Neutralizing Antibody Therapeutics for COVID-19
Abstract
1. Introduction
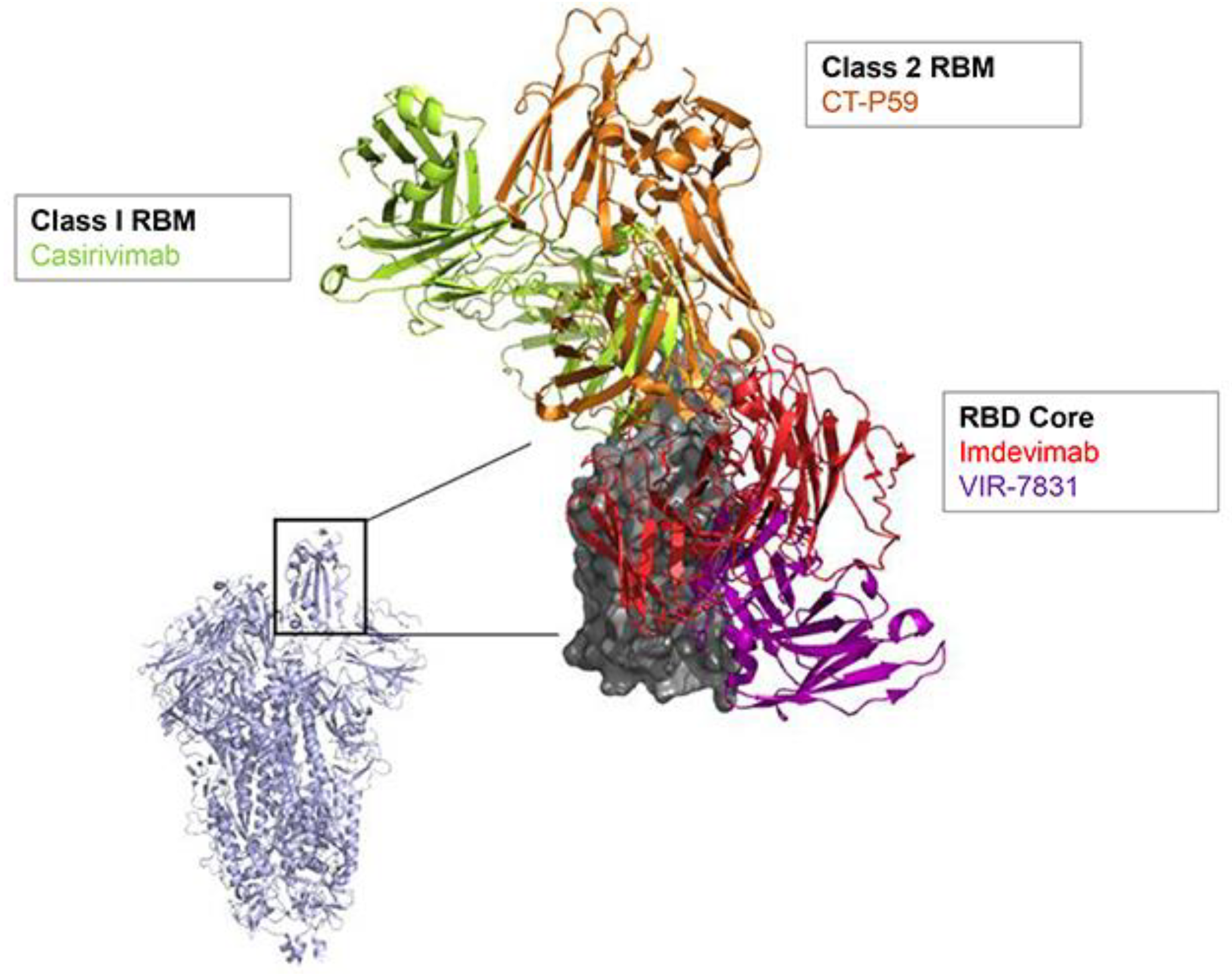
2. SARS-CoV-2 nAb Development
3. Evaluation of SARS-CoV-2 nAbs in the Treatment and Prophylaxis Settings
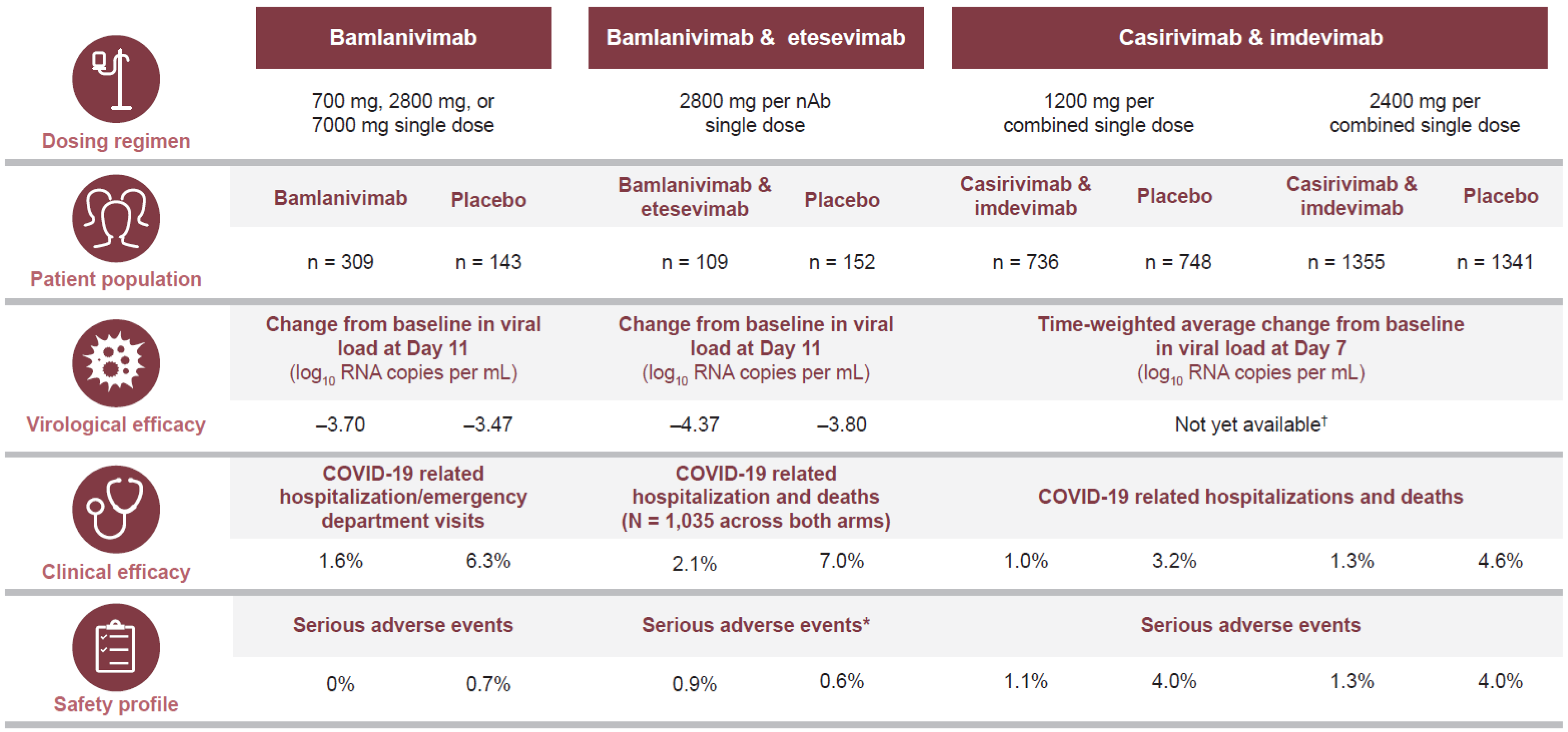
3.1. Efficacy of Treatment for Ambulatory Patients
3.2. Efficacy of Treatment for Hospitalized Patients
3.3. Efficacy of Prophylaxis
4. Susceptibility to Currently Circulating Variants
5. Approaches to Improve nAb Access and Availability
6. The Role of nAbs in Preparing for Future Novel Coronavirus Pandemics
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chotpitayasunondh, T.; Fischer, T.K.; Heraud, J.M.; Hurt, A.C.; Monto, A.S.; Osterhaus, A.; Shu, Y.; Tam, J.S. Influenza and COVID-19: What does co-existence mean? Influenza Other Respir. Viruses 2020. [Google Scholar] [CrossRef] [PubMed]
- Salzberger, B.; Buder, F.; Lampl, B.T.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B.; Hanses, F. Epidemiology of SARS-CoV-2. Infection 2021, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins University. COVID-19 Dashboard. 2020. Available online: https://coronavirus.jhu.edu/map.html (accessed on 17 February 2021).
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas Lopez, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.P.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- Skipper, C.P.; Pastick, K.A.; Engen, N.W.; Bangdiwala, A.S.; Abassi, M.; Lofgren, S.M.; Williams, D.A.; Okafor, E.C.; Pullen, M.F.; Nicol, M.R.; et al. Hydroxychloroquine in Nonhospitalized Adults with Early COVID-19: A Randomized Trial. Ann. Intern. Med. 2020, 173, 623–631. [Google Scholar] [CrossRef]
- Ahmed, S.; Karim, M.M.; Ross, A.G.; Hossain, M.S.; Clemens, J.D.; Sumiya, M.K.; Phru, C.S.; Rahman, M.; Zaman, K.; Somani, J.; et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2020, 103, 214–216. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Brau, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical pipeline. MAbs 2020, 12, 1854149. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H.; et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 460–470. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vazquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, L. COVID-19 antibodies on trial. Nat. Biotechnol. 2020, 38, 1242. [Google Scholar] [CrossRef]
- Renn, A.; Fu, Y.; Hu, X.; Hall, M.D.; Simeonov, A. Fruitful Neutralizing Antibody Pipeline Brings Hope to Defeat SARS-Cov-2. Trends Pharmacol. Sci. 2020, 41, 815–829. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Yu, X.; Wu, M.; Reichert, J.M.; Ho, M. COVID-19 antibody therapeutics tracker: A global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib. Ther. 2020, 3, 205–212. [Google Scholar] [CrossRef]
- Romero, J.R. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: Results from four years of palivizumab usage. Pediatr. Infect. Dis. J. 2003, 22 (Suppl. 2), S46–S54. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Treatment for Ebola Virus. 2020. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-treatment-ebola-virus (accessed on 30 March 2021).
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Regeneron. Regeneron’s Antibody Cocktail REGN-EB3 (inmazeb®) Is First FDA-Approved Treatment for Ebola (Zaire ebolavirus). 2020. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-antibody-cocktail-regn-eb3-inmazebr-first-fda (accessed on 30 March 2021).
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S.; Newall, A.T. Passive immunization for influenza through antibody therapies, a review of the pipeline, challenges and potential applications. Vaccine 2016, 34, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J. Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives. Adv. Biol. 2014, 157895. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2020. [Google Scholar] [CrossRef]
- Xiaojie, S.; Yu, L.; Lei, Y.; Guang, Y.; Min, Q. Neutralizing antibodies targeting SARS-CoV-2 spike protein. Stem Cell Res. 2020, 50, 102125. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.C.; Montgomery, D.; Ito, K.; Woods, R.J. Analysis of the SARS-CoV-2 spike protein glycan shield: Implications for immune recognition. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Song, G.; He, W.T.; Callaghan, S.; Anzanello, F.; Huang, D.; Ricketts, J.; Torres, J.L.; Beutler, N.; Peng, L.; Vargas, S.; et al. Cross-reactive serum and memory B cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Kamat, S. Antibodies at work in the time of severe acute respiratory syndrome coronavirus 2. Cytotherapy 2021, 23, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Asarnow, D.; Lee, W.H.; Huang, C.W.; Faust, B.; Ng, P.M.L.; Ngoh, E.Z.X.; Bohn, M.; Bulkley, D.; Pizzorno, A.; et al. Bivalent binding of a fully human IgG to the SARS-CoV-2 spike proteins reveals mechanisms of potent neutralization. bioRxiv 2020. [Google Scholar] [CrossRef]
- Murphy, A.J.; Macdonald, L.E.; Stevens, S.; Karow, M.; Dore, A.T.; Pobursky, K.; Huang, T.T.; Poueymirou, W.T.; Esau, L.; Meola, M.; et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc. Natl. Acad. Sci. USA 2014, 111, 5153–5158. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Jones, B.E.; Brown-Augsburger, P.L.; Corbett, K.S.; Westendorf, K.; Davies, J.; Cujec, T.P.; Wiethoff, C.M.; Blackbourne, J.L.; Heinz, B.A.; Foster, D.; et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020, 370, 1110–1115. [Google Scholar] [CrossRef]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front. Immunol. 2019, 10, 1296. [Google Scholar] [CrossRef]
- GSK. Vir Biotechnology and GSK Announce Global Expansion to Phase 3 of COMET-ICE Study Evaluating VIR-7831 for the Treatment of COVID-19. 2020. Available online: https://www.gsk.com/en-gb/media/press-releases/vir-biotechnology-and-gsk-announce-global-expansion-to-phase-3-of-comet-ice-study-evaluating-vir-7831-for-the-treatment-of-covid-19/ (accessed on 30 March 2021).
- AstraZeneca. COVID-19 Long-Acting AntiBody (LAAB) Combination AZD7442 Rapidly Advances into Phase III Clinical Trials. 2020. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/covid-19-long-acting-antibody-laab-combination-azd7442-rapidly-advances-into-phase-iii-clinical-trials.html (accessed on 30 March 2021).
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y.; Wang, R.; Jiao, S.; Wang, M.; Huang, W.; Shan, C.; Jiang, W.; Li, Z.; Gu, C.; et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat. Commun. 2020, 11, 5752. [Google Scholar] [CrossRef]
- Antibody Society. COVID-19 Biologics Tracker. 2021. Available online: https://www.antibodysociety.org/covid-19-biologics-tracker/ (accessed on 30 March 2021).
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2020, 21, 229–237. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 30 March 2021).
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Lilly Investors. New Data Show Treatment with Lilly’s Neutralizing Antibodies Bamlanivimab (LY-CoV555) and Etesevimab (LY-CoV016) Together Reduced Risk of COVID-19 Hospitalizations and Death by 70 Percent 2021. Available online: https://investor.lilly.com/news-releases/news-release-details/new-data-show-treatment-lillys-neutralizing-antibodies (accessed on 30 March 2021).
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0 (accessed on 30 March 2021).
- EMA. EMA Issues Advice on Use of Antibody Combination (Bamlanivimab/Etesevimab). 2021. Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-antibody-combination-bamlanivimab-etesevimab (accessed on 30 March 2021).
- Roche. New Phase III Data Shows Investigational Antibody Cocktail Casirivimab and Imdevimab Reduced Hospitalisation or Death by 70% in Non-Hospitalised Patients with COVID-19. 2021. Available online: https://www.roche.com/investors/updates/inv-update-2021-03-23.htm (accessed on 30 March 2021).
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2020, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 30 March 2021).
- EMA. EMA Starts Rolling Review of REGN-COV2 Antibody Combination (Casirivimab/Imdevimab). 2021. Available online: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-regn-cov2-antibody-combination-casirivimab-imdevimab (accessed on 30 March 2021).
- EMA. EMA Issues Advice on Use of REGN-COV2 Antibody Combination (Casirivimab/Imdevimab). 2021. Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab (accessed on 30 March 2021).
- EMA. EMA Starts Rolling Review of Eli Lilly Antibodies Bamlanivimab and Etesevimab for COVID-19. 2021. Available online: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-eli-lilly-antibodies-bamlanivimab-etesevimab-covid-19 (accessed on 30 March 2021).
- EMA. EMA Starts Rolling Review of Celltrion Antibody Regdanvimab for COVID-19. 2021. Available online: https://www.ema.europa.eu/en/news/ema-starts-rolling-review-celltrion-antibody-regdanvimab-covid-19 (accessed on 30 March 2021).
- GSK. Lilly, Vir Biotechnology and GSK Announce First Patient Dosed in Expanded BLAZE-4 Trial Evaluating Bamlanivimab (LY-CoV555) with VIR-7831 (GSK4182136) for COVID-19. 2021. Available online: https://www.gsk.com/en-gb/media/press-releases/lilly-vir-biotechnology-and-gsk-announce-first-patient-dosed-in-expanded-blaze-4-trial/ (accessed on 30 March 2021).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; von Cube, M.; Kalbhenn, J.; Utzolino, S.; Pernice, K.; Bechet, L.; Baur, J.; Lang, C.N.; Wagner, D.; Wolkewitz, M.; et al. COVID-19 in-hospital mortality and mode of death in a dynamic and non-restricted tertiary care model in Germany. PLoS ONE 2020, 15, e0242127. [Google Scholar] [CrossRef]
- Regeneron. REGN-COV2 Independent Data Monitoring Committee Recommends Holding Enrollment in Hospitalized Patients with High Oxygen Requirements and Continuing Enrollment in Patients with Low or No Oxygen Requirements. 2020. Available online: https://investor.regeneron.com/news-releases/news-release-details/regn-cov2-independent-data-monitoring-committee-recommends (accessed on 30 March 2021).
- Regeneron. Regeneron Announces Encouraging Initial Data from COVID-19 Antibody Cocktail Trial in Hospitalized Patients on Low-Flow Oxygen. 2021. Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-announces-encouraging-initial-data-covid-19-antibody (accessed on 30 March 2021).
- Clinical Trials. Lilly’s Monoclonal Antibody Fails in NIH-Sponsored ACTIV-3 Trial. 2020. Available online: https://www.clinicaltrialsarena.com/news/lilly-antibody-nih-trial/ (accessed on 30 March 2021).
- NIH. Investigational COVID-19 Therapeutics to Be Evaluated in Large Clinical Trials. 2020. Available online: https://www.nih.gov/news-events/news-releases/investigational-covid-19-therapeutics-be-evaluated-large-clinical-trials (accessed on 30 March 2021).
- Lilly Investors. Lilly’s Neutralizing Antibody Bamlanivimab (LY-CoV555) Prevented COVID-19 at Nursing Homes in the BLAZE-2 Trial, Reducing Risk by up to 80 Percent for Residents. 2021. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-prevented (accessed on 30 March 2021).
- Regeneron. Regeneron Reports Positive Interim Data with REGEN-COV™ Antibody Cocktail Used as Passive Vaccine to Prevent COVID-19. 2021. Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-reports-positive-interim-data-regen-covtm-antibody (accessed on 30 March 2021).
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Holmes, E.C.; Hurt, A.C.; Dobbie, Z.; Clinch, B.; Oxford, J.S.; Piedra, P.A. Understanding the Impact of Resistance to Influenza Antivirals. Clin. Microbiol. Rev. 2021, 34, e00224-20. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021, 371, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Regeneron. Fact Sheet for Health Care Providers. 2021. Available online: https://www.regeneron.com/sites/default/files/treatment-covid19-eua-fact-sheet-for-hcp.pdf (accessed on 30 March 2021).
- Wang, P.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; Graham, B.S.; et al. Increased Resistance of SARS-CoV-2 Variants, B.1.351 and B.1.1.7 to Antibody Neutralization. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Lambson, B.E.; de Oliveira, T.; Vermeulen, M.; van de Berg, K.; Rossouw, T.; et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wellcome Trust. Expanding Access to Monoclonal Antibody-Based Products. 2020. Available online: https://wellcome.org/sites/default/files/expanding-access-to-monoclonal-antibody-based-products.pdf (accessed on 30 March 2021).
- Lilly Investors. Lilly’s Bamlanivimab (LY-CoV555) Administered with Etesevimab (LY-CoV016) Receives FDA Emergency Use Authorization for COVID-19. 2021. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-bamlanivimab-ly-cov555-administered-etesevimab-ly-cov016 (accessed on 30 March 2021).
- Zhang, H.; Yang, Z.; Xiang, J.; Cui, Z.; Liu, J.; Liu, C. Intranasal administration of SARS-CoV-2 neutralizing human antibody prevents infection in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Limberis, M.P.; Adam, V.S.; Wong, G.; Gren, J.; Kobasa, D.; Ross, T.M.; Kobinger, G.P.; Tretiakova, A.; Wilson, J.M. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci. Transl. Med. 2013, 5, 7ra72. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (accessed on 30 March 2021).
- NIH. Therapeutic Neutralizing Monoclonal Antibodies: Report of a Summit sponsored by Operation Warp Speed and the National Institutes of Health. 2020. Available online: https://www.nih.gov/sites/default/files/research-training/initiatives/activ/20200909-mAb-summit-pub.pdf (accessed on 30 March 2021).
| nAb | Sponsor | Monotherapy or Cocktail | Administration Route | nAb Source | NCT Number | Phase |
|---|---|---|---|---|---|---|
| Ambulatory patients | ||||||
| BGB-DXP593 | BeiGene | Monotherapy | IV infusion | Convalescent plasma | NCT04551898 | Phase 2 |
| MW33 | Mabwell (Shanghai) Bioscience Co., Ltd. | Monotherapy | Unknown | Recombinant | NCT04627584 | Phase 2 |
| Bamlanivimab 1 | AbCellera/Eli Lilly and Company | Monotherapy | IV infusion | Convalescent plasma | NCT04518410 | Phase 2/3 |
| CT-P59 | Celltrion | Monotherapy | IV infusion | Convalescent plasma | NCT04602000 | Phase 2/3 |
| VIR-7831 | Vir Biotechnology, Inc. GlaxoSmithKline | Monotherapy | IV infusion | Convalescent plasma | NCT04545060 | Phase 2/3 |
| Bamlanivimab & etesevimab | AbCellera/Eli Lilly and Company | Cocktail | IV infusion | Convalescent plasma/recombinant | NCT04427501 | Phase 3 |
| Casirivimab & imdevimab 1 | Regeneron/F. Hoffmann-La Roche Ltd. | Cocktail | IV infusion | Convalescent plasma/humanized mice | NCT04425629 | Phase 1/2/3 |
| Hospitalized patients | ||||||
| Casirivimab & imdevimab | Regeneron/F. Hoffmann-La Roche Ltd. | Cocktail | IV infusion | Convalescent plasma/humanized mice | NCT04426695 | Phase 1/2/3 |
| SCTA01 | Sinocelltech Ltd. | Monotherapy | IV infusion | Recombinant | NCT04644185 | Phase 2/3 |
| VIR-7831 | Vir Biotechnology, Inc. GlaxoSmithKline | Monotherapy | IV infusion | Convalescent plasma | NCT04501978 | Phase 3 |
| BRII-196 & BRII-198 | Brii Biosciences | Cocktail | IV infusion | Convalescent plasma | NCT04501978 | Phase 3 |
| TY027 | Tychan Pte. Ltd. | Monotherapy | IV infusion and SC injection | Engineered | NCT04649515 | Phase 3 |
| Bamlanivimab | AbCellera/Eli Lilly and Company | Monotherapy | IV infusion | Convalescent plasma | NCT04501978 | Phase 3 |
| Prophylaxis | ||||||
| AZD7442 (combination of AZD8895 & AZD1061) | AstraZeneca | Cocktail | SC injection | Convalescent plasma | NCT04625725 NCT04625972 | Phase 3 Phase 3 |
| Bamlanivimab & etesevimab | AbCellera/Eli Lilly and Company | Cocktail | IV infusion | Convalescent plasma/recombinant | NCT04497987 | Phase 3 |
| Casirivimab & imdevimab | Regeneron/F. Hoffmann-La Roche Ltd. | Cocktail | SC injection | Convalescent plasma/humanized mice | NCT04452318 | Phase 3 |
| NCT Number | Comparison | Target Recruitment (N) | Primary Efficacy Outcome | Study Population |
|---|---|---|---|---|
| NCT04452318 | Casirivimab & imdevimab vs. placebo (post-exposure prophylaxis) | 2450 | Proportion of participants with RT-PCR-confirmed SARS-CoV-2 | Asymptomatic healthy contacts exposed to a household member with a SARS-CoV-2 infection, with no history of prior SARS-CoV-2 infection |
| NCT04497987 | Bamlanivimab ± etesevimab vs. placebo (post-exposure prophylaxis) | 5000 | Percentage of participants with COVID-19 within 21 days of detection | Nursing home residents or staff with a positive SARS-CoV-2 test and no prior history of SARS-CoV-2 infection or receipt of a SARS-CoV-2-specific vaccine or monoclonal antibodies |
| NCT04625725 | AZD7442 vs. placebo (pre-exposure prophylaxis) | 5000 | Incidence of SARS-CoV-2 RT-PCR positive symptomatic illness | Healthy individuals with no prior history of a positive SARS-CoV-2 diagnosis nor previous receipt of a SARS-CoV-2-specific vaccine or monoclonal antibodies |
| NCT04625972 | AZD7442 vs. placebo (post-exposure prophylaxis) | 1125 | Incidence of SARS-CoV-2 RT-PCR positive symptomatic illness | Healthy contacts with potential exposure to an individual with a SARS-CoV-2 infection, with no prior history of a positive SARS-CoV-2 diagnosis nor previous receipt of a SARS-CoV-2-specific vaccine or monoclonal antibodies |
| nAb | S1 Mutations [77,79] |
|---|---|
| Bamlanivimab | E484K |
| Etesivimab | K417N, A457V, N460T, E484K |
| Casirivimab | E406W, K417E/N, Y453F, L455Y, E484K, F486I/K/V, Y489H, Q493K |
| Imdevimab | 242–244del, E406W, N439K, N440D, K444Q, V445A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurt, A.C.; Wheatley, A.K. Neutralizing Antibody Therapeutics for COVID-19. Viruses 2021, 13, 628. https://doi.org/10.3390/v13040628
Hurt AC, Wheatley AK. Neutralizing Antibody Therapeutics for COVID-19. Viruses. 2021; 13(4):628. https://doi.org/10.3390/v13040628
Chicago/Turabian StyleHurt, Aeron C., and Adam K. Wheatley. 2021. "Neutralizing Antibody Therapeutics for COVID-19" Viruses 13, no. 4: 628. https://doi.org/10.3390/v13040628
APA StyleHurt, A. C., & Wheatley, A. K. (2021). Neutralizing Antibody Therapeutics for COVID-19. Viruses, 13(4), 628. https://doi.org/10.3390/v13040628






