Environmental Surveillance for Polioviruses in Haïti (2017–2019): The Dynamic Process for the Establishment and Monitoring of Sampling Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Review of Estimated Annual National Pol3 Coverage
2.2. Environmental Surveillance Site Selection
2.3. Sample Collection and Frequency
2.4. Environmental Sample Processing
2.5. Two-Phase Separation Method
2.6. Concentration and Filter Elution Filtration Method
2.7. Virus Isolation
3. Results
3.1. Sample Collection and Water Quality Analyses
3.2. Enterovirus Detection
3.2.1. Port Au Prince
3.2.2. Gonaïves
3.2.3. Saint Marc
3.2.4. Cap Haïtien
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassilak, S.; Orenstein, W. Challenges faced by the global polio eradication initiative. Expert Rev. Vaccines 2010, 9, 447–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Global Polio Eradication Initiative, Guidelines on Environmental Surveillance for Detection of Polioviruses. Available online: http://polioeradication.org/wp-content/uploads/2016/07/GPLN_GuidelinesES_April2015.pdf (accessed on 28 December 2020).
- Asghar, H.; Diop, O.M.; Weldegebriel, G.; Malik, F.; Shetty, S.; El Bassioni, L.; Akande, A.O.; Al Maamoun, E.; Zaidi, S.; Adeniji, A.J.; et al. Environmental Surveillance for Polioviruses in the Global Polio Eradication Initiative. J. Infect. Dis. 2014, 210, S294–S303. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, N.; Kew, O.M. From Emergence to Eradication: The Epidemiology of Poliomyelitis Deconstructed. Am. J. Epidemiol. 2010, 172, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Indicators. Available online: https://polioeradication.org/polio-today/polio-now/surveillance-indicators/ (accessed on 28 December 2020).
- Muluh, T.J.; Hamisu, A.W.; Craig, K.; Mkanda, P.; Andrew, E.; Adeniji, J.; Akande, A.; Musa, A.; Ayodeji, I.; Nicksy, G.; et al. Contribution of Environmental Surveillance Toward Interruption of Poliovirus Transmission in Nigeria, 2012–2015. J. Infect. Dis. 2016, 213, S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Hamisu, A.W.; Blake, I.M.; Sume, G.; Braka, F.; Jimoh, A.; Dahiru, H.; Bonos, M.; Dankoli, R.; Bello, A.M.; Yusuf, K.M.; et al. Characterizing Environmental Surveillance Sites in Nigeria and Their Sensitivity to Detect Poliovirus and Other Enteroviruses. J. Infect. Dis. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, K.M.; Verity, R.; Durry, E.; Asghar, H.; Sharif, S.; Zaidi, S.Z.; Wadood, M.Z.M.; Diop, O.M.; Okayasu, H.; Safdar, R.M.; et al. Population sensitivity of acute flaccid paralysis and environmental surveillance for serotype 1 poliovirus in Pakistan: An observational study. BMC Infect. Dis. 2018, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Cowger, T.L.; Burns, C.C.; Sharif, S.; Iber, J.; Henderson, E.; Malik, F.; Zaidi, S.S.Z.; Shaukat, S.; Rehman, L.; Pallansch, M.A.; et al. The role of supplementary environmental surveillance to complement acute flaccid paralysis surveillance for wild poliovirus in Pakistan—2011–2013. PLoS ONE 2017, 12, e0180608. [Google Scholar] [CrossRef] [PubMed]
- Lickness, J.S.; Gardner, T.; Diop, O.M.; Chavan, S.; Jorba, J.; Ahmed, J.; Gumede, N.; Johnson, T.; Butt, O.; Asghar, H.; et al. Surveillance to Track Progress Toward Polio Eradication—Worldwide, 2018–2019. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, C.; Alleman, M.M.; Ehrhardt, D.; Wiesen, E.; Burns, C.C.; Liu, H.; Ewetola, R.; Seakamela, L.; Mdodo, R.; Ndoutabe, M.; et al. Update on Vaccine-Derived Poliovirus Outbreaks—Democratic Republic of the Congo and Horn of Africa, 2017–2018. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Quddus, A.; Sutter, R.; Vertefeuille, J.F.; Wenger, J.; Wassilak, S.G.; et al. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2018–June 2019. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Alleman, M.M.; Jorba, J.; Greene, S.A.; Diop, O.M.; Iber, J.; Tallis, G.; Goel, A.; Wiesen, E.; Wassilak, S.G.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, July 2019–February 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 489–495. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Polio Endgame Strategy, 2019–2023. Available online: Polioeradica-tion.org/wp-content/uploads/2019/06/english-polio-endgame-strategy.pdf (accessed on 26 December 2020).
- Kroiss, S.J.; Ahmadzai, M.; Ahmed, J.; Alam, M.M.; Chabot-Couture, G.; Famulare, M.; Mahamud, A.; McCarthy, K.A.; Mercer, L.D.; Muhammad, S.; et al. Assessing the sensitivity of the polio environmental surveillance system. PLoS ONE 2018, 13, e0208336. [Google Scholar] [CrossRef] [PubMed]
- Blake, I.M.; Pons-Salort, M.; Molodecky, N.A.; Diop, O.M.; Chenoweth, P.; Bandyopadhyay, A.S.; Zaffran, M.; Sutter, R.W.; Grassly, N.C. Type 2 Poliovirus Detection after Global Withdrawal of Trivalent Oral Vaccine. N. Engl. J. Med. 2018, 379, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Coulliette-Salmond, A.D.; Alleman, M.M.; Wilnique, P.; Rey-Benito, G.; Wright, H.B.; Hecker, J.W.; Miles, S.; Penaranda, S.; Lafontant, D.; Corvil, S.; et al. Haiti Poliovirus Environmental Surveillance. Am. J. Trop. Med. Hyg. 2019, 101, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Vinjé, J.; Gregoricus, N.; Martin, J.; Gary, J.H.E.; Cáceres, V.M.; Venczel, L.; Macadam, A.; Dobbins, J.G.; Burns, C.; Wait, D.; et al. Isolation and Characterization of Circulating Type 1 Vaccine-Derived Poliovirus from Sewage and Stream Waters in Hispaniola. J. Infect. Dis. 2004, 189, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.; Morris-Glasgow, V.; Landaverde, M.; Burns, C.; Shaw, J.; Garib, Z.; André, J.; Blackman, E.; Freeman, C.J.; Jorba, J.; et al. Outbreak of Poliomyelitis in Hispaniola Associated with Circulating Type 1 Vaccine-Derived Poliovirus. Science 2002, 296, 356–359. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and UNICEF. Haïti: WHO and UNICEF Estimates of Immunization Coverage: 2019 Revision. Available online: https://www.who.int/immunization/monitoring_surveillance/data/hti.pdf (accessed on 28 December 2020).
- Pan American Health Organization. Regional Plan for Expansion of Environmental Surveillance of Poliovirus. In Proceedings of the Meeting of Ad hoc Small Working Group of Polio, Gloria Rey-Benito, PAHO, FGL/IM, Amman, Jordan, 29–30 January 2015. [Google Scholar]
- List of Cities in Haïti. Available online: https://en.wikipedia.org/wiki/List_of_cities_in_Haïti (accessed on 28 December 2020).
- Population of Cities in Haïti. 2020. Available online: https://worldpopulationreview.com/countries/cities/Haïti (accessed on 28 December 2020).
- Novel-t, PATH, Environmental Surveillance, Supporting Polio Eradication. Available online: https://www.es.world/#!/catalog (accessed on 28 December 2020).
- Gerloff, N.; Sun, H.; Mandelbaum, M.; Maher, C.; Nix, W.A.; Zaidi, S.; Shaukat, S.; Seakamela, L.; Nalavade, U.P.; Sharma, D.K.; et al. Diagnostic Assay Development for Poliovirus Eradication. J. Clin. Microbiol. 2017, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary. Available online: https://apps.who.int/immunization_monitoring/globalsummary/schedules?sc%5Br%5D%5B%5D=AMRO&sc%5Bc%5D%5B%5D=HTI&sc%5Bd%5D=&sc%5Bv%5D%5B%5D=OPV&sc%5BOK%5D=OK (accessed on 28 December 2020).
- Rey-Benito, G.; (Pan American Health Organization). Personal communication, 2020.
- Njile, D.K.; Sadeuh-Mba, S.A.; Endegue-Zanga, M.-C.; Mengouo, M.N.; Djoumetio, M.D.; Pouth, F.B.B.; Diop, O.M.; Njouom, R. Detection and characterization of polioviruses originating from urban sewage in Yaounde and Douala, Cameroon 2016–2017. BMC Res. Notes 2019, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Intercountry Support Team for Central Africa; World Health Organization Regional Office for Africa Libreville, Gabon. Mise à Jour Hebdomadaire sur l’Initiative d’Eradication de la Polio en Afrique Centrale (Weekly Update for the Polio Eradication Initiative in Central Africa). 2020. Available online: https://www.afro.who.int/ (accessed on 18 February 2021).
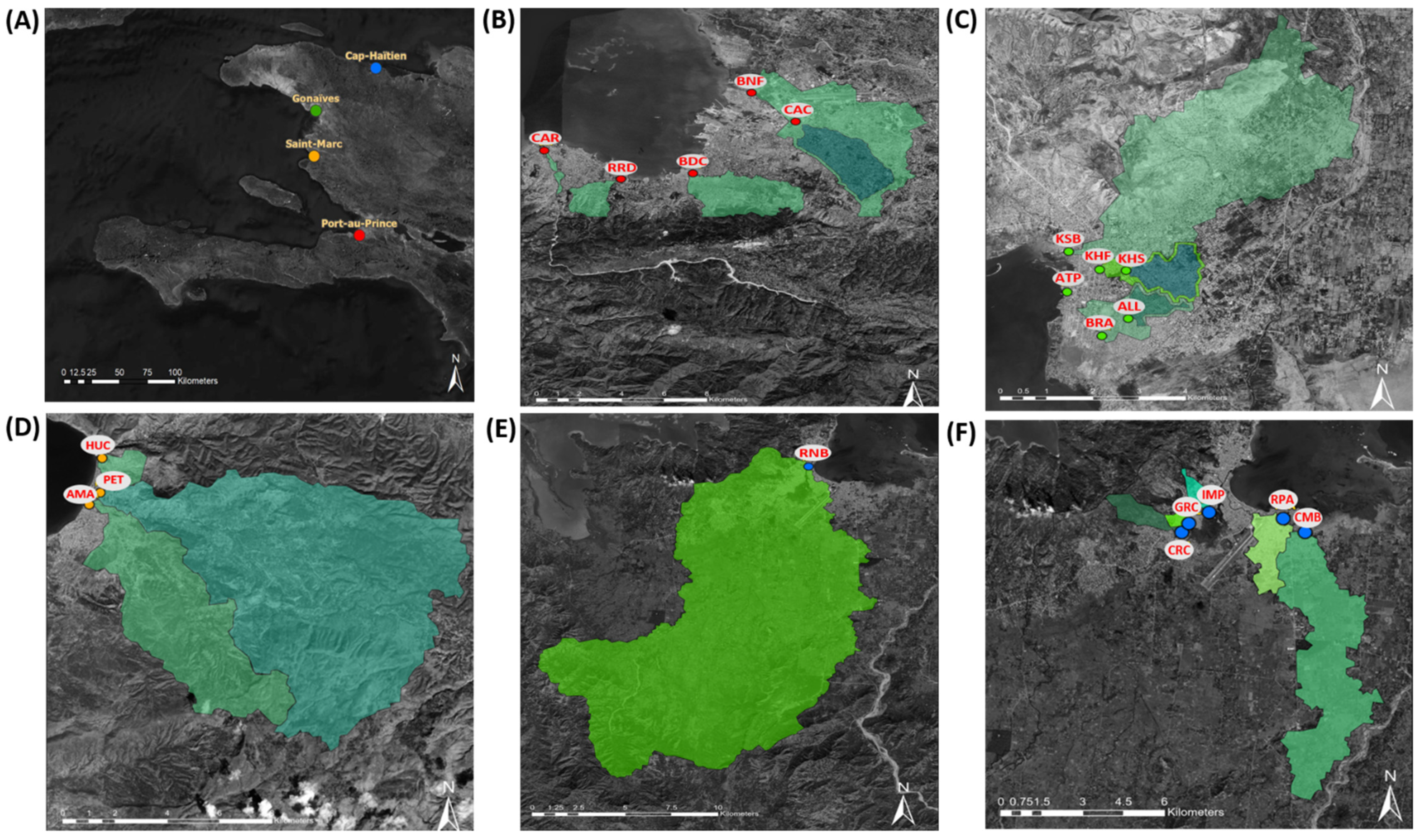

| City | Site Code | Total Sampling Events | Geographic Coordinates | Estimated Watershed Population | Additional Site Details |
|---|---|---|---|---|---|
| Port au Prince | BNF | 34 | 18.5815, −72.3291 | 347,237 * | Downstream from CAC [17] |
| BDC | 34 | 18.5383, −72.3539 | 339,624 * | [17] | |
| MAC | 1 | 18.6611, −72.1870 | Unknown † | [17] | |
| RRD | 33 | 18.5345, −72.3843 | 67,320 | Open canal near intersection Rue du Dr Dehoux and Harry Truman Blvd | |
| CAC | 4 | 18.5658, −72.3105 | 139,987 | Upstream from BNF, open canal Saint George | |
| CAR | 4 | 18.5500, −72.4166 | 9361 | Open canal Rivière Froide on Route de Rails | |
| Gonaïves | KHF | 16 | 19.4534, −72.6900 | 20,749 * | [17] |
| KSB | 5 | 19.4575, −72.6960 | 82,123 * | [17] | |
| ATP | 1 | 19.4483, −72.6963 | Unknown † | [17] | |
| ALL | 4 | 19.4422, −72.6845 | 10,034 | Upstream from BRA, open canal near intersection of Ave Leon Legros and Ruelle Patience | |
| BRA | 31 | 19.4383, −72.6896 | 20,241 | Downstream from ALL, open canal near intersection Blvd de l’Avenir and Bas Mavignole | |
| KHS | 4 | 19.4532, −72.6849 | 17,878 | Upstream from KHF, open canal near intersection Avenue Roland 1 and Rue Polkos | |
| Saint Marc | AMA | 19 | 19.1059, −72.7022 | 50,744 | Open canal at intersection Rivière Saint Marc and Avenue Maurepas ‡ |
| HUC | 16 | 19.1224, −72.6978 | 5655 | Open canal near intersection Impasse Hucar and Rue les Boutin | |
| PET | 6 | 19.1102, −72.6984 | 49,372 | Open canal at intersection Rivière Saint Marc and Rue Petion ‡ | |
| Cap Haïtien | CRC | 21 | 19.7336, −72.2178 | 8810 | Open canal along Ruelle Caporis, drains into Rivière du Haut du Cap |
| RPA | 21 | 19.7383, −72.1844 | 22,468 | Open canal along Ruelle Patience | |
| GRC | 3 | 19.7367, −72.2155 | 5455 | Open canal along Grand Rue (Ruelle) Champin, drains into Rivière du Haut du Cap | |
| IMP | 5 | 19.7405, −72.2088 | 9626 | Open canal along footpath south of Impasse Petion, drains into Rivière du Haut du Cap | |
| RNB | 5 | 19.7504, −72.2046 | 225,762 | Site is Rivière du Haut du Cap under the Route National 3 bridge | |
| CMB | 5 | 19.7336, −72.1772 | 21,580 | Open canal adjacent to the Voix Evangélique d’Haïti (4VEH) radio transmission station |
| City | Site Code | Total Sampling Events (% enterovirus Isolation) Mar 2017–Dec 2019 | Total Sampling Events (% enterovirus Isolation) Mar 2016– Feb 2017 [17] | Estimated Watershed Population | Additional Considerations | Status |
|---|---|---|---|---|---|---|
| Port au Prince | BNF | 34 (94) | 12 (100) | 347,237 † | Periodic SL poliovirus isolation; represents a watershed of northern Port au Prince | Retained |
| BDC | 34 (94) | 12 (92) | 339,624 † | Periodic SL poliovirus isolation; represents a watershed between BNF and RRD | Retained | |
| MAC | 1 (100) | 12 (75) | Unknown ‡ | High levels of particulate matter in samples; population represented was unknown | Terminated | |
| RRD | 33 (91) | N/A | 67,321 | Periodic SL poliovirus isolation; represents a watershed of southern Port au Prince | Retained | |
| CAC | 4 (100) | N/A | 139,987 | Upstream from BNF; smaller watershed population than BNF | Terminated | |
| CAR | 4 (50) | N/A | 9361 | Very small estimated watershed population | Terminated | |
| Gonaïves | KHF | 16 (50) | 12 (58) | 20,749 † | § | Terminated |
| KSB | 5 (0) | 12 (25) | 82,123 † | § | Terminated | |
| ATP | 1 (0) | 12 (0) | Unknown ‡ | § | Terminated | |
| ALL | 4 (50) | N/A | 10,034 | Upstream from BRA; smaller watershed population than BRA | Terminated | |
| BRA | 31 (84) | N/A | 20,241 | Periodic SL poliovirus isolation; downstream from ALL; larger watershed population than ALL | Retained | |
| KHS | 4 (0) | N/A | 17,878 | § | Terminated | |
| Saint Marc | AMA | 19 (90) | N/A | 50,744 | Periodic SL poliovirus isolation; represents a watershed of southern Saint Marc | Retained |
| HUC | 16 (50) | N/A | 5655 | Canal was periodically dry | Terminated | |
| PET | 6 (67) | N/A | 49,372 | Wide canal with large volume of water; represents a watershed between AMA and HUC | Retained | |
| Cap Haïtien | CRC | 21 (81) | N/A | 8810 | Periodic SL poliovirus isolation, represents a watershed of western Cap Haïtien | Retained |
| RPA | 21 (67) | N/A | 22,468 | Periodic SL poliovirus isolation, represents a watershed of eastern Cap Haïtien | Retained | |
| GRC | 3 (100) | N/A | 5455 | Very small estimated watershed population | Terminated | |
| IMP | 5 (100) | N/A | 9626 | Represents a watershed of western Cap Haïtien | Retained | |
| RNB | 5 (20) | N/A | 225,762 | Possible influence of high salinity due to proximity to sea | Terminated | |
| CMB | 5 (80) | N/A | 21,580 | Represents a watershed of eastern Cap Haïtien | Retained |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alleman, M.M.; Coulliette-Salmond, A.D.; Wilnique, P.; Belgasmi-Wright, H.; Sayyad, L.; Wong, K.; Gue, E.; Barrais, R.; Rey-Benito, G.; Burns, C.C.; et al. Environmental Surveillance for Polioviruses in Haïti (2017–2019): The Dynamic Process for the Establishment and Monitoring of Sampling Sites. Viruses 2021, 13, 505. https://doi.org/10.3390/v13030505
Alleman MM, Coulliette-Salmond AD, Wilnique P, Belgasmi-Wright H, Sayyad L, Wong K, Gue E, Barrais R, Rey-Benito G, Burns CC, et al. Environmental Surveillance for Polioviruses in Haïti (2017–2019): The Dynamic Process for the Establishment and Monitoring of Sampling Sites. Viruses. 2021; 13(3):505. https://doi.org/10.3390/v13030505
Chicago/Turabian StyleAlleman, Mary M., Angela D. Coulliette-Salmond, Pierre Wilnique, Hanen Belgasmi-Wright, Leanna Sayyad, Kimberly Wong, Edmund Gue, Robert Barrais, Gloria Rey-Benito, Cara C. Burns, and et al. 2021. "Environmental Surveillance for Polioviruses in Haïti (2017–2019): The Dynamic Process for the Establishment and Monitoring of Sampling Sites" Viruses 13, no. 3: 505. https://doi.org/10.3390/v13030505
APA StyleAlleman, M. M., Coulliette-Salmond, A. D., Wilnique, P., Belgasmi-Wright, H., Sayyad, L., Wong, K., Gue, E., Barrais, R., Rey-Benito, G., Burns, C. C., & Vega, E. (2021). Environmental Surveillance for Polioviruses in Haïti (2017–2019): The Dynamic Process for the Establishment and Monitoring of Sampling Sites. Viruses, 13(3), 505. https://doi.org/10.3390/v13030505







