Characterization of the GBoV1 Capsid and Its Antibody Interactions
Abstract
1. Introduction
2. Methods
2.1. Virus-Like Particle Production and Purification
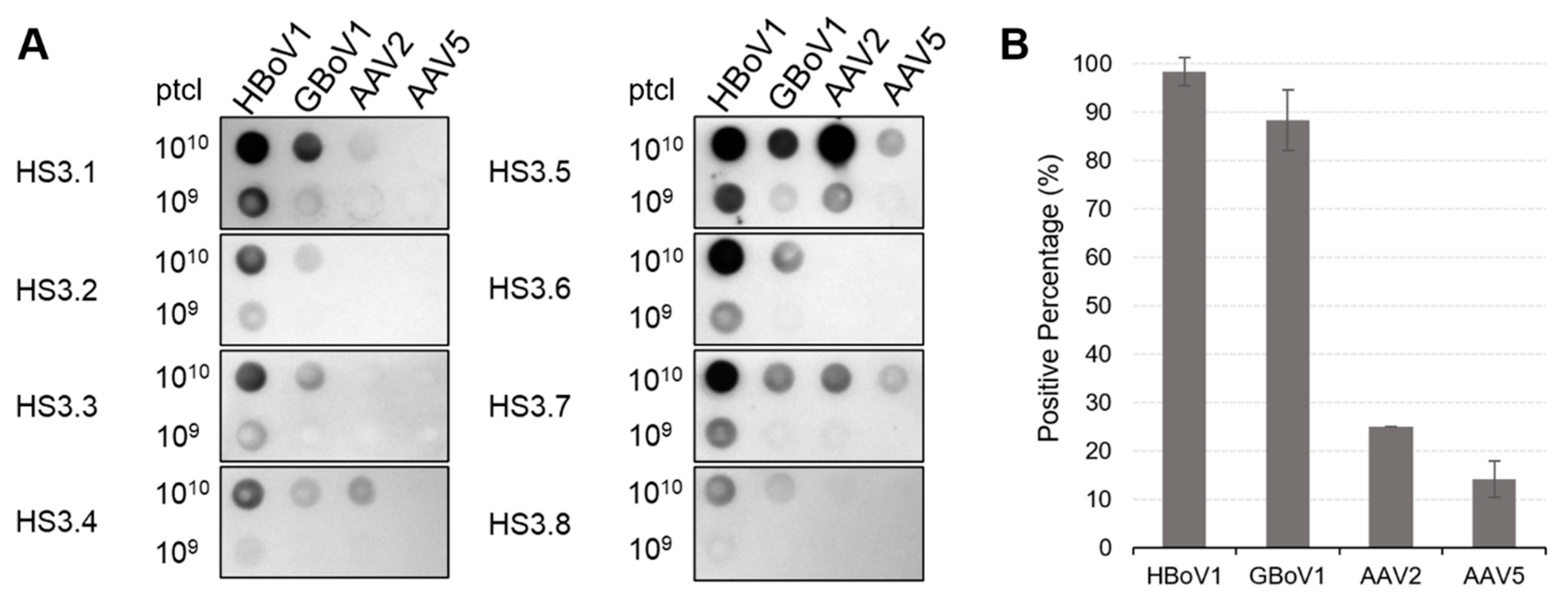
2.2. Dot Immunoblot Analysis
2.3. Generation of Fab Fragments
2.4. Preparation of GBoV1-Fab Complexes and GBoV1 VLPs for Cryo-EM Data Collection
2.5. Cryo-EM Data Collection
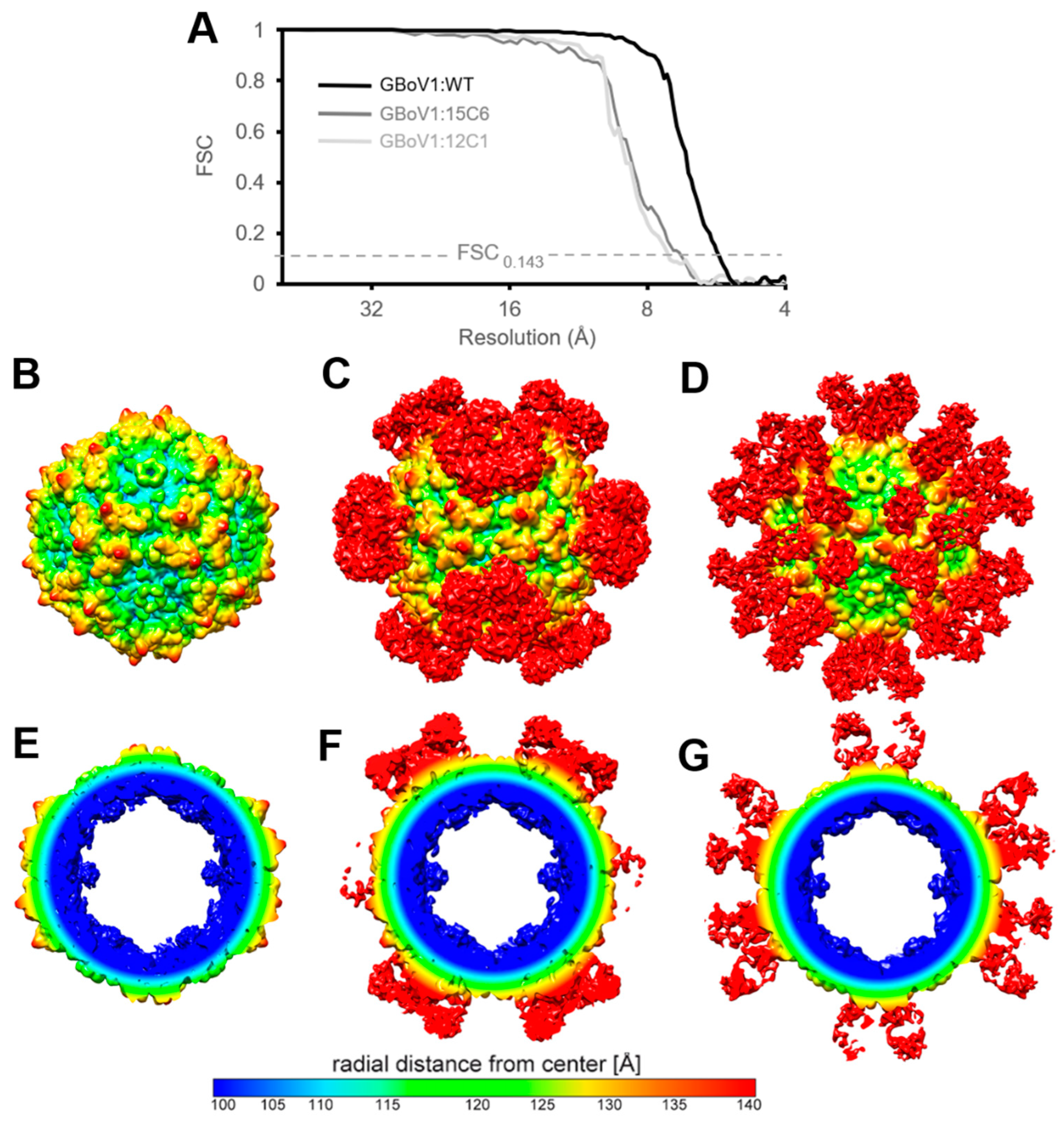
2.6. 3D Particle Reconstruction
2.7. Model Building and Structure Refinement
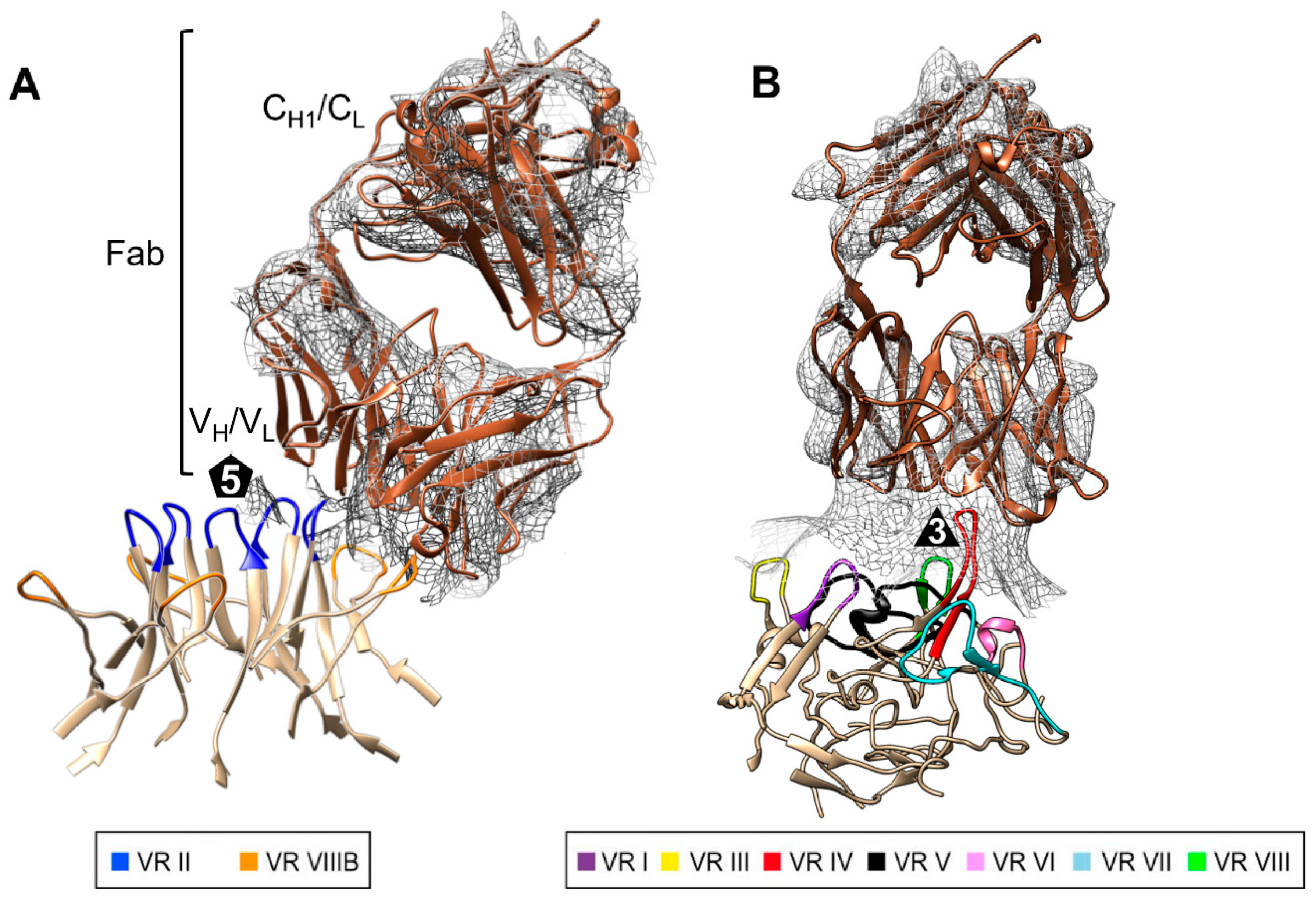
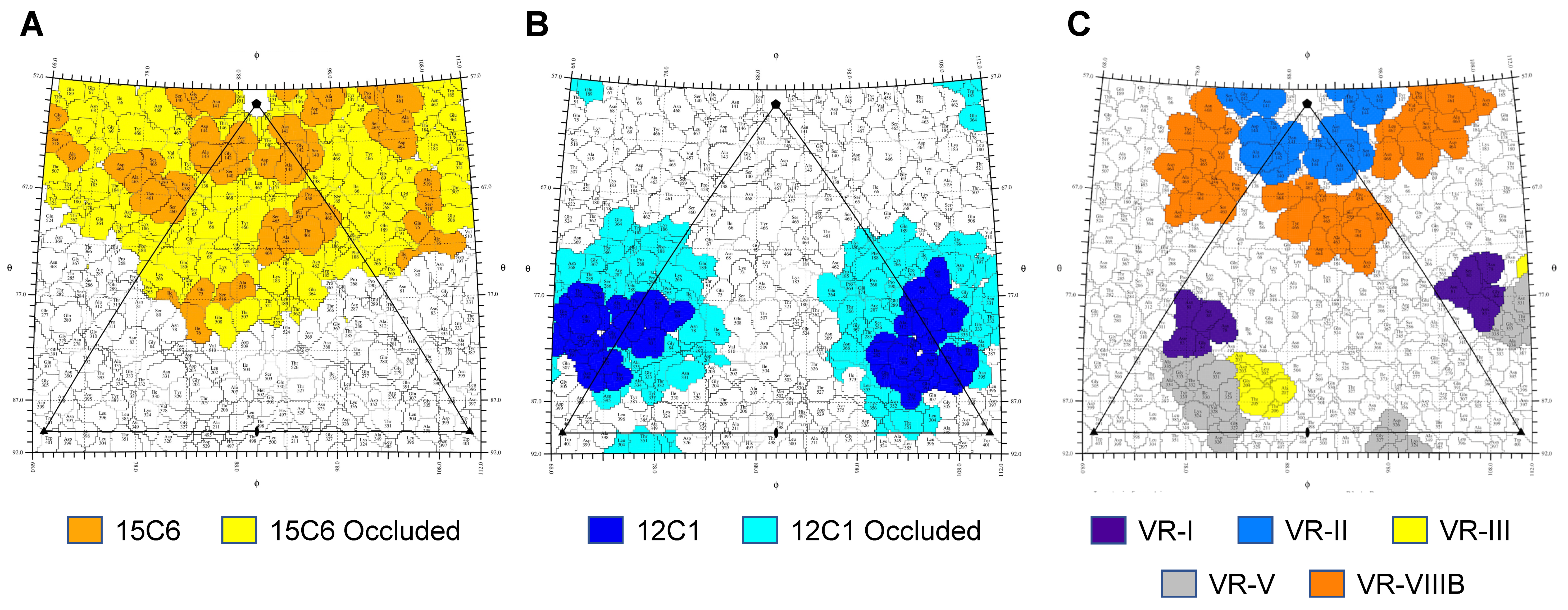
2.8. Antibody Epitope Mapping
2.9. Sequence and Structural Comparison
2.10. Structure Accession Numbers
3. Results & Discussion
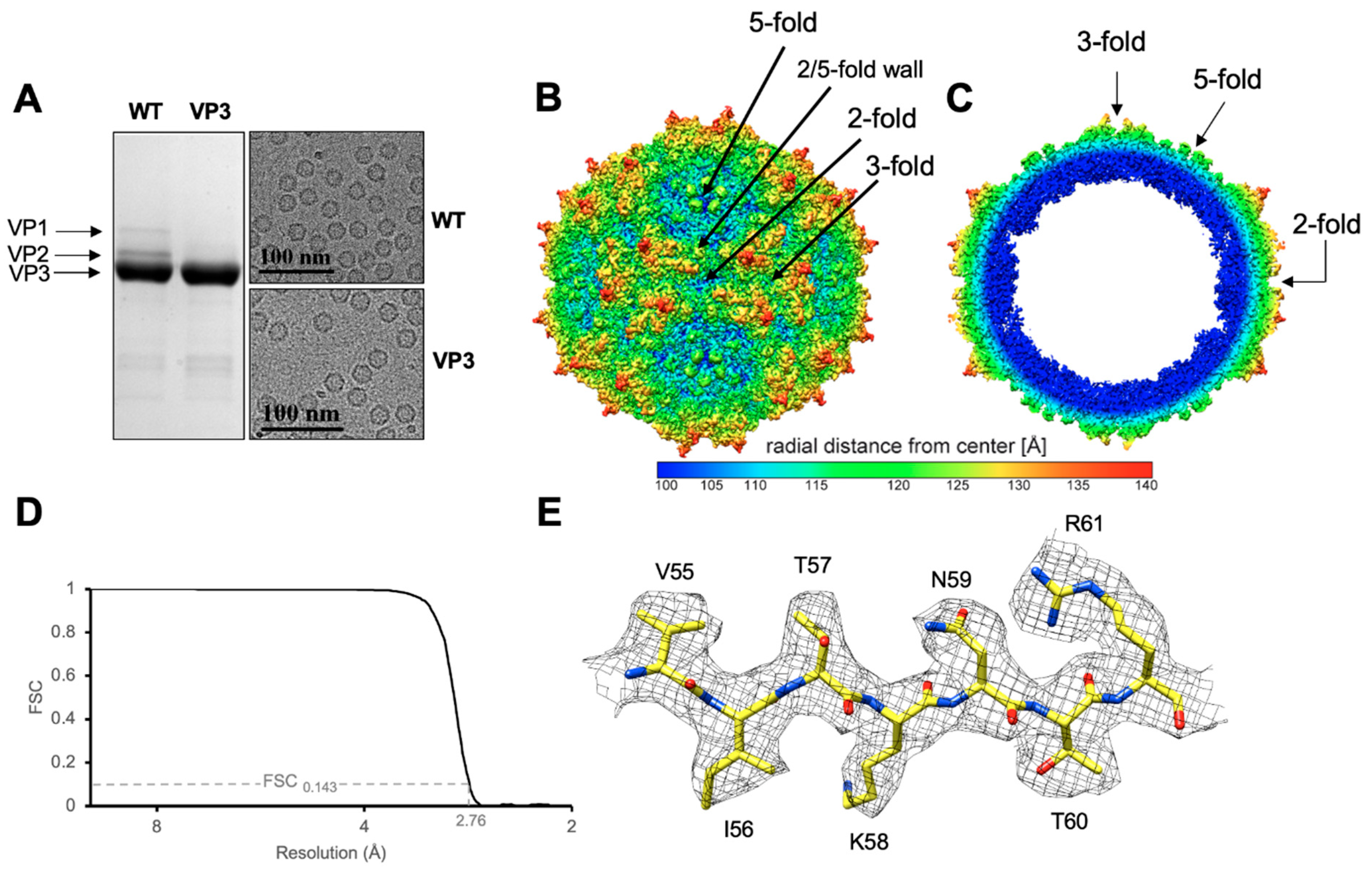
3.1. GBoV1 Shares Conserved Capsid Features with the HBoVs
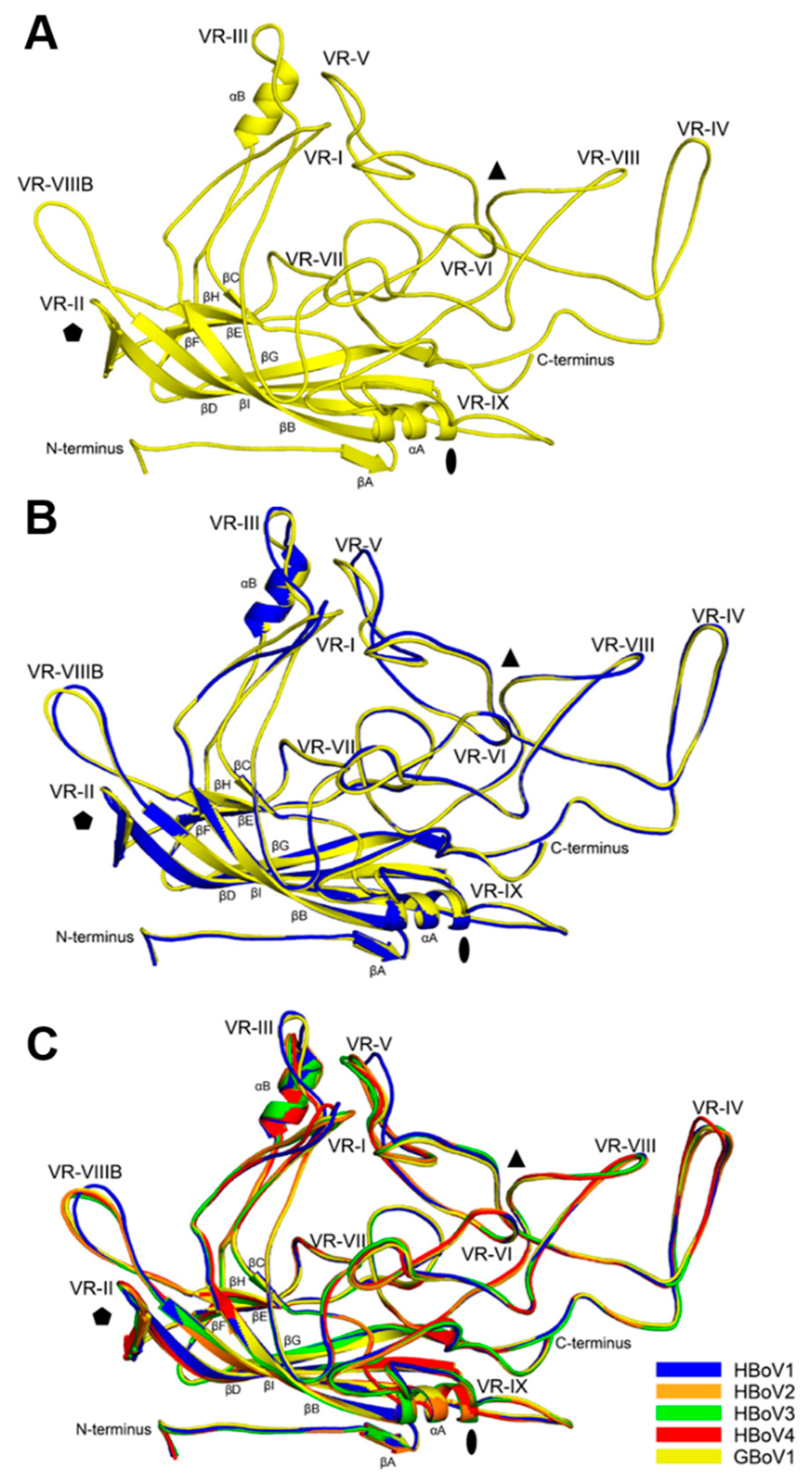
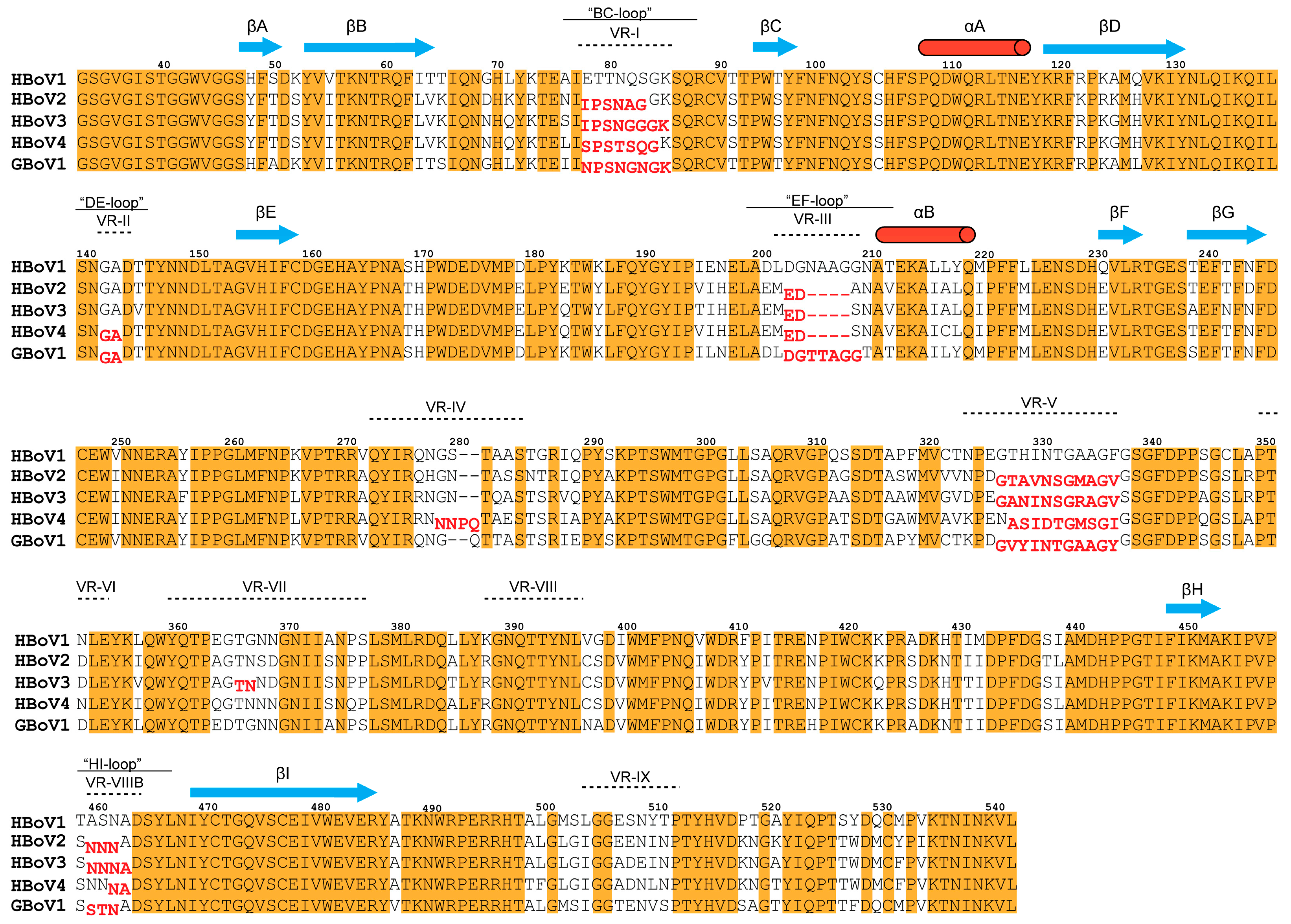
3.2. Structural Differences between GBoV1 and the HBoVs Are Localized to the Variable Regions
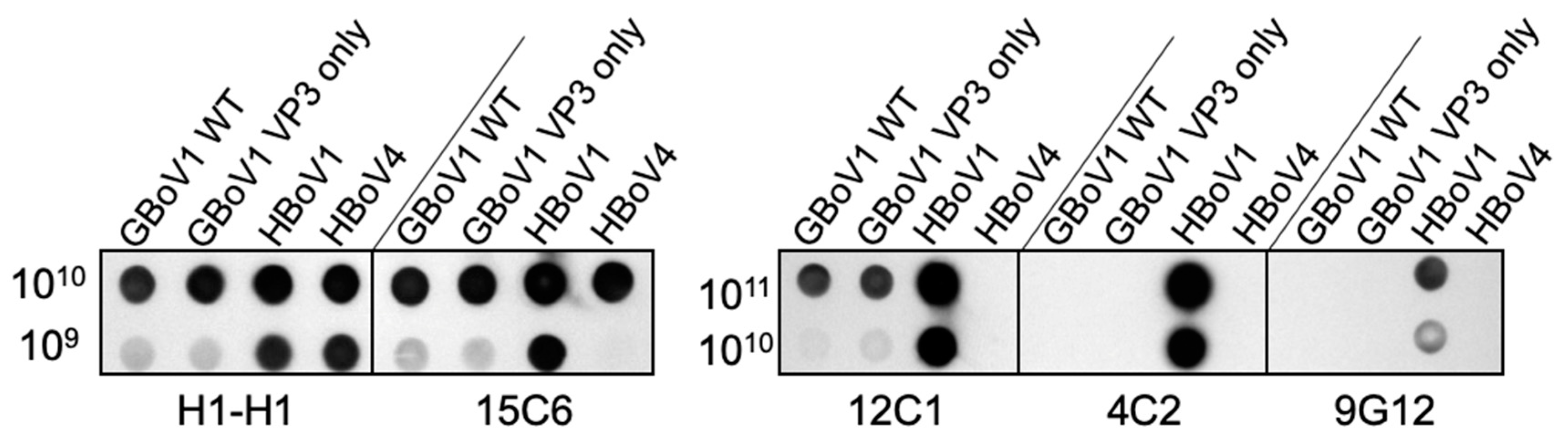
3.3. The GBoV1 Capsid Differs Antigenically to the HBoV1 Capsid
3.4. HBoV1 and GBoV1 Share Similar Rates of Seropositivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV virus taxonomy profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Bates, R.C.; Storz, J.; Reed, D.E. Isolation and comparison of bovine parvoviruses. J. Infect. Dis. 1972, 126, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Abixanti, F.R.; Warfield, M.S. Recovery of a hemadsorbing virus (HADEN) from the gastrointestinal tract of calves. Virology 1961, 14, 288–289. [Google Scholar] [CrossRef]
- Lanave, G.; Martella, V.; Farkas, S.L.; Marton, S.; Fehér, E.; Bodnar, L.; Lavazza, A.; Decaro, N.; Buonavoglia, C.; Bányai, K. Novel bocaparvoviruses in rabbits. Vet. J. 2015, 206, 131–135. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Yeung, H.C.; Li, K.S.M.; Lam, C.S.F.; Cai, J.P.; Yuen, M.C.; Wang, M.; Zheng, B.J.; Woo, P.C.Y.; Yuen, K.Y. Identification and genomic characterization of a novel rat bocavirus from brown rats in China. Infect. Genet. Evol. 2017, 47, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, F.; Xiu, L.; Liu, Y.; Yang, J.; Yao, L.; Peng, J. Identification and characterization of a novel rodent bocavirus from different rodent species in China. Emerg. Microbes Infect. 2018, 7, 48. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M.; et al. Human Bocaviruses Are Highly Diverse, Dispersed, Recombination Prone and Prevalent in Enteric Infections. J. Infect. Dis. 2010, 201, 1633–1643. [Google Scholar] [CrossRef]
- Arthur, J.L.; Higgins, G.D.; Davidson, G.P.; Givney, R.C.; Ratcliff, R.M. A Novel Bocavirus Associated with Acute Gastroenteritis in Australian Children. PLoS Pathog. 2009, 5, e1000391. [Google Scholar] [CrossRef]
- Kapoor, A.; Mehta, N.; Esper, F.; Poljsak-Prijatelj, M.; Quan, P.L.; Qaisar, N.; Delwart, E.; Lipkin, W.I. Identification and characterization of a new bocavirus species in gorillas. PLoS ONE 2010, 5, 11948. [Google Scholar] [CrossRef]
- Huang, Q.; Deng, X.; Yan, Z.; Cheng, F.; Luo, Y.; Shen, W.; Lei-Butters, D.C.M.; Chen, A.Y.; Li, Y.; Tang, L.; et al. Establishment of a Reverse Genetics System for Studying Human Bocavirus in Human Airway Epithelia. PLoS Pathog. 2012, 8, 1002899. [Google Scholar] [CrossRef]
- Dijkman, R.; Koekkoek, S.M.; Molenkamp, R.; Schildgen, O.; van der Hoek, L. Human Bocavirus Can Be Cultured in Differentiated Human Airway Epithelial Cells. J. Virol. 2009, 83, 7739–7748. [Google Scholar] [CrossRef]
- Zou, W.; Cheng, F.; Shen, W.; Engelhardt, J.F.; Yan, Z.; Qiu, J. Nonstructural Protein NP1 of Human Bocavirus 1 Plays a Critical Role in the Expression of Viral Capsid Proteins. J. Virol. 2016, 90, 4658–4669. [Google Scholar] [CrossRef]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [PubMed]
- Gurda, B.L.; Parent, K.N.; Bladek, H.; Sinkovits, R.S.; DiMattia, M.A.; Rence, C.; Castro, A.; McKenna, R.; Olson, N.; Brown, K.; et al. Human Bocavirus Capsid Structure: Insights into the Structural Repertoire of the Parvoviridae. J. Virol. 2010, 84, 5880–5889. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Pénzes, J.J.; Agbandje-Mckenna, M. Twenty-Five Years of Structural Parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef]
- Zádori, Z.; Szelei, J.; Lacoste, M.C.; Li, Y.; Gariépy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A Viral Phospholipase A2 Is Required for Parvovirus Infectivity. Dev. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef]
- Qu, X.W.; Liu, W.P.; Qi, Z.Y.; Duan, Z.J.; Zheng, L.S.; Kuang, Z.Z.; Zhang, W.J.; Hou, Y. De Phospholipase A2-like activity of human bocavirus VP1 unique region. Biochem. Biophys. Res. Commun. 2008, 365, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, S.; Lux, K.; Uhrig, S.; Kreppel, F.; Hösel, M.; Coutelle, O.; Ogris, M.; Hallek, M.; Büning, H. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology 2011, 409, 77–83. [Google Scholar] [CrossRef]
- Bleker, S.; Sonntag, F.; Kleinschmidt, J.A. Mutational Analysis of Narrow Pores at the Fivefold Symmetry Axes of Adeno-Associated Virus Type 2 Capsids Reveals a Dual Role in Genome Packaging and Activation of Phospholipase A2 Activity. J. Virol. 2005, 79, 2528–2540. [Google Scholar] [CrossRef]
- Mani, B.; Baltzer, C.; Valle, N.; Almendral, J.M.; Kempf, C.; Ros, C. Low pH-Dependent Endosomal Processing of the Incoming Parvovirus Minute Virus of Mice Virion Leads to Externalization of the VP1 N-Terminal Sequence (N-VP1), N-VP2 Cleavage and Uncoating of the Full-Length Genome. J. Virol. 2006, 80, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Garrison, J.; Ilyas, M.; Chipman, P.; McKenna, R.; Kantola, K.; Söderlund-Venermo, M.; Kučinskaitė-Kodzė, I.; Žvirblienė, A.; Agbandje-McKenna, M. Mapping Antigenic Epitopes on the Human Bocavirus Capsid. J. Virol. 2016, 90, 4670–4680. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Kailasan, S.; Garrison, J.; Ilyas, M.; Chipman, P.; Kantola, K.; Janssen, M.E.; Spear, J.; Sousa, D.; McKenna, R.; et al. Structural Insights into Human Bocaparvoviruses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Ng, R.; Agbandje-Mckenna, M. Parvoviruses: Structure and infection. Future Virol. 2012, 7, 253–278. [Google Scholar] [CrossRef]
- Luo, M.; Mietzsch, M.; Chipman, P.; Song, K.; Xu, C.; Spear, J.; Sousa, D.; McKenna, R.; Soderlund-Venermo, M.; Agbandje-Mckenna, M. pH-Induced Conformational Changes of Human Bocavirus Capsids. J. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Halder, S.; Gurda, B.; Bladek, H.; Chipman, P.R.; McKenna, R.; Brown, K.; Agbandje-McKenna, M. Structure of an Enteric Pathogen, Bovine Parvovirus. J. Virol. 2015, 89, 2603–2614. [Google Scholar] [CrossRef]
- Deng, X.; Yan, Z.; Luo, Y.; Xu, J.; Cheng, F.; Li, Y.; Engelhardt, J.F.; Qiu, J. In Vitro Modeling of Human Bocavirus 1 Infection of Polarized Primary Human Airway Epithelia. J. Virol. 2013, 87, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Keiser, N.W.; Song, Y.; Deng, X.; Cheng, F.; Qiu, J.; Engelhardt, J.F. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol. Ther. 2013, 21, 2181–2194. [Google Scholar] [CrossRef]
- Aitken, M.L.; Moss, R.B.; Waltz, D.A.; Dovey, M.E.; Tonelli, M.R.; McNamara, S.C.; Gibson, R.L.; Ramsey, B.W.; Carter, B.J.; Reynolds, T.C. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001, 12, 1907–1916. [Google Scholar] [CrossRef]
- Moss, R.B.; Milla, C.; Colombo, J.; Accurso, F.; Zeitlin, P.L.; Clancy, J.P.; Spencer, L.T.; Pilewski, J.; Waltz, D.A.; Dorkin, H.L.; et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: A randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 2007, 18, 726–732. [Google Scholar] [CrossRef]
- Mueller, C.; Flotte, T.R. Gene therapy for cystic fibrosis. Clin. Rev. Allergy Immunol. 2008, 35, 164–178. [Google Scholar] [CrossRef]
- Yan, Z.; Zou, W.; Feng, Z.; Shen, W.; Park, S.Y.; Deng, X.; Qiu, J.; Engelhardt, J.F. Establishment of a High-Yield Recombinant Adeno-Associated Virus/Human Bocavirus Vector Production System Independent of Bocavirus Nonstructural Proteins. Hum. Gene Ther. 2019, 30, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Fakhiri, J.; Schneider, M.A.; Puschhof, J.; Stanifer, M.; Schildgen, V.; Holderbach, S.; Voss, Y.; El Andari, J.; Schildgen, O.; Boulant, S.; et al. Novel Chimeric Gene Therapy Vectors Based on Adeno-Associated Virus and Four Different Mammalian Bocaviruses. Mol. Ther. Methods Clin. Dev. 2019, 12, 202–222. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Kruse, S.; Kant, R.; Venkatakrishnan, B.; Movahed, N.; Brooke, D.; Lins, B.; Bennett, A.; Potter, T.; McKenna, R.; et al. Comparative Analysis of Adeno-Associated Virus Capsid Stability and Dynamics. J. Virol. 2013, 87, 13150–13160. [Google Scholar] [CrossRef]
- Invitrogen Bac-to-Bac® Baculovirus Expression System. Manual 2015, 1–78. [CrossRef]
- Li, X.; Kantola, K.; Hedman, L.; Arku, B.; Hedman, K.; Söderlund-Venermo, M. Original antigenic sin with human bocaviruses 1–4. J. Gen. Virol. 2015, 96, 3099–3108. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Palovcak, E.; Armache, J.P.; Verba, K.A.; Cheng, Y.; Agard, D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.; Rohou, A.; Grigorieff, N. CisTEM, user-friendly software for single-particle image processing. Elife 2018, 7. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Ho, P.T.; Montiel-Garcia, D.J.; Wong, J.J.; Carrillo-Tripp, M.; Brooks, C.L.; Johnson, J.E.; Reddy, V.S. VIPERdb: A Tool for Virus Research. Annu. Rev. Virol. 2018, 477–488. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera - A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Rossmann, M.G. Interpretation of electron density with stereographic roadmap projections. J. Struct. Biol. 2007, 158, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, A.; Matthies, D.; Banerjee, S.; Merk, A.; Subramaniam, S. Structure of β-galactosidase at 3.2-Å resolution obtained by cryo-electron microscopy. Proc. Natl. Acad. Sci. USA 2014, 111, 11709–11714. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System 2020. Available online: https://pymol.org/2/ (accessed on 25 January 2021).
- Gurda, B.L.; DiMattia, M.A.; Miller, E.B.; Bennett, A.; McKenna, R.; Weichert, W.S.; Nelson, C.D.; Chen, W.-j.; Muzyczka, N.; Olson, N.H.; et al. Capsid Antibodies to Different Adeno-Associated Virus Serotypes Bind Common Regions. J. Virol. 2013, 87, 9111–9124. [Google Scholar] [CrossRef]
- DiPrimio, N.; Asokan, A.; Govindasamy, L.; Agbandje-McKenna, M.; Samulski, R.J. Surface Loop Dynamics in Adeno-Associated Virus Capsid Assembly. J. Virol. 2008, 82, 5178–5189. [Google Scholar] [CrossRef] [PubMed]
- Fakhiri, J.; Linse, K.-P.; Mietzsch, M.; Xu, M.; Schneider, M.A.; Meister, M.; Schildgen, O.; Schnitzler, P.; Soderlund-Venermo, M.; Agbandje-McKenna, M.; et al. Impact of Natural or Synthetic Singletons in the Capsid of Human Bocavirus 1 on Particle Infectivity and Immunoreactivity. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Kantola, K.; Hedman, L.; Arthur, J.; Alibeto, A.; Delwart, E.; Jartti, T.; Ruuskanen, O.; Hedman, K.; Söderlund-Venermo, M. Seroepidemiology of human bocaviruses 1-4. J. Infect. Dis. 2011, 204, 1403–1412. [Google Scholar] [CrossRef]
- Benveniste, O.; Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Montus, M.F.; Masurier, C. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1,2,5,6,8 and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef] [PubMed]
| Parameter | GBoV1 |
|---|---|
| Total no. of micrographs | 1411 |
| Defocus range (μm) | 1.08–3.19 |
| Electron dose (e−/Å2) | 60 |
| No. of frames/micrograph | 50 |
| Pixel size (Å/pixel) | 1.08 |
| No. of capsids used for final map | 168,565 |
| Resolution of final map (Å) | 2.76 |
| PHENIX model refinement statistics | |
| Residue range | 33–542 |
| Map CC | 0.877 |
| RMSD (Å) | |
| Bonds | 0.01 |
| Angles | 0.89 |
| All-atom clash score | 10.61 |
| Ramachandran plot (%) | |
| Favored | 98.4 |
| Allowed | 1.6 |
| Outliers | 0.0 |
| Rotamer outliers | 0.0 |
| No. of Cβ deviations | 0 |
| HBoV1 | HBoV2 | HBoV3 | HBoV4 | GBoV1 | |
|---|---|---|---|---|---|
| HBoV1 | 94.7 | 93.9 | 93.8 | 94.1 | |
| HBoV2 | 77.5 | 98.8 | 97.4 | 98.2 | |
| HBoV3 | 77.2 | 89.2 | 97.3 | 96.5 | |
| HBoV4 | 77.2 | 88.7 | 90.2 | 95.3 | |
| GBoV1 | 86.3 | 79.7 | 79.9 | 79.4 |
| VR-I | VR-III | VR-V | VR-II | VR-VIIIB | VR-IV | VR-VI | VR-VII | VR-VIII | VR-IX | |
|---|---|---|---|---|---|---|---|---|---|---|
| HBoV1 vs. HBoV2 | 3.1 | 3.1 | 2.9 | 1.9 | 1.8 | 1.1 | 0.7 | 1.1 | 0.7 | 1.0 |
| HBoV1 vs. HBoV3 | 2.9 | 3.2 | 3.6 | 1.3 | 2.9 | 0.9 | 0.4 | 1.3 | 0.6 | 1.3 |
| HBoV1 vs. HBoV4 | 2.8 | 3.3 | 3.0 | 2.6 | 2.5 | 2.1 | 1.1 | 1.2 | 1.2 | 1.1 |
| HBoV1 vs. GBoV1 | 3.3 | 4.5 | 3.0 | 2.1 | 2.5 | 0.9 | 0.4 | 1.1 | 0.7 | 1.1 |
| HBoV2 vs. HBoV3 | 1.8 | 1.0 | 1.5 | 1.4 | 1.6 | 0.8 | 0.8 | 0.8 | 0.9 | 0.6 |
| HBoV2 vs. HBoV4 | 2.9 | 1.2 | 1.5 | 1.2 | 1.6 | 1.6 | 0.5 | 0.8 | 1.1 | 0.7 |
| HBoV2 vs. GBoV1 | 0.8 | 3.4 | 1.2 | 0.6 | 0.6 | 0.9 | 0.6 | 0.7 | 0.7 | 0.2 |
| HBoV3 vs. HBoV4 | 2.9 | 0.9 | 1.5 | 1.4 | 1.3 | 2.0 | 0.7 | 0.9 | 1.3 | 0.6 |
| HBoV3 vs. GBoV1 | 2.0 | 3.3 | 1.8 | 1.8 | 1.7 | 0.9 | 0.5 | 0.9 | 0.9 | 0.8 |
| HBoV4 vs. GBoV1 | 3.2 | 3.8 | 1.2 | 0.9 | 1.5 | 1.8 | 0.6 | 0.8 | 0.9 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.C.; Mietzsch, M.; Singh, A.; Jimenez Ybargollin, A.; Kailasan, S.; Chipman, P.; Bhattacharya, N.; Fakhiri, J.; Grimm, D.; Kapoor, A.; et al. Characterization of the GBoV1 Capsid and Its Antibody Interactions. Viruses 2021, 13, 330. https://doi.org/10.3390/v13020330
Yu JC, Mietzsch M, Singh A, Jimenez Ybargollin A, Kailasan S, Chipman P, Bhattacharya N, Fakhiri J, Grimm D, Kapoor A, et al. Characterization of the GBoV1 Capsid and Its Antibody Interactions. Viruses. 2021; 13(2):330. https://doi.org/10.3390/v13020330
Chicago/Turabian StyleYu, Jennifer Chun, Mario Mietzsch, Amriti Singh, Alberto Jimenez Ybargollin, Shweta Kailasan, Paul Chipman, Nilakshee Bhattacharya, Julia Fakhiri, Dirk Grimm, Amit Kapoor, and et al. 2021. "Characterization of the GBoV1 Capsid and Its Antibody Interactions" Viruses 13, no. 2: 330. https://doi.org/10.3390/v13020330
APA StyleYu, J. C., Mietzsch, M., Singh, A., Jimenez Ybargollin, A., Kailasan, S., Chipman, P., Bhattacharya, N., Fakhiri, J., Grimm, D., Kapoor, A., Kučinskaitė-Kodzė, I., Žvirblienė, A., Söderlund-Venermo, M., McKenna, R., & Agbandje-McKenna, M. (2021). Characterization of the GBoV1 Capsid and Its Antibody Interactions. Viruses, 13(2), 330. https://doi.org/10.3390/v13020330







