Pittenger, N.; To, J.; Griesman, T.; Gordesky-Gold, B.; Schultz, D.C.; Cherry, S. Sirtuin Inhibitors Are Broadly Antiviral against Arboviruses. MBio 2019, 10, e01446-19. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, D.; An, H.; Zhang, H.; Jiang, C.; Yang, B. Melatonin prevents lung injury induced by hepatic ischemia-reperfusion through anti-inflammatory and anti-apoptosis effects. Int. Immunopharmacol. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Jiang, D.; Weidner, J.M.; Qing, M.; Pan, X.B.; Guo, H.; Xu, C.; Zhang, X.; Birk, A.; Chang, J.; Shi, P.Y.; et al. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J. Virol. 2010, 84, 8332–8341. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Raychoudhuri, A.; Shrivastava, S.; Steele, R.; Kim, H.; Ray, R.; Ray, R.B. ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J. Virol. 2011, 85, 12881–12889. [Google Scholar] [CrossRef]

| Primer | Forward | Reverse |
|---|---|---|
| DENV E | ATCCAGATGTCATCAGGAAAC | CCGGCTCTACTCCTATGATG |
| IFN α | TTTCTCCTGCCTGAAGGACAG | GCTCATGATTTCTGCTCTGAC |
| IFN β | GACGCCGCATTGACCATCTA | TTGGCCTTCAGGTAATGCAGA |
| IFN γ | ACTGACTTGAATGTGCAACGCA | ATCTGACTCCTTTTTCGCTTCC |
| MxA | ACCACAGAGGCTCTCAGCAT | CTCAGCTGGTCCTGGATCTC |
| ISG56 | GGGCAGACTGGCAGAAGC | TATAGCGGAAGGGATTTGAAAGC |
| GAPDH | CGACCACTTTGTCAAGCTCA | AGGGGTCTACATCGCAACTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morchang, A.; Malakar, S.; Poonudom, K.; Noisakran, S.; Yenchitsomanus, P.-t.; Limjindaporn, T. Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway. Viruses 2021, 13, 659. https://doi.org/10.3390/v13040659
Morchang A, Malakar S, Poonudom K, Noisakran S, Yenchitsomanus P-t, Limjindaporn T. Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway. Viruses. 2021; 13(4):659. https://doi.org/10.3390/v13040659
Chicago/Turabian StyleMorchang, Atthapan, Shilu Malakar, Kanchanaphan Poonudom, Sansanee Noisakran, Pa-thai Yenchitsomanus, and Thawornchai Limjindaporn. 2021. "Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway" Viruses 13, no. 4: 659. https://doi.org/10.3390/v13040659
APA StyleMorchang, A., Malakar, S., Poonudom, K., Noisakran, S., Yenchitsomanus, P.-t., & Limjindaporn, T. (2021). Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway. Viruses, 13(4), 659. https://doi.org/10.3390/v13040659






