Control of Capsid Transformations during Reovirus Entry
Abstract
:1. Introduction
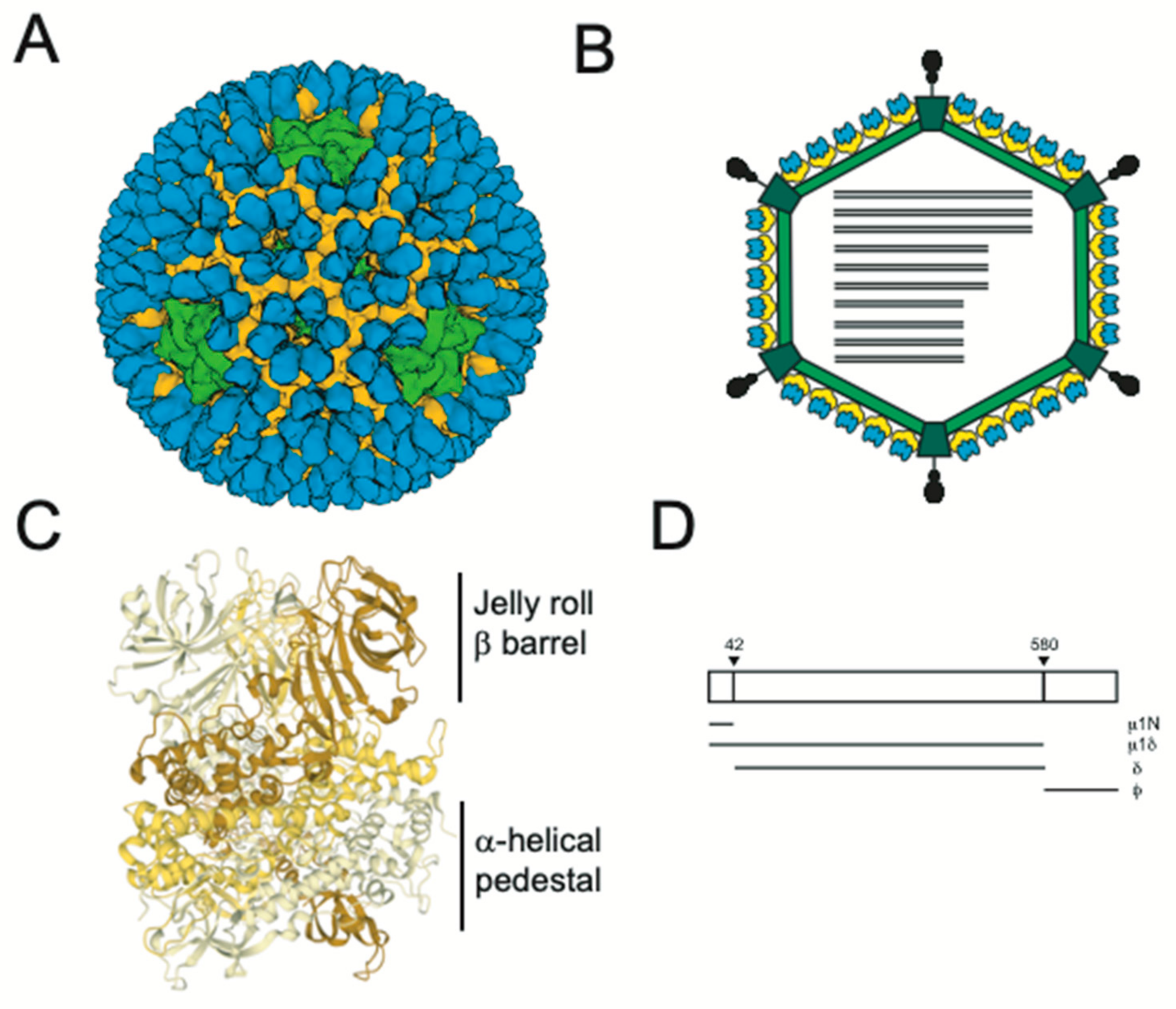
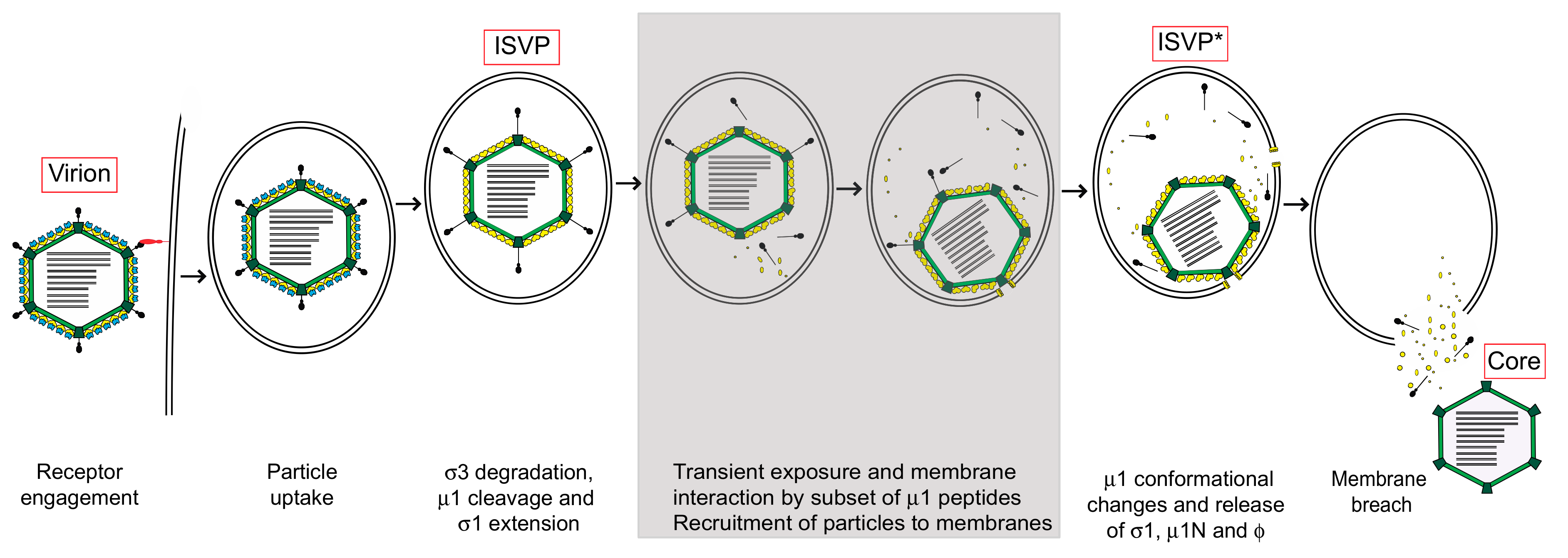
2. Structural Changes Required for Membrane Penetration Are Controlled by the μ1 Outer Capsid Protein
3. Target Membranes Control Structural Transitions in the Virus Particle
4. Role for Other Reovirus Capsid Proteins in Controlling Conformational Changes that Precede Cell Penetration
4.1. Role for σ1 and λ2
4.2. Role for σ2 and λ1
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dermody, T.S.; Parker, J.C.; Sherry, B. Orthoreoviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, pp. 1304–1346. [Google Scholar]
- Reinisch, K.M.; Nibert, M.L.; Harrison, S.C. Structure of the reovirus core at 3.6? Å resolution. Nat. Cell Biol. 2000, 404, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, Y.; Zhang, L.; Harrison, S.C.; Marinescu, D.C.; Nibert, M.L.; Baker, T.S. Features of reovirus outer capsid protein m1 revealed by electron cryomicroscopy and image reconstruction of the virion at 7.0 Å resolution. Structure 2005, 13, 1545–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Dryden, K.; Wang, G.; Yeager, M.; Nibert, M.L.; Coombs, K.M.; Furlong, D.B.; Fields, B.N.; Baker, T.S. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: Analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 1993, 122, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Mainou, B.A.; Dermody, T.S. In Search of Cathepsins: How Reovirus Enters Host Cells. DNA Cell Biol. 2012, 31, 1646–1649. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.N.; Aravamudhan, P.; De Castro, I.F.; Tenorio, R.; Risco, C.; Dermody, T.S. Ins and Outs of Reovirus: Vesicular Trafficking in Viral Entry and Egress. Trends Microbiol. 2020. [Google Scholar] [CrossRef]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Genet. 2014, 12, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Barton, E.S.; Forrest, J.; Connolly, J.L.; Chappell, J.D.; Liu, Y.; Schnell, F.J.; Nusrat, A.; Parkos, C.A.; Dermody, T.S. Junction Adhesion Molecule Is a Receptor for Reovirus. Cell 2001, 104, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.A.; Schelling, P.; Wetzel, J.D.; Johnson, E.M.; Forrest, J.C.; Wilson, G.A.R.; Aurrand-Lions, M.; Imhof, B.A.; Stehle, T.; Dermody, T.S. Junctional Adhesion Molecule A Serves as a Receptor for Prototype and Field-Isolate Strains of Mammalian Reovirus. J. Virol. 2005, 79, 7967–7978. [Google Scholar] [CrossRef] [Green Version]
- Konopka-Anstadt, J.L.; Mainou, B.A.; Sutherland, D.M.; Sekine, Y.; Strittmatter, S.M.; Dermody, T.S. The Nogo Receptor NgR1 Mediates Infection by Mammalian Reovirus. Cell Host Microbe 2014, 15, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Mainou, B.A.; Dermody, T.S. Transport to Late Endosomes Is Required for Efficient Reovirus Infection. J. Virol. 2012, 86, 8346–8358. [Google Scholar] [CrossRef] [Green Version]
- Maginnis, M.S.; Forrest, J.C.; Kopecky-Bromberg, S.A.; Dickeson, S.K.; Santoro, S.A.; Zutter, M.M.; Nemerow, G.R.; Bergelson, J.M.; Dermody, T.S. Beta1 integrin mediates internalization of mammalian reovirus. J. Virol. 2006, 80, 2760–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maginnis, M.S.; Mainou, B.A.; Derdowski, A.; Johnson, E.M.; Zent, R.; Dermody, T.S. NPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entry. J. Virol. 2008, 82, 3181–3191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravamudhan, P.; Raghunathan, K.; Konopka-Anstadt, J.L.; Pathak, A.; Sutherland, D.M.; Carter, B.D.; Dermody, T.S. Reovirus uses macropinocytosis-mediated entry and fast axonal transport to infect neurons. PLOS Pathog. 2020, 16, e1008380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, D.H.; Deussing, J.; Peters, C.; Dermody, T.S. Cathepsin L and Cathepsin B Mediate Reovirus Disassembly in Murine Fibroblast Cells. J. Biol. Chem. 2002, 277, 24609–24617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibert, M.L.; Fields, B.N. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J. Virol. 1992, 66, 6408–6418. [Google Scholar] [CrossRef] [Green Version]
- Bodkin, D.K.; Nibert, M.L.; Fields, B.N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 1989, 63, 4676–4681. [Google Scholar] [CrossRef] [Green Version]
- Bass, D.M.; Bodkin, D.; Dambrauskas, R.; Trier, J.S.; Fields, B.N.; Wolf, J.L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J. Virol. 1990, 64, 1830–1833. [Google Scholar] [CrossRef] [Green Version]
- Nygaard, R.M.; Golden, J.W.; Schiff, L.A. Impact of Host Proteases on Reovirus Infection in the Respiratory Tract. J. Virol. 2011, 86, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Liemann, S.; Chandran, K.; Baker, T.S.; Nibert, M.L.; Harrison, S.C. Structure of the Reovirus Membrane-Penetration Protein, μ1, in a Complex with Its Protector Protein, σ3. Cell 2002, 108, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Chandran, K.; Farsetta, D.L.; Nibert, M.L. Strategy for nonenveloped virus entry: A hydrophobic conformer of the reovirus membrane penetration protein m1 mediates membrane disruption. J. Virol. 2002, 76, 9920–9933. [Google Scholar] [CrossRef] [Green Version]
- Chandran, K.; Parker, J.S.; Ehrlich, M.; Kirchhausen, T.; Nibert, M.L. The delta region of outer-capsid protein m1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 2003, 77, 13361–13375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovic, T.; Agosto, M.A.; Zhang, L.; Chandran, K.; Harrison, S.C.; Nibert, M.L. Peptides released from reovirus outer capsid form membrane pores that recruit virus particles. EMBO J. 2008, 27, 1289–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Agosto, M.A.; Ivanovic, T.; King, D.S.; Nibert, M.L.; Harrison, S.C. Requirements for the formation of membrane pores by the reovirus myristoylated micro1N peptide. J. Virol. 2009, 83, 7004–7014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Chandran, K.; Nibert, M.L.; Harrison, S.C. Reovirus m1 structural rearrangements that mediate membrane penetration. J. Virol. 2006, 80, 12367–12376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, J.K.; Severson, T.F.; Chandran, K.; Gillian, A.L.; Yin, J.; Nibert, M.L. Thermostability of reovirus disassembly intermediates (ISVPs) correlates with genetic, biochemical, and thermodynamic properties of major surface protein mu1. J. Virol. 2002, 76, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Snyder, A.J.; Wang, J.C.-Y.; Danthi, P. Components of the Reovirus Capsid Differentially Contribute to Stability. J. Virol. 2018, 93, e01894-18. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, P.; Danthi, P. Determinants of Strain-Specific Differences in Efficiency of Reovirus Entry. J. Virol. 2010, 84, 12723–12732. [Google Scholar] [CrossRef] [Green Version]
- Madren, J.A.; Sarkar, P.; Danthi, P. Cell Entry-Associated Conformational Changes in Reovirus Particles Are Controlled by Host Protease Activity. J. Virol. 2012, 86, 3466–3473. [Google Scholar] [CrossRef] [Green Version]
- Thete, D.; Danthi, P. Conformational changes required for reovirus cell entry are sensitive to pH. Virology 2015, 483, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Snyder, A.J.; Danthi, P. Lipids Cooperate with the Reovirus Membrane Penetration Peptide to Facilitate Particle Uncoating. J. Biol. Chem. 2016, 291, 26773–26785. [Google Scholar] [CrossRef] [Green Version]
- Agosto, M.A.; Myers, K.S.; Ivanovic, T.; Nibert, M.L. A positive-feedback mechanism promotes reovirus particle conversion to the intermediate associated with membrane penetration. Proc. Natl. Acad. Sci. USA 2008, 105, 10571–10576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, J.K.; Agosto, M.A.; Severson, T.F.; Yin, J.; Nibert, M.L. Thermostabilizing mutations in reovirus outer-capsid protein m1 selected by heat inactivation of infectious subvirion particles. Virology 2007, 361, 412–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danthi, P.; Kobayashi, T.; Holm, G.H.; Hansberger, M.W.; Abel, T.W.; Dermody, T.S. Reovirus Apoptosis and Virulence Are Regulated by Host Cell Membrane Penetration Efficiency. J. Virol. 2007, 82, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, P.; Danthi, P. The mu1 72-96 loop controls conformational transitions during reovirus cell entry. J. Virol. 2013, 87, 13532–13542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, A.J.; Danthi, P. The reovirus mu1 340-343 loop controls entry related conformational changes. J. Virol. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danthi, P.; Coffey, C.M.; Parker, J.S.; Abel, T.W.; Dermody, T.S. Independent regulation of reovirus membrane penetration and apoptosis by the mu1 phi domain. PLoS Pathog. 2008, 4, e1000248. [Google Scholar] [CrossRef]
- Gummersheimer, S.L.; Danthi, P. Reovirus core proteins lambda1 and sigma2 promote stability of disassembly intermediates and influence early replication events. J. Virol. 2020. [Google Scholar] [CrossRef]
- Agosto, M.A.; Middleton, J.K.; Freimont, E.C.; Yin, J.; Nibert, M.L. Thermolabilizing pseudoreversions in reovirus outer-capsid protein micro 1 rescue the entry defect conferred by a thermostabilizing mutation. J. Virol. 2007, 81, 7400–7409. [Google Scholar] [CrossRef] [Green Version]
- Helenius, A. Virus entry and uncoating. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 99–118. [Google Scholar]
- Lozach, P.-Y.; Huotari, J.; Helenius, A. Late-penetrating viruses. Curr. Opin. Virol. 2011, 1, 35–43. [Google Scholar] [CrossRef]
- Snyder, A.J.; Danthi, P. Lipid Membranes Facilitate Conformational Changes Required for Reovirus Cell Entry. J. Virol. 2016, 90, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Agosto, M.A.; Ivanovic, T.; Nibert, M.L. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc. Natl. Acad. Sci. USA 2006, 103, 16496–16501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bothner, B.; Dong, X.F.; Bibbs, L.; Johnson, J.E.; Siuzdak, G. Evidence of Viral Capsid Dynamics Using Limited Proteolysis and Mass Spectrometry. J. Biol. Chem. 1998, 273, 673–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yafal, A.G.; Lee, Y.M.; Hogle, J.; Chow, M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J. Virol. 1994, 68, 3965–3970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.B.; Smith, H.Q.; Smith, T. The Dynamic Life of Virus Capsids. Viruses 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Dowd, K.A.; Post, C.B.; Pierson, T.C. Shake, rattle, and roll: Impact of the dynamics of flavivirus particles on their interactions with the host. Virology 2015, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Luisoni, S.; Suomalainen, M.; Boucke, K.; Tanner, L.B.; Wenk, M.R.; Guan, X.L.; Grzybek, M.; Coskun, Ünal; Greber, U.F. Co-option of Membrane Wounding Enables Virus Penetration into Cells. Cell Host Microbe 2015, 18, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Mohl, B.-P.; Roy, P. Entry of Bluetongue Virus Capsid Requires the Late Endosome-specific Lipid Lysobisphosphatidic Acid. J. Biol. Chem. 2016, 291, 12408–12419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Patel, A.; Celma, C.C.; Yu, X.; Roy, P.; Zhou, Z.H. Atomic model of a nonenveloped virus reveals pH sensors for a coordinated process of cell entry. Nat. Struct. Mol. Biol. 2016, 23, 74–80. [Google Scholar] [CrossRef]
- Wu, W.; Celma, C.C.; Kerviel, A.; Roy, P. Mapping the pH Sensors Critical for Host Cell Entry by a Complex Nonenveloped Virus. J. Virol. 2018, 93, e01897-18. [Google Scholar] [CrossRef] [Green Version]
- Arias, C.F.; Silva-Ayala, D.; López, S. Rotavirus Entry: A Deep Journey into the Cell with Several Exits. J. Virol. 2014, 89, 890–893. [Google Scholar] [CrossRef] [Green Version]
- Abdelhakim, A.H.; Salgado, E.N.; Fu, X.; Pasham, M.; Nicastro, D.; Kirchhausen, T.; Harrison, S.C. Structural Correlates of Rotavirus Cell Entry. PLOS Pathog. 2014, 10, e1004355. [Google Scholar] [CrossRef] [PubMed]
- Salgado, E.N.; Rodriguez, B.G.; Narayanaswamy, N.; Krishnan, Y.; Harrison, S.C. Visualization of Calcium Ion Loss from Rotavirus during Cell Entry. J. Virol. 2018, 92, e01327-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.S.; Trask, S.D.; Babyonyshev, M.; Dormitzer, P.R.; Harrison, S.C. Effect of Mutations in VP5* Hydrophobic Loops on Rotavirus Cell Entry. J. Virol. 2010, 84, 6200–6207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoder, J.D.; Trask, S.D.; Vo, P.T.; Binka, M.; Feng, N.; Harrison, S.C.; Greenberg, H.B.; Dormitzer, P.R. VP5* Rearranges when Rotavirus Uncoats. J. Virol. 2009, 83, 11372–11377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R. Fusogenic Reoviruses and Their Fusion-Associated Small Transmembrane (FAST) Proteins. Annu. Rev. Virol. 2019, 6, 341–363. [Google Scholar] [CrossRef]
- Kanai, Y.; Kawagishi, T.; Sakai, Y.; Nouda, R.; Shimojima, M.; Saijo, M.; Matsuura, Y.; Kobayashi, T. Cell–cell fusion induced by reovirus FAST proteins enhances replication and pathogenicity of non-enveloped dsRNA viruses. PLOS Pathog. 2019, 15, e1007675. [Google Scholar] [CrossRef]
- Furlong, D.B.; Nibert, M.L.; Fields, B.N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 1988, 62, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Thete, D.; Danthi, P. Protein Mismatches Caused by Reassortment Influence Functions of the Reovirus Capsid. J. Virol. 2018, 92, 20. [Google Scholar] [CrossRef] [Green Version]
- Dadonaite, B.; Vijayakrishnan, S.; Fodor, E.; Bhella, D.; Hutchinson, E.C. Filamentous influenza viruses. J. Gen. Virol. 2016, 97, 1755–1764. [Google Scholar] [CrossRef]
- Murphy, F.A.; Whitfield, S.G. Morphology and morphogenesis of arenaviruses*. Bull. World Health Organ. 1975, 52, 409–419. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gummersheimer, S.L.; Snyder, A.J.; Danthi, P. Control of Capsid Transformations during Reovirus Entry. Viruses 2021, 13, 153. https://doi.org/10.3390/v13020153
Gummersheimer SL, Snyder AJ, Danthi P. Control of Capsid Transformations during Reovirus Entry. Viruses. 2021; 13(2):153. https://doi.org/10.3390/v13020153
Chicago/Turabian StyleGummersheimer, Stephanie L., Anthony J. Snyder, and Pranav Danthi. 2021. "Control of Capsid Transformations during Reovirus Entry" Viruses 13, no. 2: 153. https://doi.org/10.3390/v13020153
APA StyleGummersheimer, S. L., Snyder, A. J., & Danthi, P. (2021). Control of Capsid Transformations during Reovirus Entry. Viruses, 13(2), 153. https://doi.org/10.3390/v13020153





