Idiopathic Chronic Diarrhea in Rhesus Macaques Is Not Associated with Enteric Viral Infections
Abstract
:1. Introduction
2. Materials and Methods
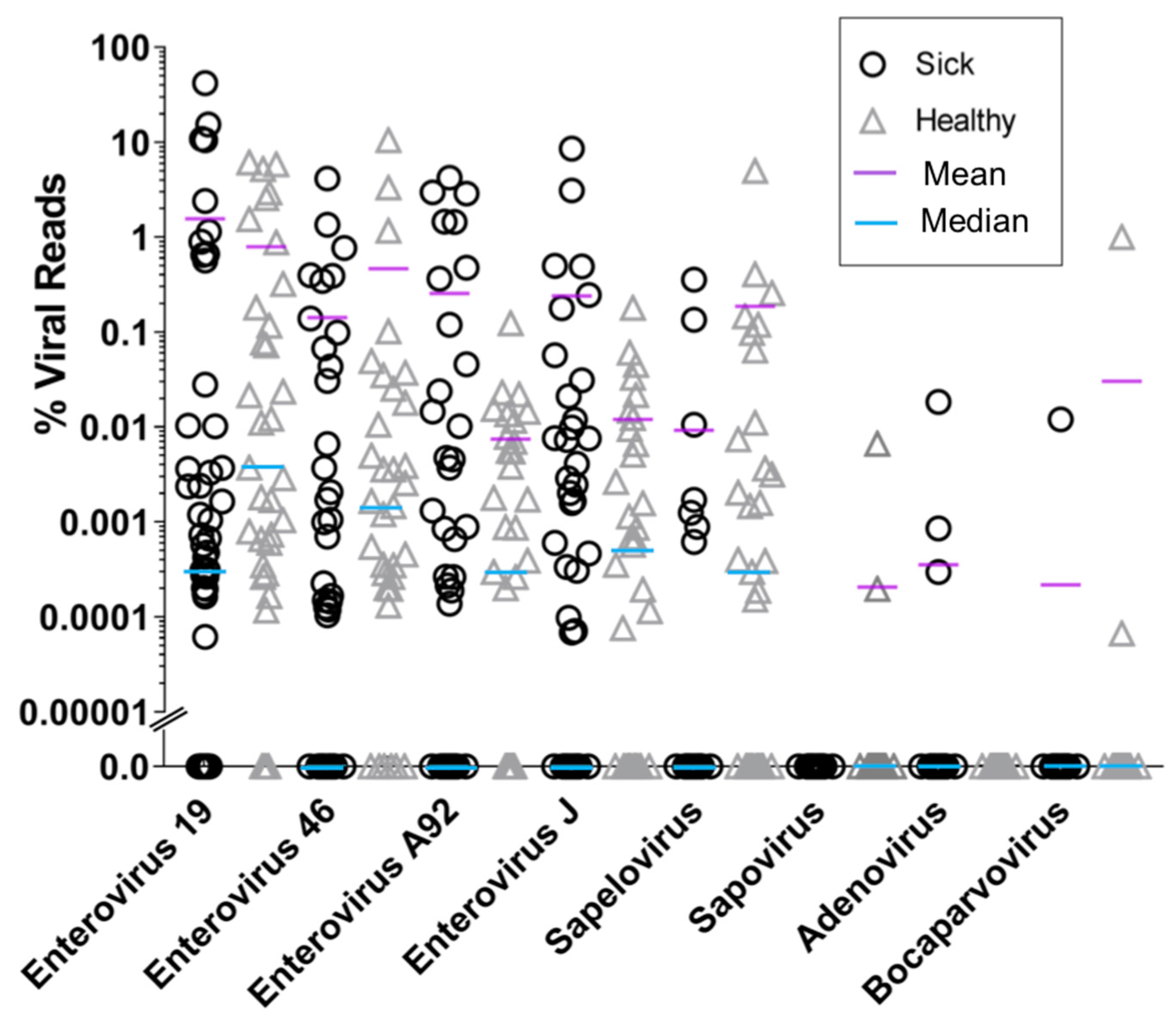
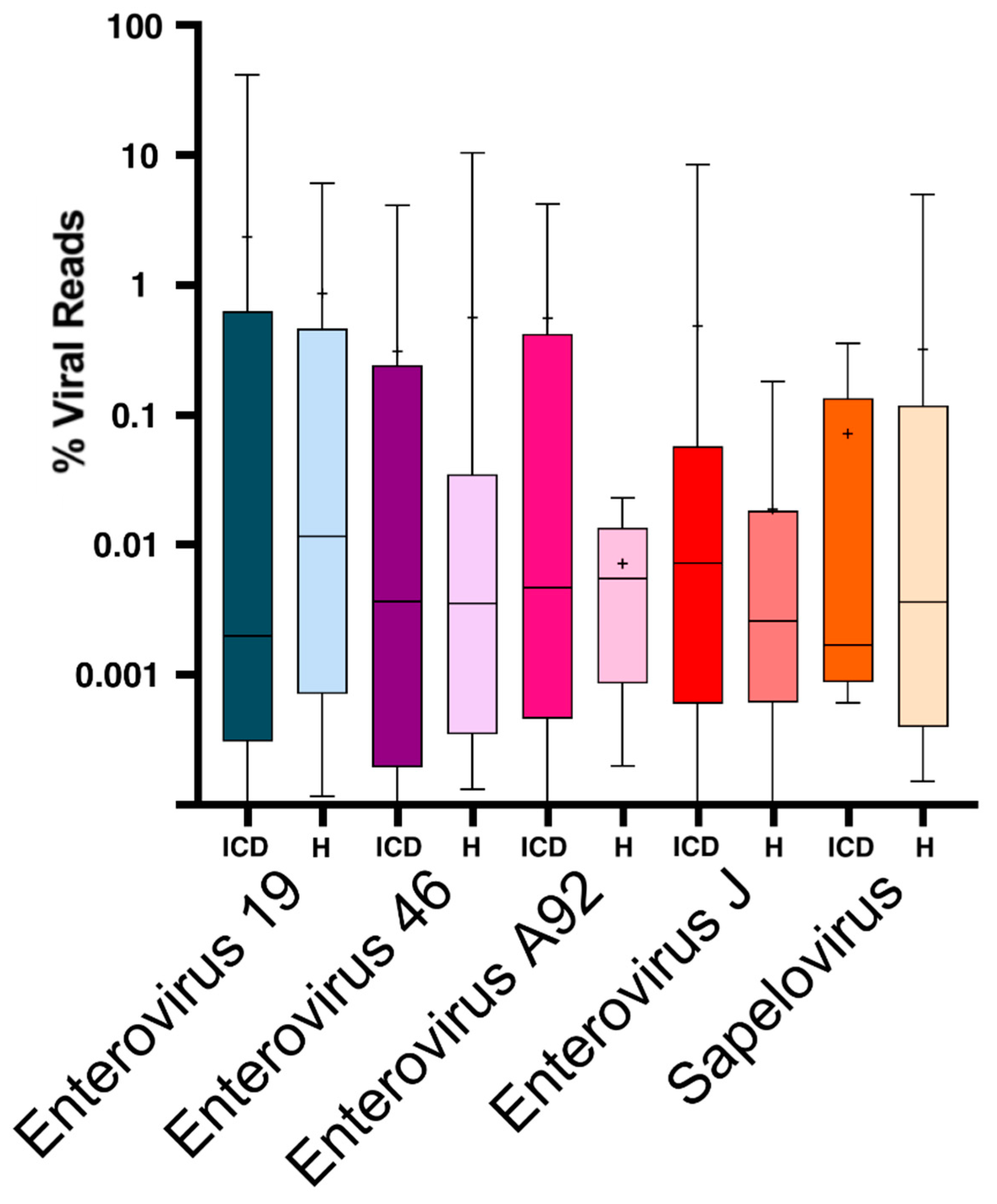
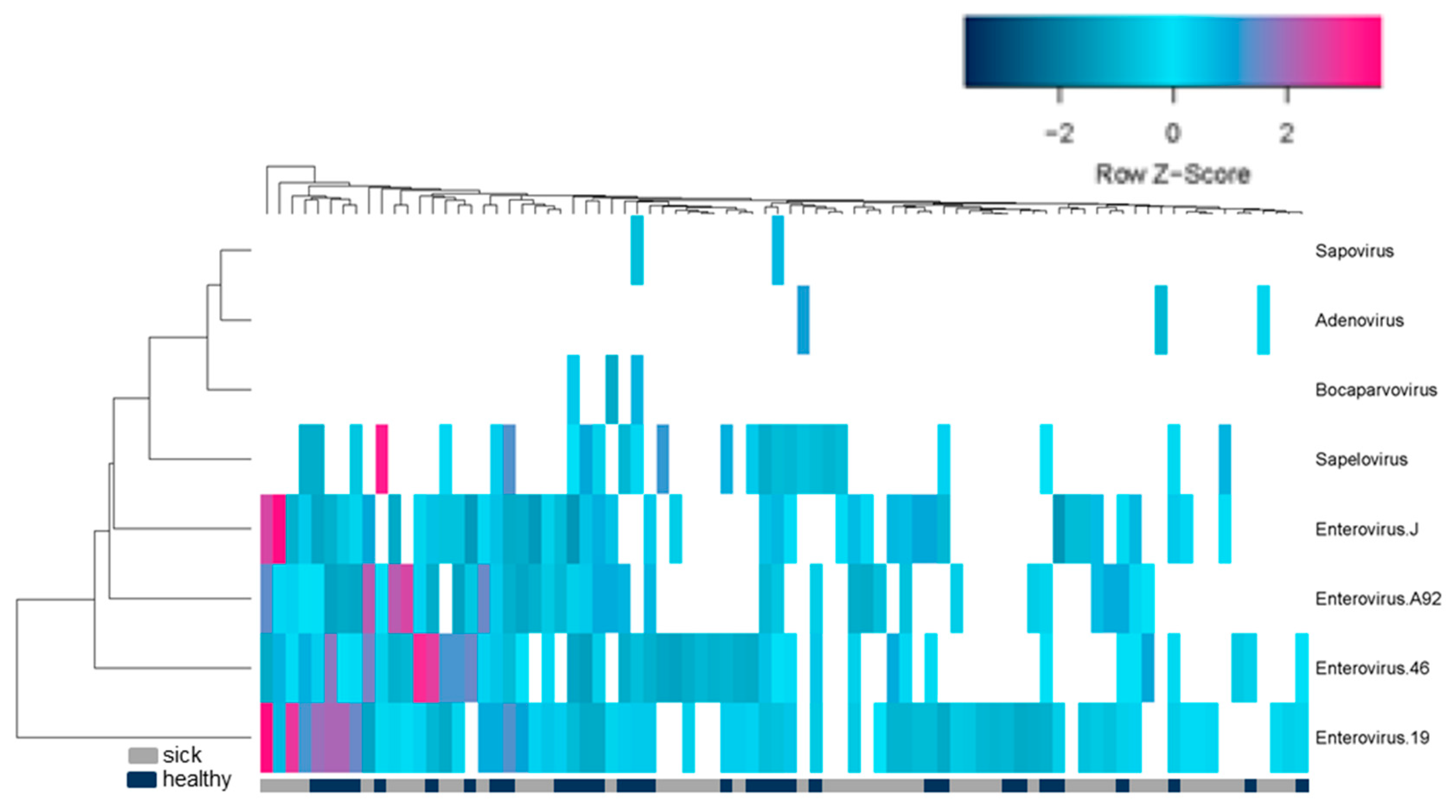
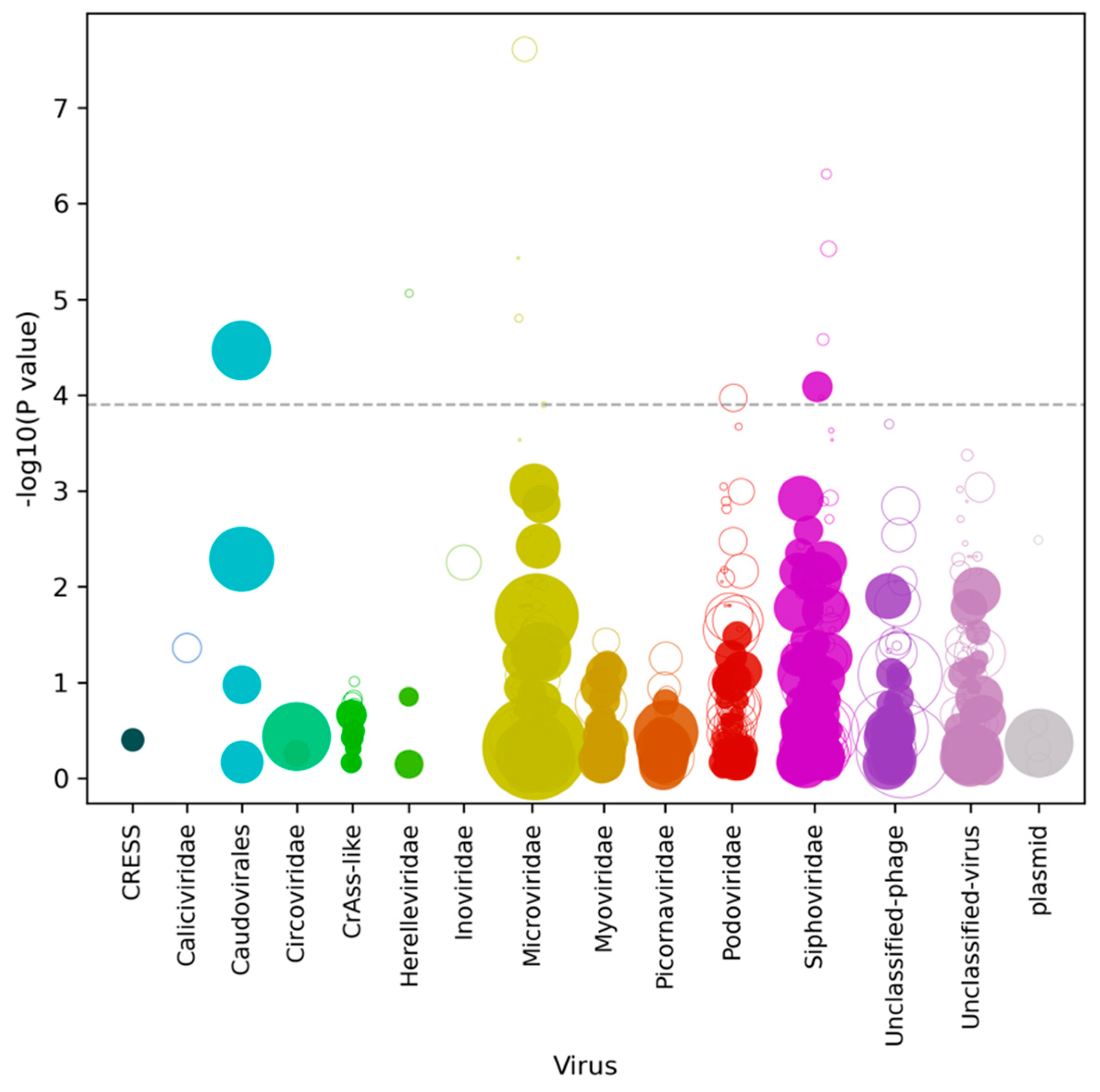
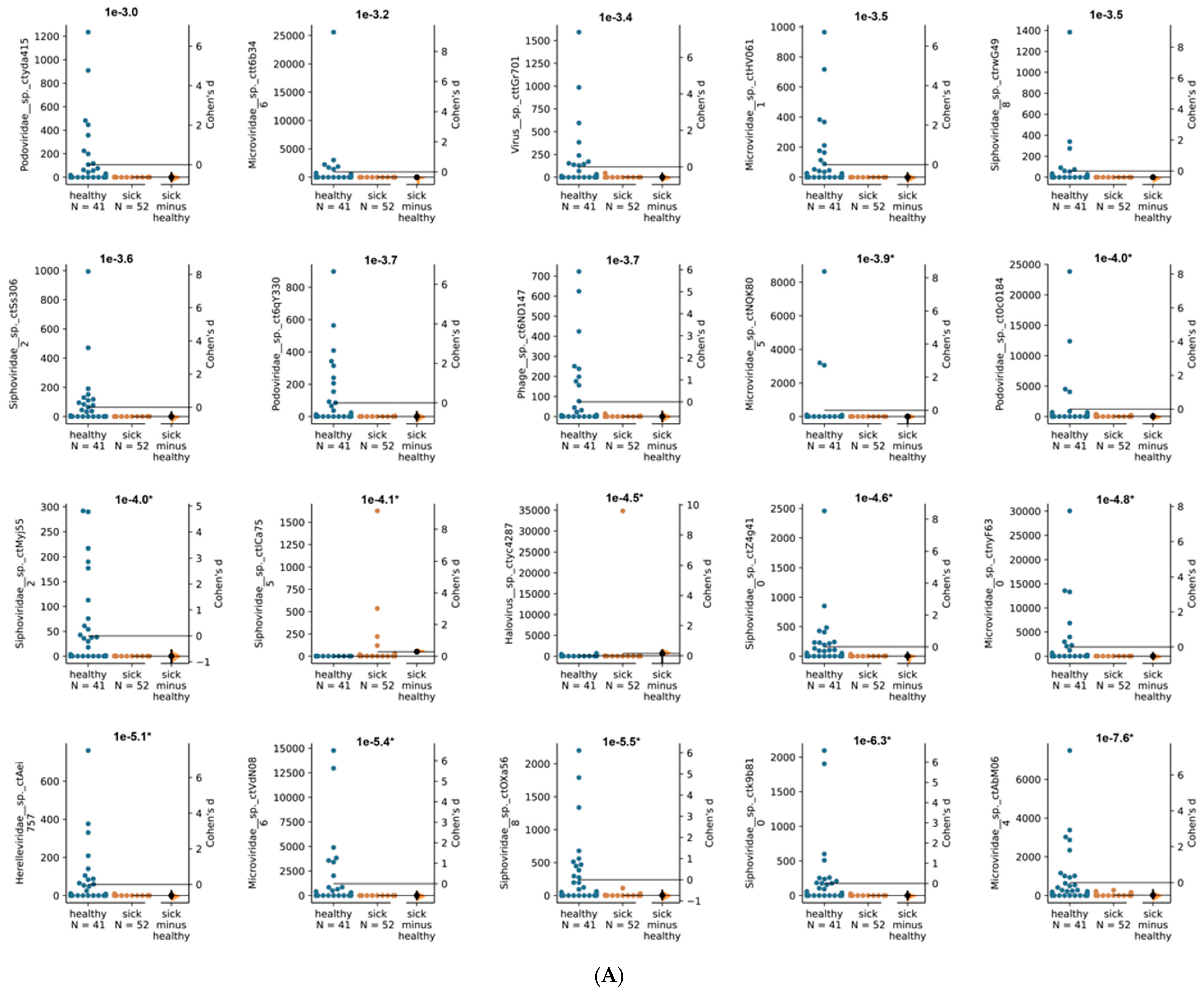
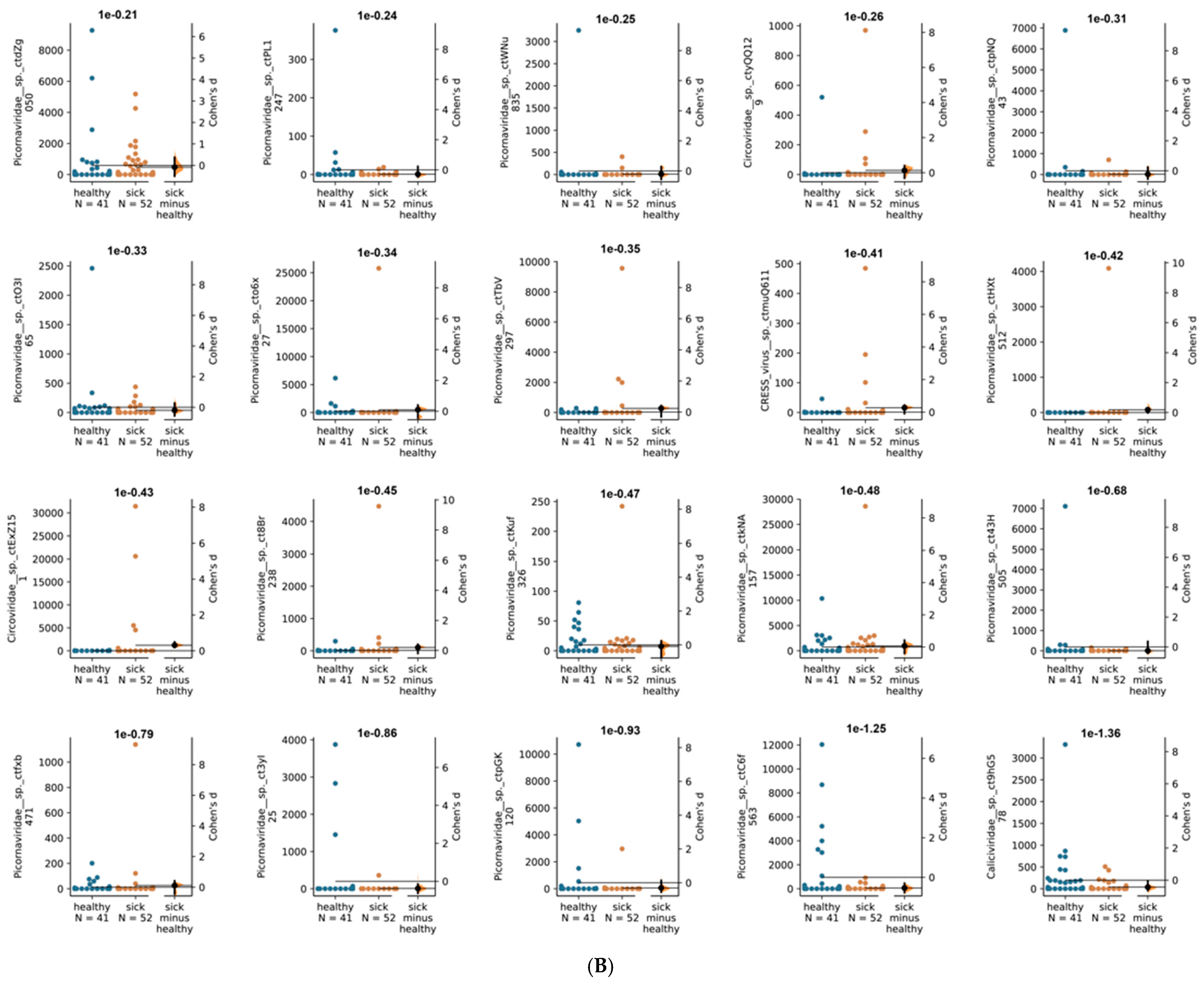
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prongay, K.; Park, B.; Murphy, S.J. Risk factor analysis may provide clues to diarrhea prevention in outdoor-housed rhesus macaques (Macaca mulatta). Am. J. Primatol. 2013, 75, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Sestak, K.; Merritt, C.K.; Borda, J.; Saylor, E.; Schwamberger, S.R.; Cogswell, F.; Didier, E.S.; Didier, P.J.; Plauche, G.; Bohm, R.P.; et al. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect. Immun. 2003, 71, 4079–4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingle, S.B.; Adgaonkar, B.D.; Ingle, C.R.H. Microscopic colitis: Common cause of unexplained nonbloody diarrhea. World J. Gastrointest. Pathophysiol. 2014, 5, 48–53. [Google Scholar] [CrossRef]
- Elmore, D.B.; Anderson, J.H.; Hird, D.W.; Sanders, K.D.; Lerche, N.W. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab. Anim. Sci. 1992, 42, 356–359. [Google Scholar]
- Blackwood, R.S.; Tarara, R.P.; Christe, K.L.; Spinner, A.; Lerche, N.W. Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comp. Med. 2008, 58, 81–87. [Google Scholar]
- Ardeshir, A.; Oslund, K.L.; Ventimiglia, F.; Yee, J.; Lerche, N.W.; Hyde, D.M. Idiopathic microscopic colitis of rhesus macaques: Quantitative assessment of colonic mucosa. Anat. Rec. 2013, 296, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardeshir, A.; Sankaran, S.; Oslund, K.L.; Hartigan-O’Connor, D.J.; Lerche, N.W.; Hyde, D.M.; Dandekar, S. Inulin treatment leads to changes in intestinal microbiota and resolution of idiopathic chronic diarrhea in rhesus macaques. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. S1), S75. [Google Scholar] [CrossRef]
- Broadhurst, M.J.; Ardeshir, A.; Kanwar, B.; Mirpuri, J.; Gundra, U.M.; Leung, J.M.; Wiens, K.E.; Vujkovic-Cvijin, I.; Kim, C.C.; Yarovinsky, F.; et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012, 8, e1003000. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in inflammatory bowel disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Russell, R.G.; Rosenkranz, S.L.; Lee, L.A.; Howard, H.; DiGiacomo, R.F.; Bronsdon, M.A.; Blakley, G.A.; Tsai, C.C.; Morton, W.R. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab. Anim. Sci. 1987, 37, 309–316. [Google Scholar]
- Wang, Y.; Tu, X.; Humphrey, C.; McClure, H.; Jiang, X.; Qin, C.; Glass, R.I.; Jiang, B. Detection of viral agents in fecal specimens of monkeys with diarrhea. J. Med. Primatol. 2007, 36, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Stuker, G.; Oshiro, L.S.; Schmidt, N.J.; Holmberg, C.A.; Anderson, J.H.; Glaser, C.A.; Henrickson, R.V. Virus detection in monkeys with diarrhea: The association of adenoviruses with diarrhea and the possible role of rotaviruses. Lab. Anim. Sci. 1979, 29, 610–616. [Google Scholar] [PubMed]
- Wang, K.Y.; Christe, K.L.; Yee, J.; Roberts, J.A.; Ardeshir, A. Rotavirus is associated with decompensated diarrhea among young rhesus macaques (Macaca mulatta). Am. J. Primatol. 2019, 81, e22948. [Google Scholar] [CrossRef] [Green Version]
- Sestak, K.; Feely, S.; Fey, B.; Dufour, J.; Hargitt, E.; Alvarez, X.; Pahar, B.; Gregoricus, N.; Vinje, J.; Farkas, T. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS ONE 2012, 7, e37973. [Google Scholar] [CrossRef]
- Handley, S.A.; Thackray, L.B.; Zhao, G.; Presti, R.; Miller, A.D.; Droit, L.; Abbink, P.; Maxfield, L.F.; Kambal, A.; Duan, E.; et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell 2012, 151, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Kapusinszky, B.; Ardeshir, A.; Mulvaney, U.; Deng, X.T.; Delwart, E. Case-control comparison of enteric viromes in captive rhesus macaques with acute or idiopathic chronic diarrhea. J. Virol. 2017, 91, e00952-17. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; McGinnis, S.; Madden, T.L. BLAST: Improvements for better sequence analysis. Nucleic Acids Res. 2006, 34, W6–W9. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucleic Acids Res. 2015, 43, e46. [Google Scholar] [CrossRef] [Green Version]
- Tisza, M.J.; Pastrana, D.V.; Welch, N.L.; Stewart, B.; Peretti, A.; Starrett, G.J.; Pang, Y.S.; Krishnamurthy, S.R.; Pesavento, P.A.; McDermott, D.H.; et al. Discovery of several thousand highly diverse circular DNA viruses. Elife 2020, 9, e51971. [Google Scholar] [CrossRef]
- Tisza, M.J.; Belford, A.K.; Domínguez-Huerta, G.; Bolduc, B.; Buck, C.B. Cenote-Taker 2 democratizes virus discovery and sequence annotation. Virus Evol. 2021, 7, veaa100. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Salzberg, S.L.; Phillippy, A.M. Using MUMmer to identify similar regions in large sequence sets. Curr. Protoc. Bioinform. 2003, 10, 10–13. [Google Scholar] [CrossRef]
- Tarailo-Graovac, M.; Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform. 2009, 4, 4–10. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond p values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]
- Kanthaswamy, S.; Elfenbein, H.A.; Ardeshir, A.; Ng, J.; Hyde, D.; Smith, D.G.; Lerche, N. Familial aggregation of chronic diarrhea disease (CDD) in rhesus macaques (Macaca mulatta). Am. J. Primatol. 2014, 76, 262–270. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delwart, E.; Tisza, M.J.; Altan, E.; Li, Y.; Deng, X.; Hartigan-O’Connor, D.J.; Ardeshir, A. Idiopathic Chronic Diarrhea in Rhesus Macaques Is Not Associated with Enteric Viral Infections. Viruses 2021, 13, 2503. https://doi.org/10.3390/v13122503
Delwart E, Tisza MJ, Altan E, Li Y, Deng X, Hartigan-O’Connor DJ, Ardeshir A. Idiopathic Chronic Diarrhea in Rhesus Macaques Is Not Associated with Enteric Viral Infections. Viruses. 2021; 13(12):2503. https://doi.org/10.3390/v13122503
Chicago/Turabian StyleDelwart, Eric, Michael J. Tisza, Eda Altan, Yanpeng Li, Xutao Deng, Dennis J. Hartigan-O’Connor, and Amir Ardeshir. 2021. "Idiopathic Chronic Diarrhea in Rhesus Macaques Is Not Associated with Enteric Viral Infections" Viruses 13, no. 12: 2503. https://doi.org/10.3390/v13122503
APA StyleDelwart, E., Tisza, M. J., Altan, E., Li, Y., Deng, X., Hartigan-O’Connor, D. J., & Ardeshir, A. (2021). Idiopathic Chronic Diarrhea in Rhesus Macaques Is Not Associated with Enteric Viral Infections. Viruses, 13(12), 2503. https://doi.org/10.3390/v13122503







