Human Astroviruses: A Tale of Two Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus Propagation
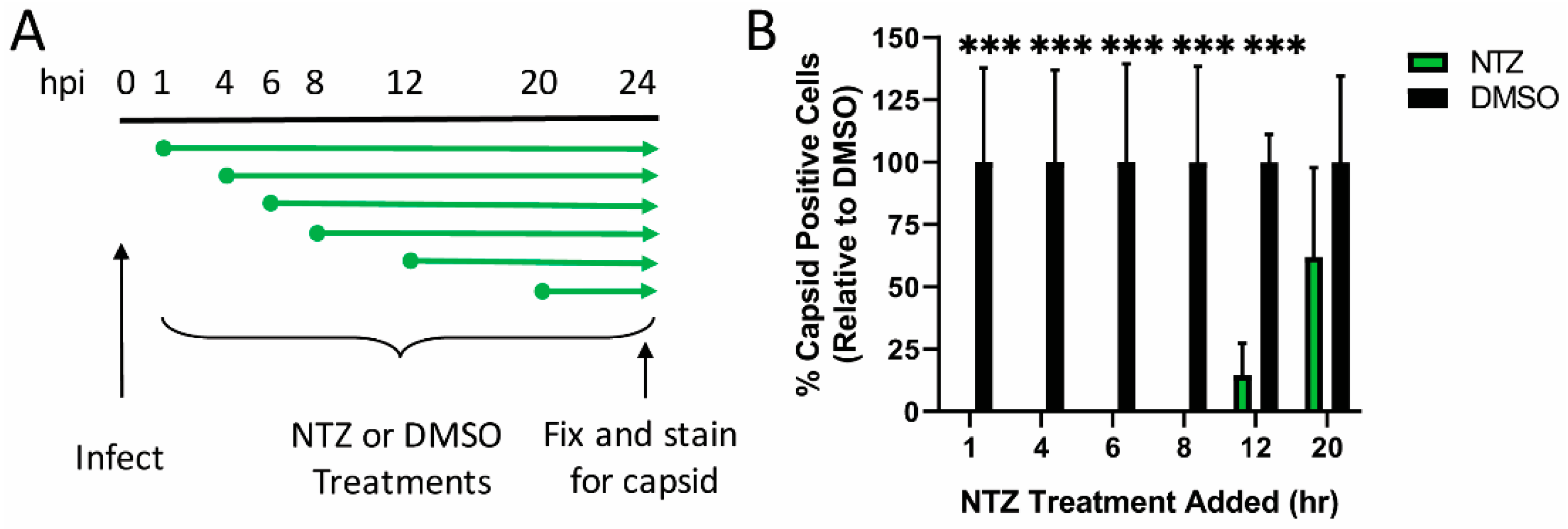
2.2. Nitazoxonide (NTZ) Treatment
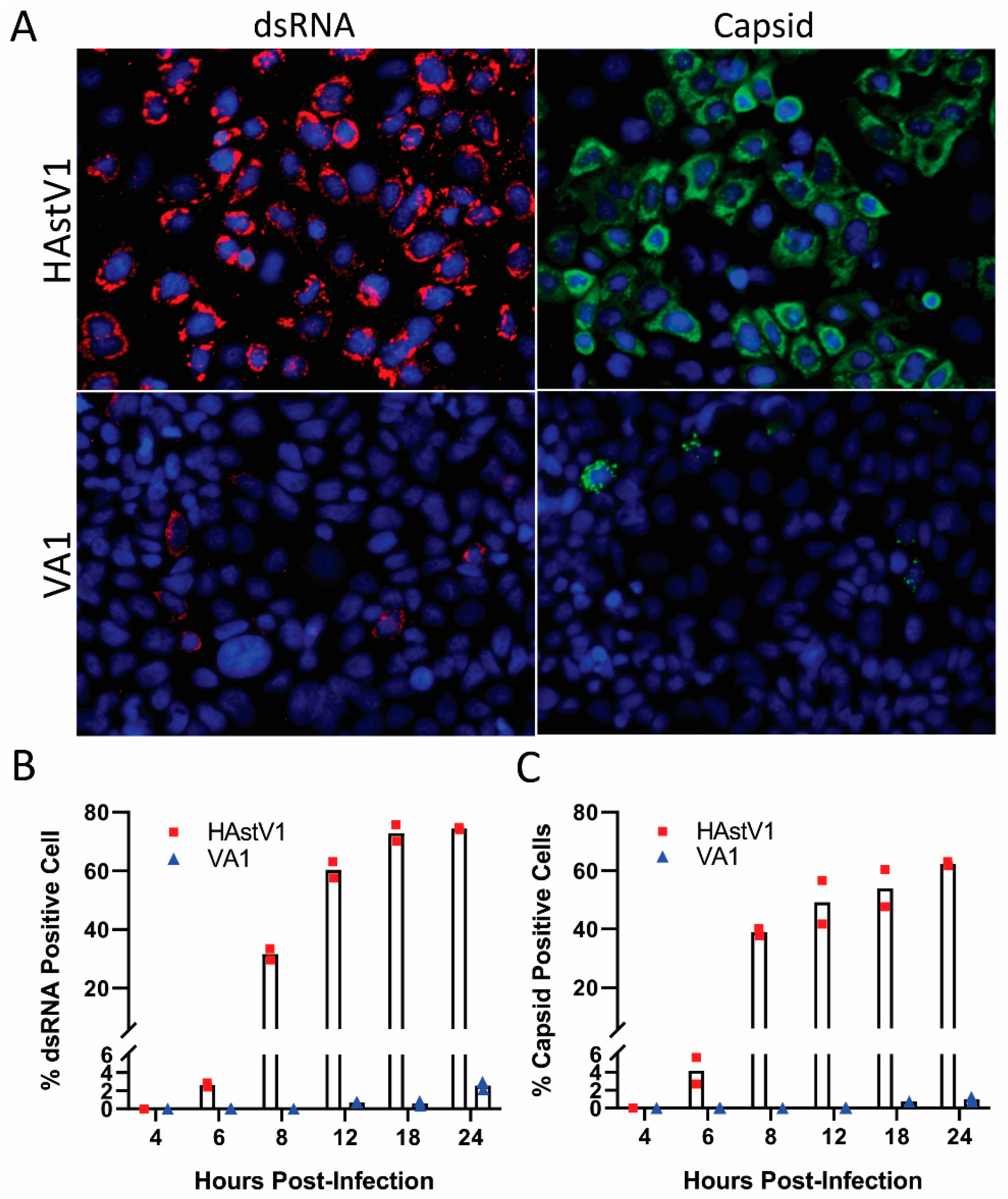
2.3. Capsid and dsRNA Staining
2.4. Viral Kinetics
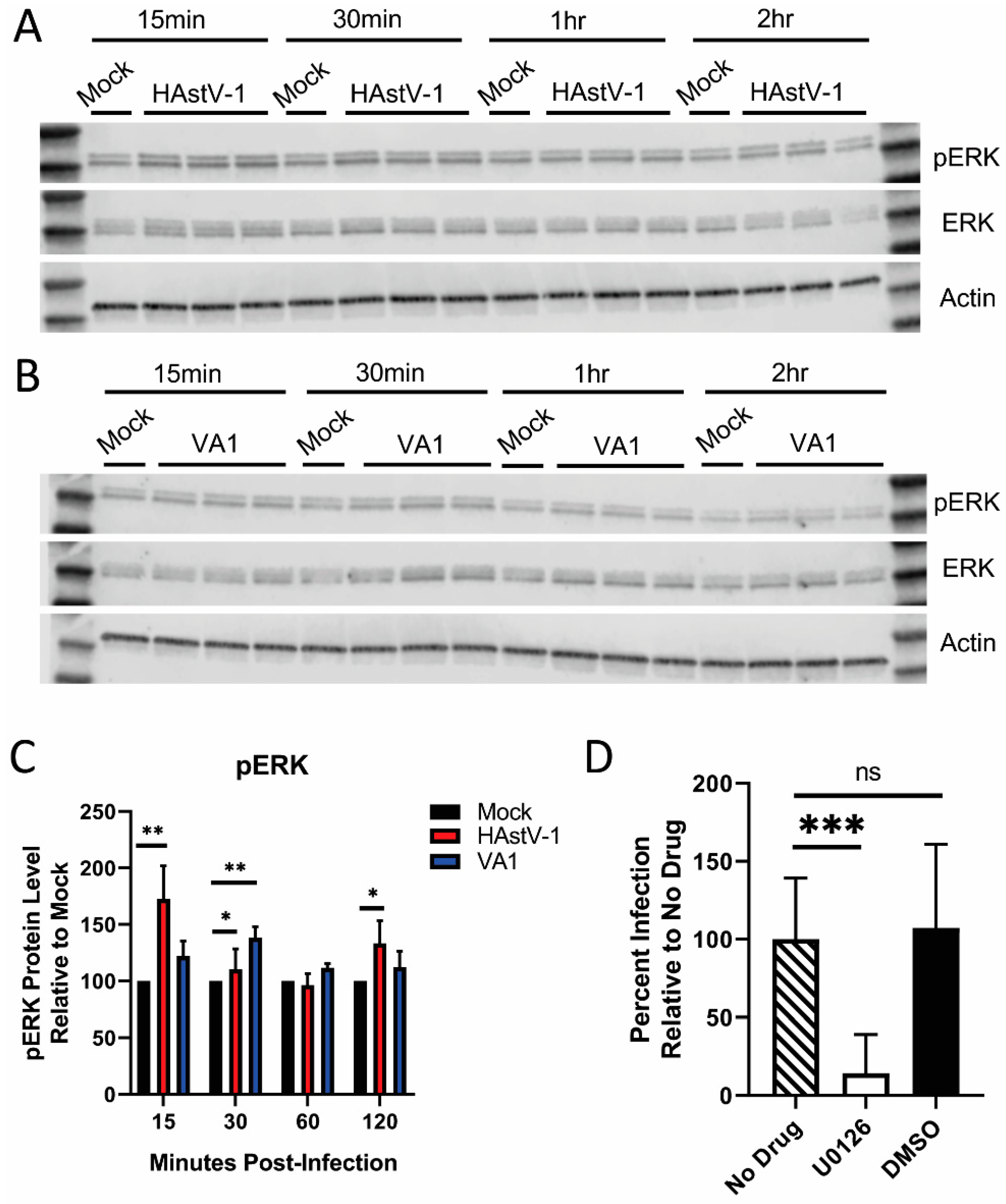
2.5. p-ERK Western Blotting
2.6. U0126 Treatment
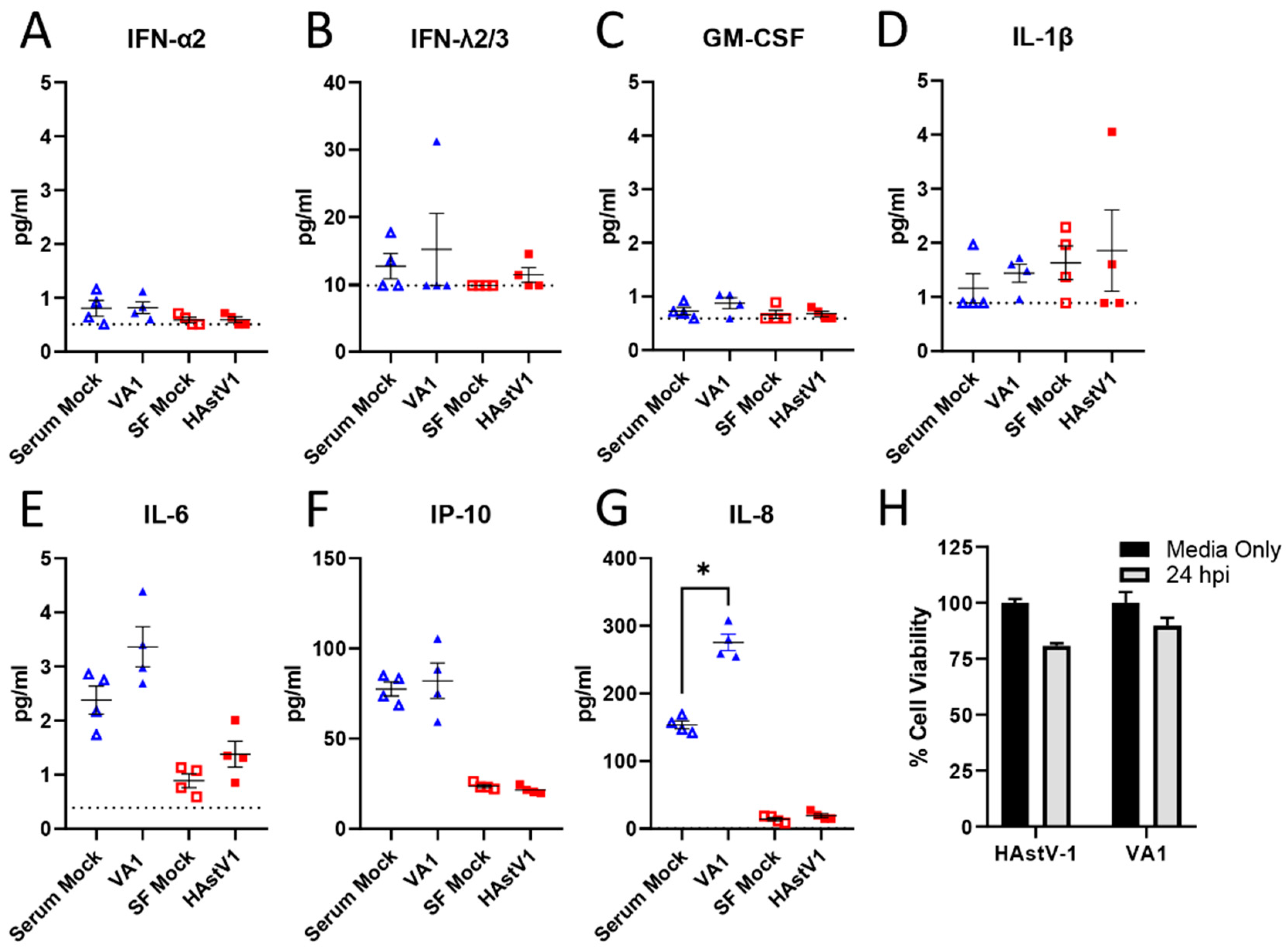
2.7. Cytokine Analysis
2.8. Cytotoxicity Assay
2.9. Occludin Staining
2.10. Transepithelial Electrical Resistance (TER)
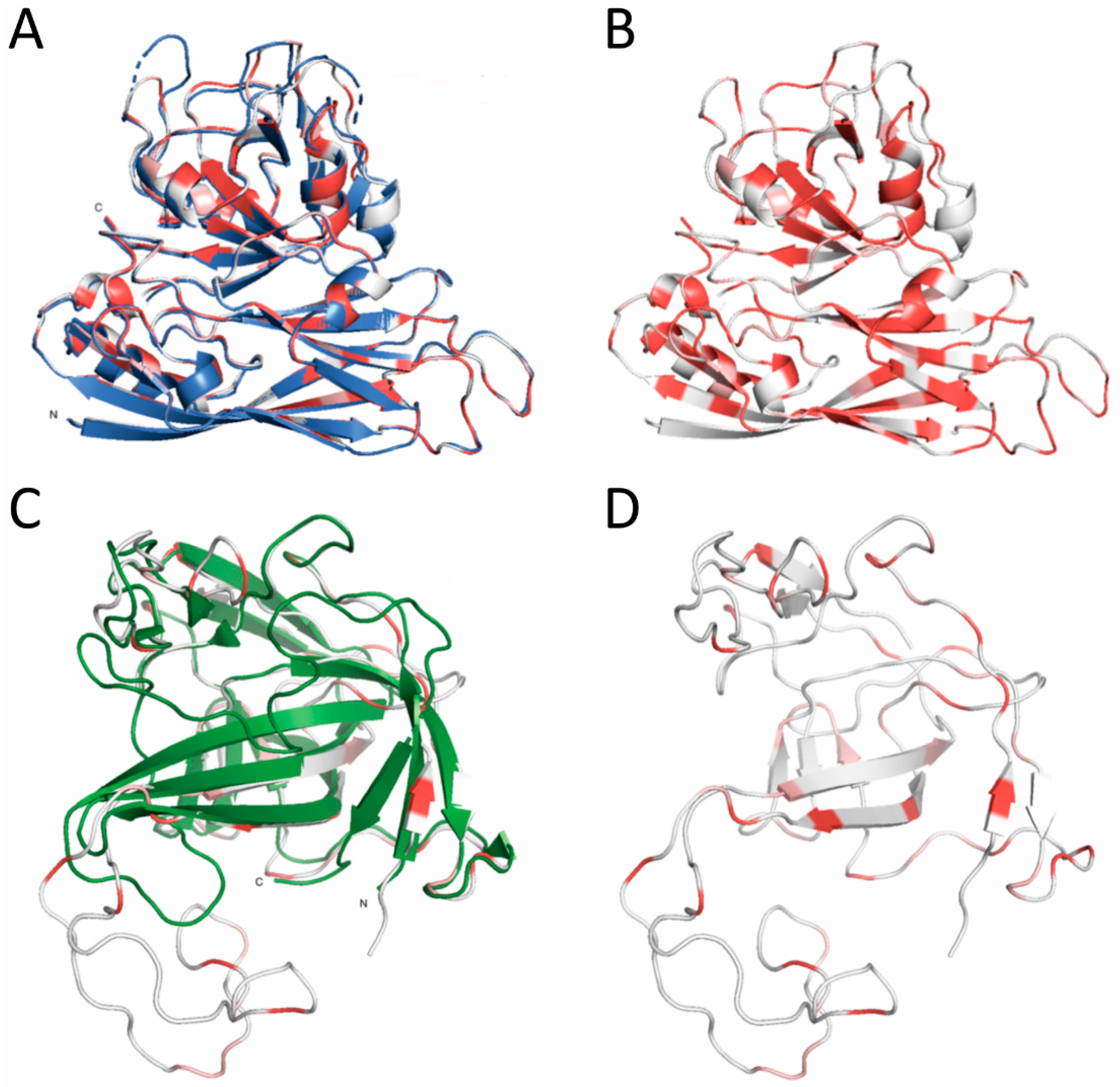
2.11. Capsid Structure Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koopmans, M.P.; Bijen, M.H.; Monroe, S.S.; Vinjé, J. Age-Stratified Seroprevalence of Neutralizing Antibodies to Astrovirus Types 1 to 7 in Humans in The Netherlands. Clin. Diagn. Lab. Immunol. 1998, 5, 33–37. [Google Scholar] [CrossRef]
- Kriston, S.; Willcocks, M.M.; Carter, M.J.; Cubitt, W.D. Seroprevalence of Astrovirus Types 1 and 6 in London, Determined Using Recombinant Virus Antigen. Epidemiol. Infect. 1996, 117, 159–164. [Google Scholar] [CrossRef]
- Mitchell, D.K.; Matson, D.O.; Cubitt, W.D.; Jackson, L.J.; Willcocks, M.M.; Pickering, L.K.; Carter, M.J. Prevalence of Antibodies to Astrovirus Types 1 and 3 in Children and Adolescents in Norfolk, Virginia. Pediatr. Infect. Dis. J. 1999, 18, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Hargest, V.; Cortez, V.; Meliopoulos, V.A.; Schultz-Cherry, S. Astrovirus Pathogenesis. Viruses 2017, 9, 22. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Holtz, L.R.; Jiang, Y.; Rajendran, P.; Franz, C.J.; Zhao, G.; Kang, G.; Wang, D. Human Stool Contains a Previously Unrecognized Diversity of Novel Astroviruses. Virol. J. 2009, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.R.; Li, Y.; Ruone, S.; Conrardy, C.; Gregoricus, N.; Toney, D.; Virgin, H.W.; Anderson, L.J.; Vinjé, J.; Wang, D.; et al. Identification of a Novel Astrovirus (Astrovirus VA1) Associated with an Outbreak of Acute Gastroenteritis. J. Virol. 2009, 83, 10836–10839. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.-L.; Cordey, S.; Brito, F.; Kaiser, L. Novel Human Astroviruses: Novel Human Diseases? J. Clin. Virol. 2016, 82, 56–63. [Google Scholar] [CrossRef]
- Vu, D.-L.; Bosch, A.; Pintó, R.M.; Guix, S. Epidemiology of Classic and Novel Human Astrovirus: Gastroenteritis and Beyond. Viruses 2017, 9, 33. [Google Scholar] [CrossRef]
- De Grazia, S.; Platia, M.A.; Rotolo, V.; Colomba, C.; Martella, V.; Giammanco, G.M. Surveillance of Human Astrovirus Circulation in Italy 2002–2005: Emergence of Lineage 2c Strains. Clin. Microbiol. Infect. 2011, 17, 97–101. [Google Scholar] [CrossRef]
- Gabbay, Y.B.; Leite, J.P.G.; Oliveira, D.S.; Nakamura, L.S.; Nunes, M.R.T.; Mascarenhas, J.D.P.; Heinemann, M.B.; Linhares, A.C. Molecular Epidemiology of Astrovirus Type 1 in Belém, Brazil, as an Agent of Infantile Gastroenteritis, over a Period of 18 Years (1982–2000): Identification of Two Possible New Lineages. Virus Res. 2007, 129, 166–174. [Google Scholar] [CrossRef]
- Guix, S.; Caballero, S.; Villena, C.; Bartolomé, R.; Latorre, C.; Rabella, N.; Simó, M.; Bosch, A.; Pintó, R.M. Molecular Epidemiology of Astrovirus Infection in Barcelona, Spain. J. Clin. Microbiol. 2002, 40, 133–139. [Google Scholar] [CrossRef]
- Méndez-Toss, M.; Griffin, D.D.; Calva, J.; Contreras, J.F.; Puerto, F.I.; Mota, F.; Guiscafré, H.; Cedillo, R.; Muñoz, O.; Herrera, I.; et al. Prevalence and Genetic Diversity of Human Astroviruses in Mexican Children with Symptomatic and Asymptomatic Infections. J. Clin. Microbiol. 2004, 42, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Ching, K.H.; Esper, F.; Iadarola, M.J.; Delwart, E.; Lipkin, W.I.; Kapoor, A. Serological Studies Confirm the Novel Astrovirus HMOAstV-C as a Highly Prevalent Human Infectious Agent. PLoS ONE 2011, 6, e22576. [Google Scholar] [CrossRef] [PubMed]
- Holtz, L.R.; Bauer, I.K.; Jiang, H.; Belshe, R.; Freiden, P.; Schultz-Cherry, S.L.; Wang, D. Seroepidemiology of Astrovirus MLB1. Clin. Vaccine Immunol. 2014, 21, 908–911. [Google Scholar] [CrossRef]
- Holtz, L.R.; Bauer, I.K.; Rajendran, P.; Kang, G.; Wang, D. Astrovirus MLB1 Is Not Associated with Diarrhea in a Cohort of Indian Children. PLoS ONE 2011, 6, e28647. [Google Scholar] [CrossRef]
- Meyer, C.T.; Bauer, I.K.; Antonio, M.; Adeyemi, M.; Saha, D.; Oundo, J.O.; Ochieng, J.B.; Omore, R.; Stine, O.C.; Wang, D.; et al. Prevalence of Classic, MLB-Clade and VA-Clade Astroviruses in Kenya and The Gambia. Virol. J. 2015, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Brito, F.; Vu, D.-L.; Turin, L.; Kilowoko, M.; Kyungu, E.; Genton, B.; Zdobnov, E.M.; D’Acremont, V.; Kaiser, L. Astrovirus VA1 Identified by Next-Generation Sequencing in a Nasopharyngeal Specimen of a Febrile Tanzanian Child with Acute Respiratory Disease of Unknown Etiology. Emerg. Microbes Infect. 2016, 5, e99. [Google Scholar] [CrossRef]
- Wunderli, W.; Meerbach, A.; Güngör, T.; Guengoer, T.; Berger, C.; Greiner, O.; Caduff, R.; Trkola, A.; Bossart, W.; Gerlach, D.; et al. Astrovirus Infection in Hospitalized Infants with Severe Combined Immunodeficiency after Allogeneic Hematopoietic Stem Cell Transplantation. PLoS ONE 2011, 6, e27483. [Google Scholar] [CrossRef]
- Koukou, G.; Niendorf, S.; Hornei, B.; Schlump, J.-U.; Jenke, A.C.; Jacobsen, S. Human Astrovirus Infection Associated with Encephalitis in an Immunocompetent Child: A Case Report. J. Med. Case Rep. 2019, 13, 341. [Google Scholar] [CrossRef]
- Quan, P.L.; Wagner, T.A.; Briese, T.; Torgerson, T.R.; Hornig, M.; Tashmukhamedova, A.; Firth, C.; Palacios, G.; Baisre-De-Leon, A.; Paddock, C.D.; et al. Astrovirus Encephalitis in Boy with X-Linked Agammaglobulinemia. Emerg. Infect. Dis. 2010, 16, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Morfopoulou, S.; Hubb, J.; Emmett, W.A.; Ip, W.; Shah, D.; Brooks, T.; Paine, S.M.L.; Anderson, G.; Virasami, A.; et al. Astrovirus VA1/HMO-C: An Increasingly Recognized Neurotropic Pathogen in Immunocompromised Patients. Clin. Infect. Dis. 2015, 60, 881–888. [Google Scholar] [CrossRef]
- Sato, M.; Kuroda, M.; Kasai, M.; Matsui, H.; Fukuyama, T.; Katano, H.; Tanaka-Taya, K. Acute Encephalopathy in an Immunocompromised Boy with Astrovirus-MLB1 Infection Detected by next Generation Sequencing. J. Clin. Virol. 2016, 78, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Naccache, S.N.; Peggs, K.S.; Mattes, F.M.; Phadke, R.; Garson, J.A.; Grant, P.; Samayoa, E.; Federman, S.; Miller, S.; Lunn, M.P.; et al. Diagnosis of Neuroinvasive Astrovirus Infection in an Immunocompromised Adult with Encephalitis by Unbiased Next-Generation Sequencing. Clin. Infect. Dis. 2015, 60, 919–923. [Google Scholar] [CrossRef]
- Frémond, M.-L.; Pérot, P.; Muth, E.; Cros, G.; Dumarest, M.; Mahlaoui, N.; Seilhean, D.; Desguerre, I.; Hébert, C.; Corre-Catelin, N.; et al. Next-Generation Sequencing for Diagnosis and Tailored Therapy: A Case Report of Astrovirus-Associated Progressive Encephalitis. J. Pediatr. Infect. Dis. Soc. 2015, 4, e53–e57. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Vu, D.-L.; Schibler, M.; L’Huillier, A.G.; Brito, F.; Docquier, M.; Posfay-Barbe, K.M.; Petty, T.J.; Turin, L.; Zdobnov, E.M.; et al. Astrovirus MLB2, a New Gastroenteric Virus Associated with Meningitis and Disseminated Infection. Emerg. Infect. Dis. 2016, 22, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Lum, S.H.; Turner, A.; Guiver, M.; Bonney, D.; Martland, T.; Davies, E.; Newbould, M.; Brown, J.; Morfopoulou, S.; Breuer, J.; et al. An Emerging Opportunistic Infection: Fatal Astrovirus (VA1/HMO-C) Encephalitis in a Pediatric Stem Cell Transplant Recipient. Transpl. Infect. Dis. 2016, 18, 960–964. [Google Scholar] [CrossRef]
- Gavier-Widén, D.; Bröjer, C.; Dietz, H.H.; Englund, L.; Hammer, A.S.; Hedlund, K.-O.; Hård af Segerstad, C.; Nilsson, K.; Nowotny, N.; Puurula, V.; et al. Investigations into Shaking Mink Syndrome: An Encephalomyelitis of Unknown Cause in Farmed Mink (Mustela Vison) Kits in Scandinavia. J. Vet. Diagn. Investig. 2004, 16, 305–312. [Google Scholar] [CrossRef]
- Blomström, A.-L.; Widén, F.; Hammer, A.-S.; Belák, S.; Berg, M. Detection of a Novel Astrovirus in Brain Tissue of Mink Suffering from Shaking Mink Syndrome by Use of Viral Metagenomics. J. Clin. Microbiol. 2010, 48, 4392–4396. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, F.; Schlottau, K.; Scholes, S.; Courtenay, A.; Hoffmann, B.; Höper, D.; Beer, M. A Novel Astrovirus Associated with Encephalitis and Ganglionitis in Domestic Sheep. Transbound. Emerg. Dis. 2017, 64, 677–682. [Google Scholar] [CrossRef]
- Hargest, V.; Sharp, B.; Livingston, B.; Cortez, V.; Schultz-Cherry, S. Astrovirus Replication Is Inhibited by Nitazoxanide In Vitro and In Vivo. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Janowski, A.B.; Bauer, I.K.; Holtz, L.R.; Wang, D. Propagation of Astrovirus VA1, a Neurotropic Human Astrovirus, in Cell Culture. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Kolawole, A.O.; Mirabelli, C.; Hill, D.R.; Svoboda, S.A.; Janowski, A.B.; Passalacqua, K.D.; Rodriguez, B.N.; Dame, M.K.; Freiden, P.; Berger, R.P.; et al. Astrovirus Replication in Human Intestinal Enteroids Reveals Multi-Cellular Tropism and an Intricate Host Innate Immune Landscape. PLoS Pathog. 2019, 15, e1008057. [Google Scholar] [CrossRef] [PubMed]
- Marvin, S.; Meliopoulos, V.; Schultz-Cherry, S. Human Astrovirus Propagation, Purification and Quantification. BIO Protoc. 2014, 4. [Google Scholar] [CrossRef]
- Janowski, A.B.; Wang, D. Infection and Propagation of Astrovirus VA1 in Cell Culture. Curr. Protoc. Microbiol. 2019, 52, e73. [Google Scholar] [CrossRef]
- Koci, M.D.; Moser, L.A.; Kelley, L.A.; Larsen, D.; Brown, C.C.; Schultz-Cherry, S. Astrovirus Induces Diarrhea in the Absence of Inflammation and Cell Death. J. Virol. 2003, 77, 11798–11808. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Schultz-Cherry, S. Suppression of Astrovirus Replication by an ERK1/2 Inhibitor. J. Virol. 2008, 82, 7475–7482. [Google Scholar] [CrossRef] [PubMed]
- York, R.L.; Yousefi, P.A.; Bogdanoff, W.; Haile, S.; Tripathi, S.; DuBois, R.M. Structural, Mechanistic, and Antigenic Characterization of the Human Astrovirus Capsid. J. Virol. 2016, 90, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Janson, G.; Paiardini, A. PyMod 3: A Complete Suite for Structural Bioinformatics in PyMOL. Bioinformatics 2020. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Meliopoulos, V.A.; Marvin, S.A.; Freiden, P.; Moser, L.A.; Nighot, P.; Ali, R.; Blikslager, A.; Reddivari, M.; Heath, R.J.; Koci, M.D.; et al. Oral Administration of Astrovirus Capsid Protein Is Sufficient To Induce Acute Diarrhea In Vivo. mBio 2016, 7. [Google Scholar] [CrossRef]
- Gray, E.W.; Angus, K.W.; Snodgrass, D.R. Ultrastructure of the Small Intestine in Astrovirus-Infected Lambs. J. Gen. Virol. 1980, 49, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Woode, G.N.; Bridger, J.C. Isolation of Small Viruses Resembling Astroviruses and Caliciviruses from Acute Enteritis of Calves. J. Med. Microbiol. 1978, 11, 441–452. [Google Scholar] [CrossRef]
- Sebire, N.J.; Malone, M.; Shah, N.; Anderson, G.; Gaspar, H.B.; Cubitt, W.D. Pathology of Astrovirus Associated Diarrhoea in a Paediatric Bone Marrow Transplant Recipient. J. Clin. Pathol. 2004, 57, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Carter, M.; Schultz-Cherry, S. Astrovirus Increases Epithelial Barrier Permeability Independently of Viral Replication. J. Virol. 2007, 81, 11937–11945. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Kirkwood, C.D.; Wang, D. Complete Genome Sequence of a Highly Divergent Astrovirus Isolated from a Child with Acute Diarrhea. Virol. J. 2008, 5, 117. [Google Scholar] [CrossRef]
- Janowski, A.B.; Klein, R.S.; Wang, D. Differential In Vitro Infection of Neural Cells by Astroviruses. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Modelska, K.; Pittet, J.F.; Folkesson, H.G.; Courtney Broaddus, V.; Matthay, M.A. Acid-Induced Lung Injury. Protective Effect of Anti-Interleukin-8 Pretreatment on Alveolar Epithelial Barrier Function in Rabbits. Am. J. Respir. Crit. Care Med. 1999, 160, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Haffizulla, J.; Hartman, A.; Hoppers, M.; Resnick, H.; Samudrala, S.; Ginocchio, C.; Bardin, M.; Rossignol, J.-F. Effect of Nitazoxanide in Adults and Adolescents with Acute Uncomplicated Influenza: A Double-Blind, Randomised, Placebo-Controlled, Phase 2b/3 Trial. Lancet Infect. Dis. 2014, 14, 609–618. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.A.; Jones, S.A.; et al. Existing Drugs as Broad-Spectrum and Potent Inhibitors for Zika Virus by Targeting NS2B-NS3 Interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.-F.; El-Gohary, Y.M. Nitazoxanide in the Treatment of Viral Gastroenteritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Aliment. Pharmacol. Ther. 2006, 24, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.-F. Nitazoxanide: A First-in-Class Broad-Spectrum Antiviral Agent. Antivir. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, D.M.; Koo, H.L.; Adachi, J.A.; Viola, G.M. Norovirus Gastroenteritis Successfully Treated with Nitazoxanide. J. Infect. 2011, 63, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, K.; Gluud, C.; Grevstad, B.; Jakobsen, J.C. Nitazoxanide for Chronic Hepatitis C. Cochrane Database Syst. Rev. 2014, CD009182. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.B.; Shitu, Z.; Mostafa, A. Drug Repurposing of Nitazoxanide: Can It Be an Effective Therapy for COVID-19? J. Genet. Eng. Biotechnol. 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Janowski, A.B.; Dudley, H.; Wang, D. Antiviral Activity of Ribavirin and Favipiravir against Human Astroviruses. J. Clin. Virol. 2020, 123, 104247. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargest, V.; Davis, A.E.; Tan, S.; Cortez, V.; Schultz-Cherry, S. Human Astroviruses: A Tale of Two Strains. Viruses 2021, 13, 376. https://doi.org/10.3390/v13030376
Hargest V, Davis AE, Tan S, Cortez V, Schultz-Cherry S. Human Astroviruses: A Tale of Two Strains. Viruses. 2021; 13(3):376. https://doi.org/10.3390/v13030376
Chicago/Turabian StyleHargest, Virginia, Amy E. Davis, Shaoyuan Tan, Valerie Cortez, and Stacey Schultz-Cherry. 2021. "Human Astroviruses: A Tale of Two Strains" Viruses 13, no. 3: 376. https://doi.org/10.3390/v13030376
APA StyleHargest, V., Davis, A. E., Tan, S., Cortez, V., & Schultz-Cherry, S. (2021). Human Astroviruses: A Tale of Two Strains. Viruses, 13(3), 376. https://doi.org/10.3390/v13030376





