Maternal HPV Infection: Effects on Pregnancy Outcome
Abstract
1. Introduction
1.1. Cutaneous HPV
1.2. Mucosal HPV
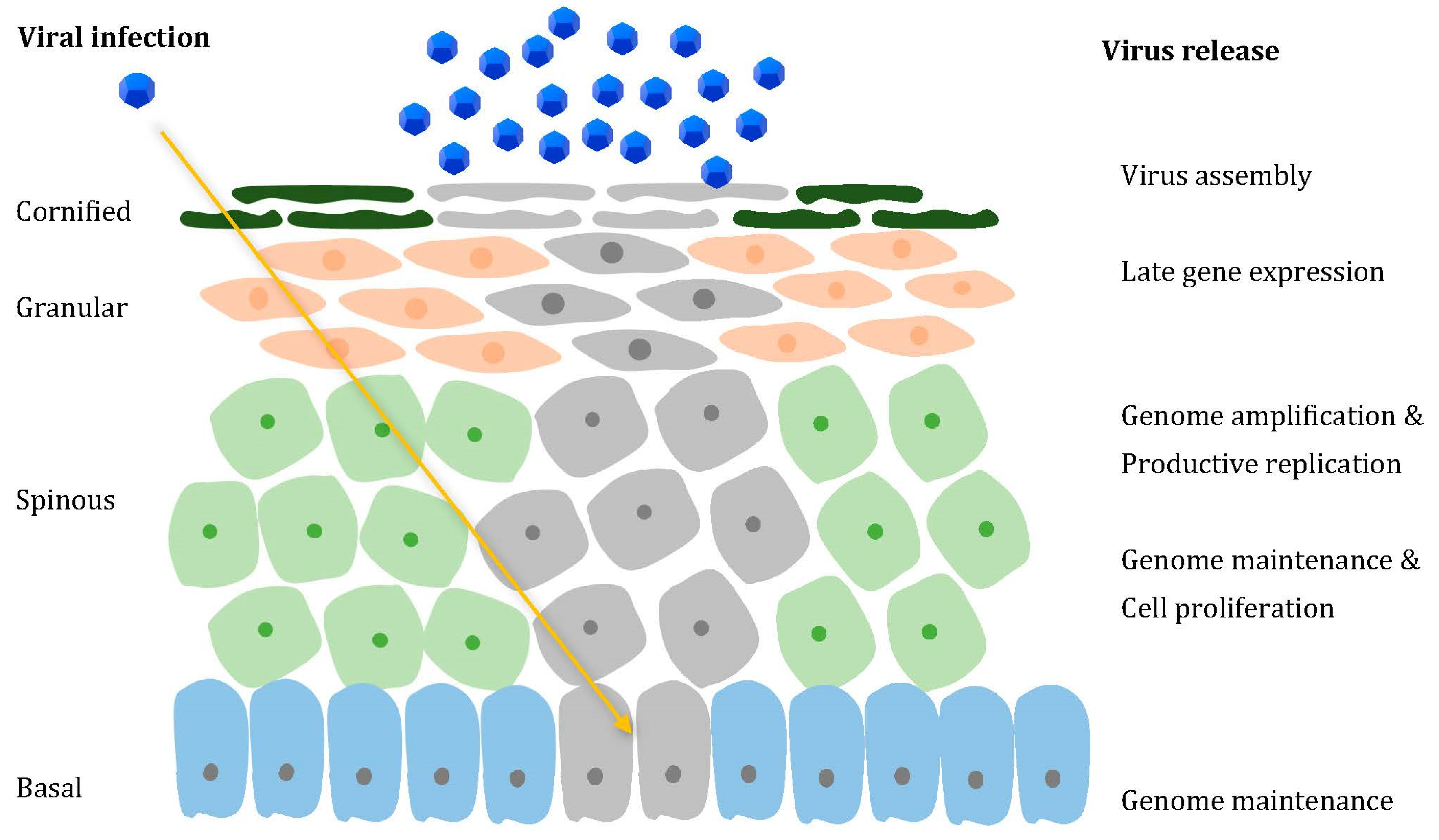
2. How Does HPV Operate
3. HPV in Pregnant Women
4. HPV and Pregnancy Outcomes
4.1. Preterm Birth
4.2. Miscarriage
4.3. Preeclampsia
4.4. Intrauterine Growth Restriction
4.5. Premature Rupture of Membranes
4.6. Fetal Death
5. Immunization of Pregnant Women
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McQuillan, G.; Kruszon-Moran, D.; Markowitz, L.E.; Unger, E.R.; Paulose-Ram, R. Prevalence of HPV in Adults Aged 18–69: United States, 2011–2014; NCHS Data Brief; National Center for Health Statistics: Atlanta, GA, USA, 2017; pp. 1–8.
- Sehnal, B.; Zikan, M.; Nipcova, M.; Dusek, L.; Cibula, D.; Slama, J. The association among cervical, anal, and oral HPV infections in high-risk and low-risk women. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100061. [Google Scholar] [CrossRef]
- Otter, S.; Whitaker, S.; Chatterjee, J.; Stewart, A. The Human Papillomavirus as a Common Pathogen in Oropharyngeal, Anal and Cervical Cancers. Clin. Oncol. 2019, 31, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.-Y.; Choi, S.; Kim, D.S.; Oh, Y.L. Carcinogenic risk of human papillomavirus (HPV) genotypes and potential effects of HPV vaccines in Korea. Sci. Rep. 2019, 9, 12556. [Google Scholar] [CrossRef]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Braaten, K.P.; Laufer, M.R. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev. Obstet. Gynecol. 2008, 1, 2–10. [Google Scholar] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Chan, P.K.S.; et al. Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology 2018, 516, 86–101. [Google Scholar] [CrossRef]
- Mühr, L.S.A.; Eklund, C.; Dillner, J. Towards quality and order in human papillomavirus research. Virology 2018, 519, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Johansson, H.; Madsen, K.G.; Kofoed, K. The nasal mucosa contains a large spectrum of human papillomavirus types from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2013, 208, 1335–1341. [Google Scholar] [CrossRef]
- Hampras, S.S.; Rollison, D.E.; Giuliano, A.R.; McKay-Chopin, S.; Minoni, L.; Sereday, K.; Gheit, T.; Tommasino, M. Prevalence and Concordance of Cutaneous Beta Human Papillomavirus Infection at Mucosal and Cutaneous Sites. J. Infect. Dis. 2017, 216, 92–96. [Google Scholar] [CrossRef]
- Accardi, R.; Gheit, T. Cutaneous HPV and skin cancer. Presse Med. 2014, 43, e435–e443. [Google Scholar] [CrossRef] [PubMed]
- Ramoz, N.; Rueda, L.A.; Bouadjar, B.; Montoya, L.S.; Orth, G.; Favre, M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, D.; Chen, Z.; Dunne, A.; Ostoloza, J.; McKinney, S.; Sun, C.; Schlecht, N.F.; Fatahzadeh, M.; Herrero, R.; Schiffman, M.; et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2011, 204, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.G.; Gheit, T.; Latini, A.; Benevolo, M.; Torres, M.; Smelov, V.; McKay-Chopin, S.; Giglio, A.; Cristaudo, A.; Zaccarelli, M.; et al. Alpha, beta and gamma Human Papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J. Infect. 2015, 71, 74–84. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Plasmeijer, E.I.; Struijk, L.; Bouwes Bavinck, J.N.; Feltkamp, M.C. Epidemiology of cutaneous human papillomavirus infections. Cancer Treat. Res. 2009, 146, 143–157. [Google Scholar] [CrossRef]
- de Koning, M.N.C.; Weissenborn, S.J.; Abeni, D.; Bouwes Bavinck, J.N.; Euvrard, S.; Green, A.C.; Harwood, C.A.; Naldi, L.; Neale, R.; Nindl, I.; et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J. Gen. Virol. 2009, 90, 1611–1621. [Google Scholar] [CrossRef]
- de Koning, M.N.C.; Struijk, L.; Bavinck, J.N.B.; Kleter, B.; Ter Schegget, J.; Quint, W.G.V.; Feltkamp, M.C.W. Betapapillomaviruses frequently persist in the skin of healthy individuals. J. Gen. Virol. 2007, 88, 1489–1495. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Ma, Y.; Gheit, T.; McKay-Chopin, S.; Farhat, S.; Widdice, L.E.; Tommasino, M. Prevalence and Transmission of Beta and Gamma Human Papillomavirus in Heterosexual Couples. Open Forum Infect. Dis. 2017, 4, ofw216. [Google Scholar] [CrossRef]
- Bouwes Bavinck, J.N.; Neale, R.E.; Abeni, D.; Euvrard, S.; Green, A.C.; Harwood, C.A.; de Koning, M.N.; Naldi, L.; Nindl, I.; Pawlita, M.; et al. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res. 2010, 70, 9777–9786. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, S.; Neale, R.E.; Waterboer, T.; Abeni, D.; Bavinck, J.N.; Green, A.C.; Harwood, C.A.; Euvrard, S.; Feltkamp, M.C.; de Koning, M.N.; et al. Beta-papillomavirus DNA loads in hair follicles of immunocompetent people and organ transplant recipients. Med. Microbiol. Immunol. 2012, 201, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.M.; Waterboer, T.; Sehr, P.; Rother, A.; Reidel, U.; Boeing, H.; Bravo, I.G.; Schlehofer, J.; Gärtner, B.C.; Pawlita, M. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008, 4, e1000091. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly Zwald, F.; Brown, M. Skin cancer in solid organ transplant recipients: Advances in therapy and management: Part I. Epidemiology of skin cancer in solid organ transplant recipients. J. Am. Acad. Dermatol. 2011, 65, 253–261. [Google Scholar] [CrossRef]
- Asgari, M.M.; Ray, G.T.; Quesenberry, C.P., Jr.; Katz, K.A.; Silverberg, M.J. Association of Multiple Primary Skin Cancers With Human Immunodeficiency Virus Infection, CD4 Count, and Viral Load. JAMA Dermatol. 2017, 153, 892–896. [Google Scholar] [CrossRef]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Faust, H.; Andersson, K.; Luostarinen, T.; Gislefoss, R.E.; Dillner, J. Cutaneous Human Papillomaviruses and Squamous Cell Carcinoma of the Skin: Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 721–724. [Google Scholar] [CrossRef]
- Orth, G. Host defenses against human papillomaviruses: Lessons from epidermodysplasia verruciformis. Curr. Top. Microbiol. Immunol. 2008, 321, 59–83. [Google Scholar] [CrossRef]
- Ryndock, E.J.; Meyers, C. A risk for non-sexual transmission of human papillomavirus? Expert Rev. Anti-Infect. Ther. 2014, 12, 1165–1170. [Google Scholar] [CrossRef]
- Weinstock, H.S.; Kreisel, K.M.; Spicknall, I.H.; Chesson, H.W.; Miller, W.C. STI Prevalence, Incidence, and Costs in the United States: New Estimates, New Approach. Sex. Transm. Dis. 2021, 48, 207. [Google Scholar] [CrossRef]
- Valasoulis, G.; Pouliakis, A. The Influence of Sexual Behavior and Demographic Characteristics in the Expression of HPV-Related Biomarkers in a Colposcopy Population of Reproductive Age Greek Women. Biology 2021, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Agency for Research on Cancer. IARC Monograohs on the Evaluation of Carcinogenic Risks to Humans. In Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; IARC Publications: Lyon, France, 2010; Volume 94. [Google Scholar]
- Bertolotti, A.; Milpied, B.; Fouéré, S.; Dupin, N.; Cabié, A.; Derancourt, C. Local Management of Anogenital Warts in Non-immunocompromised Adults: A Systematic Review and Meta-analyses of Randomized Controlled Trials. Dermatol. Ther. 2019, 9, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papilomavirus DNA. Virol. J. 2009, 6, 83. [Google Scholar] [CrossRef]
- Loenenbach, A.; Poethko-Müller, C.; Pawlita, M.; Thamm, M.; Harder, T.; Waterboer, T.; Schröter, J.; Deleré, Y.; Wichmann, O.; Wiese-Posselt, M. Mucosal and cutaneous Human Papillomavirus seroprevalence among adults in the prevaccine era in Germany—Results from a nationwide population-based survey. Int. J. Infect. Dis. 2019, 83, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Niccolai, P.; Ortiz, A.M.; Sheth, S.S.; Shapiro, E.D.; Niccolai, L.M.; Brandt, C.A. Natural Language Processing for Surveillance of Cervical and Anal Cancer and Precancer: Algorithm Development and Split-Validation Study. JMIR Med. Inform. 2020, 8, e20826. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Seo, S.S.; Kim, M.K.; Lee, D.O.; Chung, Y.K.; Lim, M.C.; Kim, J.Y.; Lee, C.W.; Park, S.Y. Synergistic effect of viral load and alcohol consumption on the risk of persistent high-risk human papillomavirus infection. PLoS ONE 2014, 9, e104374. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Sedjo, R.L.; Roe, D.J.; Harri, R.; Baldwi, S.; Papenfuss, M.R.; Abrahamsen, M.; Inserra, P. Clearance of oncogenic human papillomavirus (HPV) infection: Effect of smoking (United States). Cancer Causes Control 2002, 13, 839–846. [Google Scholar] [CrossRef]
- Wang, S.S.; Hildesheim, A.; Gao, X.; Schiffman, M.; Herrero, R.; Bratti, M.C.; Sherman, M.E.; Barnes, W.A.; Greenberg, M.D.; McGowan, L.; et al. Comprehensive analysis of human leukocyte antigen class I alleles and cervical neoplasia in 3 epidemiologic studies. J. Infect. Dis. 2002, 186, 598–605. [Google Scholar] [CrossRef]
- Carreon, J.D.; Martin, M.P.; Hildesheim, A.; Gao, X.; Schiffman, M.; Herrero, R.; Bratti, M.C.; Sherman, M.E.; Zaino, R.J.; Carrington, M.; et al. Human leukocyte antigen class I and II haplotypes and risk of cervical cancer. Tissue Antigens 2005, 66, 321–324. [Google Scholar] [CrossRef]
- Chan, P.K.; Cheung, J.L.; Cheung, T.H.; Lin, C.K.; Tam, A.O.; Chan, D.P.; Zhou, D.X.; Lo, K.W.; Yim, S.F.; Siu, S.S. HLA-B alleles, high-risk HPV infection and risk for cervical neoplasia in southern Chinese women. Int. J. Cancer 2006, 118, 1430–1435. [Google Scholar] [CrossRef]
- Bernal-Silva, S.; Granados, J.; Gorodezky, C.; Aláez, C.; Flores-Aguilar, H.; Cerda-Flores, R.M.; Guerrero-González, G.; Valdez-Chapa, L.D.; Morales-Casas, J.; González-Guerrero, J.F.; et al. HLA-DRB1 Class II antigen level alleles are associated with persistent HPV infection in Mexican women; a pilot study. Infect. Agents Cancer 2013, 8, 31. [Google Scholar] [CrossRef]
- Chan, D.P.; Cheung, T.H.; Tam, A.O.; Cheung, J.L.; Yim, S.F.; Lo, K.W.; Siu, N.S.; Zhou, D.X.; Chan, P.K. Risk association between human leukocyte antigen-A allele and high-risk human papillomavirus infection for cervical neoplasia in Chinese women. J. Infect. Dis. 2005, 192, 1749–1756. [Google Scholar] [CrossRef]
- Ghaderi, M.; Nikitina, L.; Peacock, C.S.; Hjelmström, P.; Hallmans, G.; Wiklund, F.; Lenner, P.; Blackwell, J.M.; Dillner, J.; Sanjeevi, C.B. Tumor necrosis factor a-11 and DR15-DQ6 (B*0602) haplotype increase the risk for cervical intraepithelial neoplasia in human papillomavirus 16 seropositive women in Northern Sweden. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1067–1070. [Google Scholar]
- Govan, V.A.; Constant, D.; Hoffman, M.; Williamson, A.L. The allelic distribution of -308 Tumor Necrosis Factor-alpha gene polymorphism in South African women with cervical cancer and control women. BMC Cancer 2006, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Human Papillomaviruses; World Health Organization: Geneva, Switzerland, 2007; Volume 90. [Google Scholar]
- Wilson, V.G.; West, M.; Woytek, K.; Rangasamy, D. Papillomavirus E1 proteins: Form, function, and features. Virus Genes 2002, 24, 275–290. [Google Scholar] [CrossRef]
- Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef]
- Fehrmann, F.; Laimins, L.A. Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene 2003, 22, 5201–5207. [Google Scholar] [CrossRef] [PubMed]
- Egawa, K. Do human papillomaviruses target epidermal stem cells? Dermatology 2003, 207, 251–254. [Google Scholar] [CrossRef]
- Flores, E.R.; Allen-Hoffmann, B.L.; Lee, D.; Sattler, C.A.; Lambert, P.F. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 1999, 262, 344–354. [Google Scholar] [CrossRef][Green Version]
- Peh, W.L.; Middleton, K.; Christensen, N.; Nicholls, P.; Egawa, K.; Sotlar, K.; Brandsma, J.; Percival, A.; Lewis, J.; Liu, W.J.; et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 2002, 76, 10401–10416. [Google Scholar] [CrossRef] [PubMed]
- Klingelhutz, A.J.; Roman, A. Cellular transformation by human papillomaviruses: Lessons learned by comparing high- and low-risk viruses. Virology 2012, 424, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Felsani, A.; Mileo, A.M.; Paggi, M.G. Retinoblastoma family proteins as key targets of the small DNA virus oncoproteins. Oncogene 2006, 25, 5277–5285. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Crum, C.P.; Münger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. USA 2011, 108, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Gewin, L.C.; Myers, H.; Luo, W.; Grandori, C.; Katzenellenbogen, R.A.; McDougall, J.K. Regulation of telomerase by human papillomaviruses. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zanier, K.; ould M’hamed ould Sidi, A.; Boulade-Ladame, C.; Rybin, V.; Chappelle, A.; Atkinson, A.; Kieffer, B.; Travé, G. Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53. Structure 2012, 20, 604–617. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.; Sun, Y.; Wang, Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol. Infect. 2014, 142, 1567–1578. [Google Scholar] [CrossRef]
- Luo, D.; Peng, M.; Wei, X.; Pan, D.; Xue, H.; Xu, Y.; Dong, B. Prevalence of Human Papillomavirus and Genotype Distribution in Pregnant and Non-Pregnant Women in China. Risk Manag. Healthc. Policy 2021, 14, 3147–3157. [Google Scholar] [CrossRef]
- Gomez, L.M.; Ma, Y.; Ho, C.; McGrath, C.M.; Nelson, D.B.; Parry, S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum. Reprod. 2008, 23, 709–715. [Google Scholar] [CrossRef]
- Srinivas, S.K.; Ma, Y.; Sammel, M.D.; Chou, D.; McGrath, C.; Parry, S.; Elovitz, M.A. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am. J. Obstet. Gynecol. 2006, 195, 797–802. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.S.; Norwitz, E.R.; Koo, J.N.; Oh, I.H.; Park, J.W.; Kim, S.M.; Kim, Y.H.; Park, C.W.; Song, Y.S. Risk of vertical transmission of human papillomavirus throughout pregnancy: A prospective study. PLoS ONE 2013, 8, e66368. [Google Scholar] [CrossRef]
- Slatter, T.L.; Hung, N.G.; Clow, W.M.; Royds, J.A.; Devenish, C.J.; Hung, N.A. A clinicopathological study of episomal papillomavirus infection of the human placenta and pregnancy complications. Mod. Pathol. 2015, 28, 1369–1382. [Google Scholar] [CrossRef]
- Armbruster-Moraes, E.; Ioshimoto, L.M.; Leao, E.; Zugaib, M. Detection of human papillomavirus deoxyribonucleic acid sequences in amniotic fluid during different periods of pregnancy. Am. J. Obstet. Gynecol. 1993, 169, 1074. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grénman, S.E.; Järvenkylä, M.E.; Syrjänen, K.J.; Syrjänen, S.M. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: Experience from the Finnish HPV Family Study. Clin. Infect. Dis. 2005, 41, 1728–1733. [Google Scholar] [CrossRef]
- Koskimaa, H.M.; Waterboer, T.; Pawlita, M.; Grénman, S.; Syrjänen, K.; Syrjänen, S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012, 160, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Varlas, V.N.; Duică, F.; Antoniadis, P.; Danila, C.A.; Cretoiu, D.; Suciu, N.; Crețoiu, S.M.; Voinea, S.C. Pregnancy-Related Extracellular Vesicles Revisited. Int. J. Mol. Sci. 2021, 22, 3904. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 2627. [Google Scholar] [CrossRef]
- von Knebel Doeberitz, M.; Bauknecht, T.; Bartsch, D.; zur Hausen, H. Influence of chromosomal integration on glucocorticoid-regulated transcription of growth-stimulating papillomavirus genes E6 and E7 in cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 1991, 88, 1411–1415. [Google Scholar] [CrossRef]
- Mittal, R.; Pater, A.; Pater, M.M. Multiple human papillomavirus type 16 glucocorticoid response elements functional for transformation, transient expression, and DNA-protein interactions. J. Virol. 1993, 67, 5656–5659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, H.; Chiriva-Internati, M.; Korourian, S.; Lowery, C.L.; Carey, M.J.; Smith, C.V.; Hermonat, P.L. Display of complete life cycle of human papillomavirus type 16 in cultured placental trophoblasts. Virology 2001, 290, 99–105. [Google Scholar] [CrossRef]
- You, H.; Liu, Y.; Carey, M.J.; Lowery, C.L.; Hermonat, P.L. Defective 3A trophoblast-endometrial cell adhesion and altered 3A growth and survival by human papillomavirus type 16 oncogenes. Mol. Cancer Res. 2002, 1, 25–31. [Google Scholar] [PubMed]
- You, H.; Liu, Y.; Agrawal, N.; Prasad, C.K.; Chiriva-Internati, M.; Lowery, C.L.; Kay, H.H.; Hermonat, P.L. Infection, replication, and cytopathology of human papillomavirus type 31 in trophoblasts. Virology 2003, 316, 281–289. [Google Scholar] [CrossRef]
- Boulenouar, S.; Weyn, C.; Van Noppen, M.; Moussa Ali, M.; Favre, M.; Delvenne, P.O.; Bex, F.; Noël, A.; Englert, Y.; Fontaine, V. Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis 2010, 31, 473–480. [Google Scholar] [CrossRef]
- Wetherill, L.F.; Holmes, K.K.; Verow, M.; Müller, M.; Howell, G.; Harris, M.; Fishwick, C.; Stonehouse, N.; Foster, R.; Blair, G.E.; et al. High-risk human papillomavirus E5 oncoprotein displays channel-forming activity sensitive to small-molecule inhibitors. J. Virol. 2012, 86, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Castaño-Rodriguez, C.; Aguilella, V.M.; Enjuanes, L. Relevance of Viroporin Ion Channel Activity on Viral Replication and Pathogenesis. Viruses 2015, 7, 3552–3573. [Google Scholar] [CrossRef]
- Værnesbranden, M.R.; Wiik, J.; Sjøborg, K.; Staff, A.C.; Carlsen, K.C.L.; Haugen, G.; Hedlin, G.; Hilde, K.; Nordlund, B.; Nystrand, C.F.; et al. Maternal human papillomavirus infections at mid-pregnancy and delivery in a Scandinavian mother-child cohort study. Int. J. Infect. Dis. 2021, 108, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Nobbenhuis, M.A.E.; Helmerhorst, T.J.M.; van den Brule, A.J.C.; Rozendaal, L.; Bezemer, P.D.; Voorhorst, F.J.; Meijer, C.J.L.M. High-risk human papillomavirus clearance in pregnant women: Trends for lower clearance during pregnancy with a catch-up postpartum. Br. J. Cancer 2002, 87, 75–80. [Google Scholar] [CrossRef]
- Veress, G.; Csiky-Mészáros, T.; Kónya, J.; Czeglédy, J.; Gergely, L. Follow-up of human papillomavirus (HPV) DNA and local anti-HPV antibodies in cytologically normal pregnant women. Med. Microbiol. Immunol. 1996, 185, 139–144. [Google Scholar] [CrossRef]
- Gibbs, R.S. The relationship between infections and adverse pregnancy outcomes: An overview. Ann. Periodontol. 2001, 6, 153–163. [Google Scholar] [CrossRef]
- Conde-Ferráez, L.; May, A.D.A.C.; Carrillo-Martínez, J.R.; Ayora-Talavera, G.; del Refugio González-Losa, M. Human papillomavirus infection and spontaneous abortion: A case-control study performed in Mexico. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 468–473. [Google Scholar] [CrossRef]
- Subramaniam, A.; Lees, B.F.; Becker, D.A.; Tang, Y.; Khan, M.J.; Edwards, R.K. Evaluation of Human Papillomavirus as a Risk Factor for Preterm Birth or Pregnancy-Related Hypertension. Obstet. Gynecol. 2016, 127, 233–240. [Google Scholar] [CrossRef]
- Aldhous, M.C.; Bhatia, R.; Pollock, R.; Vragkos, D.; Cuschieri, K.; Cubie, H.A.; Norman, J.E.; Stock, S.J. HPV infection and pre-term birth: A data-linkage study using Scottish Health Data. Wellcome Open Res. 2019, 4, 48. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Mo, Y.; Luo, Q.M.; Huo, S.T.; He, W.Q.; Chen, Q. The Risk of Human Papillomavirus Infection for Spontaneous Abortion, Spontaneous Preterm Birth, and Pregnancy Rate of Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2018, 83, 417–427. [Google Scholar] [CrossRef]
- Cho, G.; Min, K.J.; Hong, H.R.; Kim, S.; Hong, J.H.; Lee, J.K.; Oh, M.J.; Kim, H. High-risk human papillomavirus infection is associated with premature rupture of membranes. BMC Pregnancy Childbirth 2013, 13, 173. [Google Scholar] [CrossRef]
- Niyibizi, J.; Zanré, N.; Mayrand, M.-H.; Trottier, H. The association between adverse pregnancy outcomes and maternal human papillomavirus infection: A systematic review protocol. Syst. Rev. 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.H.; Li, M.; Scheil, W.; Roder, D. Human papillomavirus infection and intrauterine growth restriction: A data-linkage study. J. Matern.-Fetal Neonatal Med. 2019, 32, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Zanré, N.; Mayrand, M.-H.; Trottier, H. Association Between Maternal Human Papillomavirus Infection and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 221, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Cotton-Caballero, A.; Dudley, D.; Ferguson, J.; Pettit, K.; Boyle, A. Maternal Human Papillomavirus Infection Increases the Risk of Premature Rupture of Membranes [19M]. Obstet. Gynecol. 2017, 129, S137. [Google Scholar] [CrossRef]
- Caballero, A.; Dudley, D.; Ferguson, J.; Pettit, K.; Boyle, A. Maternal Human Papillomavirus and Preterm Premature Rupture of Membranes: A Retrospective Cohort Study. J. Women Health 2019, 28, 606–611. [Google Scholar] [CrossRef]
- Huang, Q.T.; Zhong, M.; Gao, Y.F.; Huang, L.P.; Huang, Q.; Wang, W.; Wang, Z.J.; Yu, Y.H. Can HPV vaccine have other health benefits more than cancer prevention? A systematic review of association between cervical HPV infection and preterm birth. J. Clin. Virol. 2014, 61, 321–328. [Google Scholar] [CrossRef]
- Zuo, Z.; Goel, S.; Carter, J.E. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. Am. J. Clin. Pathol. 2011, 136, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Mayrand, M.-H.; Audibert, F.; Monnier, P.; Brassard, P.; Laporte, L.; Lacaille, J.; Zahreddine, M.; Bédard, M.-J.; Girard, I.; et al. Association Between Human Papillomavirus Infection Among Pregnant Women and Preterm Birth. JAMA Network Open 2021, 4, e2125308. [Google Scholar] [CrossRef] [PubMed]
- Wiik, J.; Nilsson, S.; Kärrberg, C.; Strander, B.; Jacobsson, B.; Sengpiel, V. Associations of treated and untreated human papillomavirus infection with preterm delivery and neonatal mortality: A Swedish population-based study. PLoS Med. 2021, 18, e1003641. [Google Scholar] [CrossRef]
- Ambühl, L.M.M.; Leonhard, A.K.; Widen Zakhary, C.; Jørgensen, A.; Blaakaer, J. Human papillomavirus infects placental trophoblast and Hofbauer cells, but appears not to play a causal role in miscarriage and preterm labor. Acta Obstet. Gynecol. Scand. 2017, 96, 1188–1196. [Google Scholar] [CrossRef]
- Ambühl, L.M.M.; Baandrup, U.; Dybkær, K.; Blaakær, J.; Uldbjerg, N.; Sørensen, S. Human Papillomavirus Infection as a Possible Cause of Spontaneous Abortion and Spontaneous Preterm Delivery. Infect. Dis. Obstet. Gynecol. 2016, 2016, 3086036. [Google Scholar] [CrossRef]
- Nimrodi, M.; Kleitman, V.; Wainstock, T.; Gemer, O.; Meirovitz, M.; Maymon, E.; Benshalom-Tirosh, N.; Erez, O. The association between cervical inflammation and histologic evidence of HPV in PAP smears and adverse pregnancy outcome in low risk population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 160–165. [Google Scholar] [CrossRef]
- McDonnold, M.; Dunn, H.; Hester, A.; Pacheco, L.D.; Hankins, G.D.; Saade, G.R.; Costantine, M.M. High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am. J. Obstet. Gynecol. 2014, 210, 138.e1–138.e5. [Google Scholar] [CrossRef]
- Giambanco, L.; Iannone, V.; Borriello, M.; Montalto, A. Papillomavirus infection and preterm birth. Chronicle of a broken relationship? case series and review of the literature. PAMJ Clin. Med. 2020, 3, 133. [Google Scholar] [CrossRef]
- Gibb, W.; Challis, J.R. Mechanisms of term and preterm birth. J. Obstet. Gynaecol. Can. 2002, 24, 874–883. [Google Scholar] [CrossRef]
- Zeitlin, J.; Szamotulska, K.; Drewniak, N.; Mohangoo, A.D.; Chalmers, J.; Sakkeus, L.; Irgens, L.; Gatt, M.; Gissler, M.; Blondel, B.; et al. Preterm birth time trends in Europe: A study of 19 countries. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Kogan, M.D.; Alexander, G.R.; Dattani, N.; Kramer, M.S.; Macfarlane, A.; Wen, S.W. The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: An international study. Am. J. Public Health 2002, 92, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Fanczal, E.; Berecz, B.; Szijártó, A.; Gasparics, Á.; Varga, P. The Prognosis of Preterm Infants Born at the Threshold of Viability: Fog Over the Gray Zone—Population-Based Studies of Extremely Preterm Infants. Med. Sci. Monit. 2020, 26, e92694. [Google Scholar] [CrossRef] [PubMed]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Womens Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Maternal obesity influences birth weight more than gestational diabetes. BMC Pregnancy Childbirth 2021, 21, 111. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Lauderdale, D.S.; Lantos, J.D. Medically induced preterm birth and the associations between prenatal care and infant mortality. Ann. Epidemiol. 2013, 23, 435–440. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. 3), 17–42. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Henderson, J.J.; McWilliam, O.A.; Newnham, J.P.; Pennell, C.E. Preterm birth aetiology 2004-2008. Maternal factors associated with three phenotypes: Spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J. Matern.-Fetal Neonatal Med. 2012, 25, 642–647. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am. J. Obstet. Gynecol. 2006, 195, 1557–1563. [Google Scholar] [CrossRef]
- Roescher, A.M.; Timmer, A.; van der Laan, M.E.; Erwich, J.J.H.M.; Bos, A.F.; Kooi, E.M.W.; Verhagen, E.A. In preterm infants, ascending intrauterine infection is associated with lower cerebral tissue oxygen saturation and higher oxygen extraction. Pediatric Res. 2015, 77, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T.; Espinoza, J. Micronutrients and Intrauterine Infection, Preterm Birth and the Fetal Inflammatory Response Syndrome. J. Nutr. 2003, 133, 1668S–1673S. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef]
- Wenstrom, K.D.; Andrews, W.W.; Bowles, N.E.; Towbin, J.A.; Hauth, J.C.; Goldenberg, R.L. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet. Gynecol. 1998, 92, 420–424. [Google Scholar] [CrossRef]
- Tavares Da Silva, F.; Gonik, B.; McMillan, M.; Keech, C.; Dellicour, S.; Bhange, S.; Tila, M.; Harper, D.M.; Woods, C.; Kawai, A.T.; et al. Stillbirth: Case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2016, 34, 6057–6068. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, M.; Ye, X.; Chen, F.; Li, Y.; Li, N.; Wu, W.; Sun, J. A cross-sectional survey of pregnant women’s knowledge of chromosomal aneuploidy and microdeletion and microduplication syndromes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Devall, A.J.; Coomarasamy, A. Sporadic pregnancy loss and recurrent miscarriage. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 30–39. [Google Scholar] [CrossRef]
- Pillai, R.N.; Konje, J.C.; Richardson, M.; Tincello, D.G.; Potdar, N. Prediction of miscarriage in women with viable intrauterine pregnancy—A systematic review and diagnostic accuracy meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 1984, 6, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Worda, C.; Huber, A.; Hudelist, G.; Schatten, C.; Leipold, H.; Czerwenka, K.; Eppel, W. Prevalence of cervical and intrauterine human papillomavirus infection in the third trimester in asymptomatic women. J. Soc. Gynecol. Investig. 2005, 12, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hermonat, P.L.; Han, L.; Wendel, P.J.; Quirk, J.G.; Stern, S.; Lowery, C.L.; Rechtin, T.M. Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes 1997, 14, 13–17. [Google Scholar] [CrossRef]
- Sarkola, M.E.; Grénman, S.E.; Rintala, M.A.; Syrjänen, K.J.; Syrjänen, S.M. Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet. Gynecol. Scand. 2008, 87, 1181–1188. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Rao, H. Maternal-fetal transmission of human papillomavirus. Chin. Med. J. 1998, 111, 726–727. [Google Scholar] [PubMed]
- Bober, L.; Guzowski, G.; Moczulska, H.; Sieroszewski, P. Influence of human Papilloma Virus (hPV) infection on early pregnancy. Ginekol. Pol. 2019, 90, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Pietropolli, A.; Fabbri, G.; Capogna, M.V.; Perno, C.F.; Piccione, E. Recurrent miscarriage and cervical human papillomavirus infection. Am. J. Reprod. Immunol. 2013, 70, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sugiura-Ogasawara, M.; Ebara, T.; Yamada, Y.; Shoji, N.; Matsuki, T.; Kano, H.; Kurihara, T.; Omori, T.; Tomizawa, M.; Miyata, M.; et al. Adverse pregnancy and perinatal outcome in patients with recurrent pregnancy loss: Multiple imputation analyses with propensity score adjustment applied to a large-scale birth cohort of the Japan Environment and Children’s Study. Am. J. Reprod. Immunol. 2019, 81, e13072. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Redman, C.W.G.; Global Pregnancy, C. Global Pregnancy Collaboration symposium: Prepregnancy and very early pregnancy antecedents of adverse pregnancy outcomes: Overview and recommendations. Placenta 2017, 60, 103–109. [Google Scholar] [CrossRef]
- Dekker, G.A.; Sibai, B.M. Etiology and pathogenesis of preeclampsia: Current concepts. Am. J. Obstet. Gynecol. 1998, 179, 1359–1375. [Google Scholar] [CrossRef]
- Nevis, I.F.; Reitsma, A.; Dominic, A.; McDonald, S.; Thabane, L.; Akl, E.A.; Hladunewich, M.; Akbari, A.; Joseph, G.; Sia, W.; et al. Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin. J. Am. Soc. Nephrol. 2011, 6, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Villar, J.; Lindheimer, M. Maternal infection and risk of preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2008, 198, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Minassian, C.; Thomas, S.L.; Williams, D.J.; Campbell, O.; Smeeth, L. Acute maternal infection and risk of pre-eclampsia: A population-based case-control study. PLoS ONE 2013, 8, e73047. [Google Scholar] [CrossRef]
- Xiong, X.; Buekens, P.; Fraser, W.D.; Beck, J.; Offenbacher, S. Periodontal disease and adverse pregnancy outcomes: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cota, L.O.; Guimarães, A.N.; Costa, J.E.; Lorentz, T.C.; Costa, F.O. Association between maternal periodontitis and an increased risk of preeclampsia. J. Periodontol. 2006, 77, 2063–2069. [Google Scholar] [CrossRef]
- Mazor-Dray, E.; Levy, A.; Schlaeffer, F.; Sheiner, E. Maternal urinary tract infection: Is it independently associated with adverse pregnancy outcome? J. Matern.-Fetal Neonatal Med. 2009, 22, 124–128. [Google Scholar] [CrossRef]
- von Dadelszen, P.; Magee, L.A.; Krajden, M.; Alasaly, K.; Popovska, V.; Devarakonda, R.M.; Money, D.M.; Patrick, D.M.; Brunham, R.C. Levels of antibodies against cytomegalovirus and Chlamydophila pneumoniae are increased in early onset pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 725–730. [Google Scholar] [CrossRef]
- Heine, R.P.; Ness, R.B.; Roberts, J.M. Seroprevalence of antibodies to Chlamydia pneumoniae in women with preeclampsia. Obstet. Gynecol. 2003, 101, 221–226. [Google Scholar] [CrossRef] [PubMed]
- UstUn, Y.; Engin-UstUn, Y.; Ozkaplan, E.; Otlu, B.; Sait TekerekoGlu, M. Association of Helicobacter pylori infection with systemic inflammation in preeclampsia. J. Matern.-Fetal Neonatal Med. 2010, 23, 311–314. [Google Scholar] [CrossRef]
- Aksoy, H.; Ozkan, A.; Aktas, F.; Borekci, B. Helicobacter pylori seropositivity and its relationship with serum malondialdehyde and lipid profile in preeclampsia. J. Clin. Lab. Anal. 2009, 23, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef] [PubMed]
- Peleg, D.; Kennedy, C.M.; Hunter, S.K. Intrauterine growth restriction: Identification and management. Am. Fam. Physician 1998, 58, 453–460. [Google Scholar]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Bendix, I.; Miller, S.L.; Winterhager, E. Editorial: Causes and Consequences of Intrauterine Growth Restriction. Front. Endocrinol. 2020, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Wardinger, J.E.; Ambati, S. Placental Insufficiency. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Suhag, A.; Berghella, V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Mandelbrot, L. Fetal varicella—Diagnosis, management, and outcome. Prenatal. Diagn. 2012, 32, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Genç, M.; Ledger, W.J. Syphilis in pregnancy. Sex. Transm. Infect. 2000, 76, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Morales-Prieto, D.M.; Favaro, R.R.; Schneider, H.; Markert, U.R. Molecular Principles of Intrauterine Growth Restriction in Plasmodium Falciparum Infection. Front. Endocrinol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Kishore, J.; Misra, R.; Paisal, A.; Pradeep, Y. Adverse reproductive outcome induced by Parvovirus B19 and TORCH infections in women with high-risk pregnancy. J. Infect. Dev. Ctries. 2011, 5, 868–873. [Google Scholar] [CrossRef]
- Karowicz-Bilińska, A. The latent infection of human papilloma virus in pregnat woman and colonization of placenta—Preliminary report. Ginekol. Pol. 2008, 78, 966–970. [Google Scholar]
- Ford, J.H. Preconception risk factors and SGA babies: Papilloma virus, omega 3 and fat soluble vitamin deficiencies. Early Hum. Dev. 2011, 87, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Hong, P.L. Premature Rupture Of Membranes. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Assefa, N.E.; Berhe, H.; Girma, F.; Berhe, K.; Berhe, Y.Z.; Gebreheat, G.; Werid, W.M.; Berhe, A.; Rufae, H.B.; Welu, G. Risk factors of premature rupture of membranes in public hospitals at Mekele city, Tigray, a case control study. BMC Pregnancy Childbirth 2018, 18, 386. [Google Scholar] [CrossRef]
- Mandel, D.; Oron, T.; Mimouni, G.S.; Littner, Y.; Dollberg, S.; Mimouni, F.B. The Effect of Prolonged Rupture of Membranes on Circulating Neonatal Nucleated Red Blood Cells. J. Perinatol. 2005, 25, 690–693. [Google Scholar] [CrossRef][Green Version]
- Sirak, B.; Mesfin, E. Maternal and perinatal outcome of pregnancies with preterm premature rupture of membranes (pprom) at tikur anbessa specialized teaching hospital, addis ababa, ethiopia. Ethiop. Med. J. 2014, 52, 165–172. [Google Scholar]
- Mercer, B.; Milluzzi, C.; Collin, M. Periviable birth at 20 to 26 weeks of gestation: Proximate causes, previous obstetric history and recurrence risk. Am. J. Obstet. Gynecol. 2005, 193, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Solleti, V.; Jain, G.; Das, A.; Shama Prasada, K.; Acharya, S.; Satyamoorthy, K. Human Papillomavirus (HPV) Infection in Early Pregnancy: Prevalence and Implications. Infect. Dis. Obstet. Gynecol. 2019, 2019, 4376902. [Google Scholar] [CrossRef] [PubMed]
- Korteweg, F.J.; Erwich, J.; Holm, J.P.; Ravisé, J.M.; van der Meer, J.; Veeger, N.; Timmer, A. Diverse placental pathologies as the main causes of fetal death. Obstet. Gynecol. 2009, 114, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Achievements in Public Health, 1900–1999: Changes in the Public Health System. JAMA 2000, 283, 735–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gidengil, C.; Chen, C.; Parker, A.M.; Nowak, S.; Matthews, L. Beliefs around childhood vaccines in the United States: A systematic review. Vaccine 2019, 37, 6793–6802. [Google Scholar] [CrossRef]
- Marshall, H.; McMillan, M.; Andrews, R.M.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccin. Immunother. 2016, 12, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Gidengil, C.; Goetz, M.B.; Newberry, S.; Maglione, M.; Hall, O.; Larkin, J.; Motala, A.; Hempel, S. Safety of vaccines used for routine immunization in the United States: An updated systematic review and meta-analysis. Vaccine 2021, 39, 3696–3716. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017—Recommendations. Vaccine 2017, 35, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, E.; Sudre, P.; Chastonay, P. Introducing a HPV Vaccination Program: The Experience in the State of Geneva, Switzerland (2007–2009). World J. Vaccines 2011, 1, 11–14. [Google Scholar] [CrossRef]
- World Health Organization. Human papillomavirus vaccines: WHO position paper, October 2014—Recommendations. Vaccine 2015, 33, 4383–4384. [Google Scholar] [CrossRef] [PubMed]
- Yuill, S.; Egger, S.; Smith, M.; Velentzis, L.; Wrede, C.D.; Bateson, D.; Canfell, K. Has Human Papillomavirus (HPV) Vaccination Prevented Adverse Pregnancy Outcomes? Population-Level Analysis After 8 Years of a National HPV Vaccination Program in Australia. J. Infect. Dis. 2020, 222, 499–508. [Google Scholar] [CrossRef]
- Magaña-León, C.; Oros, C.; López-Revilla, R. Human papillomavirus types in non-cervical high-grade intraepithelial neoplasias and invasive carcinomas from San Luis Potosí, Mexico: A retrospective cross-sectional study. Infect. Agents Cancer 2015, 10, 33. [Google Scholar] [CrossRef][Green Version]
- Giraldo, P.C.; Sanches, J.M.; Sparvolli, L.G.; Amaral, R.; Migliorini, I.; Gil, C.D.; Taddei, C.R.; Witkin, S.S.; Discacciati, M.G. Relationship between Papillomavirus vaccine, vaginal microbiome, and local cytokine response: An exploratory research. Braz. J. Microbiol. 2021, 52, 2363–2371. [Google Scholar] [CrossRef]
- WHO Position on HPV Vaccines. Vaccine 2009, 27, 7236–7237. [CrossRef]
- Poland, G.A.; Jacobson, R.M.; Koutsky, L.A.; Tamms, G.M.; Railkar, R.; Smith, J.F.; Bryan, J.T.; Cavanaugh, P.F., Jr.; Jansen, K.U.; Barr, E. Immunogenicity and Reactogenicity of a Novel Vaccine for Human Papillomavirus 16: A 2-Year Randomized Controlled Clinical Trial. Mayo Clin. Proc. 2005, 80, 601–610. [Google Scholar] [CrossRef]
- Human Medicines European Public Assessment Report (EPAR). Gardasil 9: Human papillomavirus 9-valent vaccine (recombinant, adsorbed), Condylomata Acuminata, Papillomavirus Infections, Immunization, Uterine Cervical Dysplasia. Case Med. Res. 2019, 32, 414–421. [Google Scholar] [CrossRef]
- Liu, G.; Kong, L.; Du, P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.L.; Tunis, M.C.; Ismail, S. Summary of the NACI Update on the recommended use of Human Papillomavirus (HPV) vaccine: Nine-valent HPV vaccine two-dose immunization schedule and the use of HPV vaccines in immunocompromised populations. Can. Commun. Dis. Rep. 2017, 43, 138–142. [Google Scholar] [CrossRef]
- Munn, M.S.; Kay, M.; Page, L.C.; Duchin, J.S. Completion of the Human Papillomavirus Vaccination Series Among Adolescent Users and Nonusers of School-Based Health Centers. Public Health Rep. 2019, 134, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bonde, U.; Joergensen, J.S.; Lamont, R.F.; Mogensen, O. Is HPV vaccination in pregnancy safe? Hum. Vaccines Immunother. 2016, 12, 1960–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McNamara, M.; Batur, P.; Walsh, J.M.E.; Johnson, K.M. HPV Update: Vaccination, Screening, and Associated Disease. J. Gen. Intern. Med. 2016, 31, 1360–1366. [Google Scholar] [CrossRef]
- Valasoulis, G.; Pouliakis, A.; Michail, G.; Kottaridi, C.; Spathis, A.; Kyrgiou, M.; Paraskevaidis, E.; Daponte, A. Alterations of HPV-Related Biomarkers after Prophylactic HPV Vaccination. A Prospective Pilot Observational Study in Greek Women. Cancers 2020, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Bevis, K.S.; Biggio, J.R. Cervical conization and the risk of preterm delivery. Am. J. Obstet. Gynecol. 2011, 205, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sedgh, G.; Hussain, R. Unintended Pregnancy: Worldwide Levels, Trends, and Outcomes. Stud. Fam. Plan. 2010, 41, 241–250. [Google Scholar] [CrossRef]
- Eckert, L.O.N. Human Papillomavirus Vaccination: ACOG Committee Opinion Summary, Number 809. Obstet. Gynecol. 2020, 136, E15–E21. [Google Scholar]
- El-shrqawy, E.; El-Nemer, A. Unintended Pregnancy: Associated Factors and outcomes among pregnant women. Mansoura Nurs. J. 2020, 7, 227–240. [Google Scholar] [CrossRef]
- Kuehn, B.M. Pandemic Contributes to Worse Pregnancy Outcomes Worldwide. JAMA 2021, 325, 1931. [Google Scholar] [CrossRef] [PubMed]
- Klima, C. Unintended pregnancy Consequences and solutions for a worldwide problem. J. Nurse-Midwifery 1998, 43, 483–491. [Google Scholar] [CrossRef]
- Ward, D.; Thorsen, N.M.; Frisch, M.; Valentiner-Branth, P.; Mølbak, K.; Hviid, A. A cluster analysis of serious adverse event reports after human papillomavirus (HPV) vaccination in Danish girls and young women, September 2009 to August 2017. Euro Surveill. 2019, 24, 1800380. [Google Scholar] [CrossRef] [PubMed]
- Scheller, N.M.; Svanström, H.; Pasternak, B.; Arnheim-Dahlström, L.; Sundström, K.; Fink, K.; Hviid, A. Quadrivalent HPV Vaccination and Risk of Multiple Sclerosis and Other Demyelinating Diseases of the Central Nervous System. JAMA 2015, 313, 54. [Google Scholar] [CrossRef] [PubMed]
- Scheller, N.M.; Pasternak, B.; Svanström, H.; Hviid, A. Quadrivalent Human Papillomavirus Vaccine and the Risk of Venous Thromboembolism. JAMA 2014, 312, 187. [Google Scholar] [CrossRef][Green Version]
- Arnheim-Dahlstrom, L.; Pasternak, B.; Svanstrom, H.; Sparen, P.; Hviid, A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: Cohort study. BMJ 2013, 347, f5906. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, O.A.; Befano, B.L.; Gonzalez, P.; Rodríguez, A.C.; Herrero, R.; Schiller, J.T.; Kreimer, A.R.; Schiffman, M.; Hildesheim, A.; Wilcox, A.J.; et al. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: Long term observational follow-up in the Costa Rica HPV Vaccine Trial. BMJ 2015, 7, 351. [Google Scholar] [CrossRef]
- Garland, S.M.; Ault, K.A.; Gall, S.A.; Paavonen, J.; Sings, H.L.; Ciprero, K.L.; Saah, A.; Marino, D.; Ryan, D.; Radley, D.; et al. Pregnancy and Infant Outcomes in the Clinical Trials of a Human Papillomavirus Type 6/11/16/18 Vaccine. Obstet. Gynecol. 2009, 114, 1179–1188. [Google Scholar] [CrossRef]
- Dana, A.; Buchanan, K.M.; Goss, M.A.; Seminack, M.M.; Shields, K.E.; Korn, S.; Cunningham, M.L.; Haupt, R.M. Pregnancy Outcomes From the Pregnancy Registry of a Human Papillomavirus Type 6/11/16/18 Vaccine. Obstet. Gynecol. Surv. 2010, 65, 169–170. [Google Scholar] [CrossRef]
- Wacholder, S.; Chen, B.E.; Wilcox, A.; Macones, G.; Gonzalez, P.; Befano, B.; Hildesheim, A.; Rodriguez, A.C.; Solomon, D.; Herrero, R.; et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: Pooled analysis of two randomised controlled trials. BMJ 2010, 340, c712. [Google Scholar] [CrossRef] [PubMed]
- Narducci, A.; Einarson, A.; Bozzo, P. Human papillomavirus vaccine and pregnancy. Can. Fam. Physician Med. Fam. Can. 2012, 58, 268–269. [Google Scholar]
| Genus | Biological and Clinical Aspects |
|---|---|
| Alpha- papillomavirus | Mucosal and cutaneous lesions Comprised of 14 species (α1–α14) that include 65 HPV types [8] Molecular genotyping of HPV L1 gene in low-risk and high-risk groups |
| Beta- papillomavirus | Cutaneous lesions Comprised of 5 species (β1–β5) that include 54 HPV types [9] Reports of beta HPV in mucosal epithelia [10,11] Etiological role in non-melanoma skin cancer [12] Promotes the development of cSCC in EV patients [13] |
| Gamma- papillomavirus | Cutaneous lesions Comprised of 27 species (γ1–γ5) that include 98 HPV types [9] Reports of gamma HPV in mucosal epithelia [14,15] |
| Mu- papillomavirus | Cutaneous lesions Includes 3 HPV types [16] |
| Nu- papillomavirus | Cutaneous lesions Includes 1 HPV type [16] |
| Authors, Year | Study Type | Sample | HPV Detection | HPV Type | HPV History (e.g., Previous History of CIN, Genital Warts) | Conclusions |
| Cotton-Caballero et al., 2017 [91,92] | Retrospective cohort study (2153 pregnant women) | Cervical samples | Cervical cytology HPV genotyping | HR-HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) | Patients with treated cervical dysplasia (conization, loop electrosurgical excision procedure, and cryotherapy) were included and adjusted for | HR-HPV infection led to an increase in PPROM and preterm birth resulting from PPROM, but not preterm birth without PPROM |
| Huang et al., 2014 [93] | Systematic review (8 studies) | Cervical samples | Cervical cytology HPV DNA testing (ISH, PCR) | HR-HPV LR-HPV undefined | Two studies in the meta-analysis included adjustment for prior cervical procedures | HR-HPV-infected women had an overall 2.55-fold increased risk of delivering prematurely |
| Zuo et al., 2011 [94] | Retrospective study (2480 cases) | Cervical samples Placental tissue | Cervical cytology Reflex HPV DNA testing via RNA-DNA hybrids Pathologic examination of the placenta | HR-HPV | Not specified | HR-HPV-related changes in cervical cytology were associated with preterm birth and placental abnormalities |
| Gomez et al., 2008 [62] | Case–control study (108 cases) | Placental tissue | HPV DNA testing (PCR) followed by HPV type confirmation via DNA sequencing | HR-HPV (types 16, 18) LR-HPV (types 6, 11) | Not specified | HR-HPV infection was correlated with placental abnormalities and preterm delivery HR-HPV infection did not increase the risk of preeclampsia |
| Niyibizi et al., 2021 [95] | Prospective cohort study (899 pregnant women) | Vaginal secretion samples Placental tissue | HPV DNA testing and genotyping (PCR) | HR-HPV LR-HPV | 7.1% of women had previously undergone treatment for CIN | Persistent vaginal HPV-16/18 infection and placental HPV infection were associated with an increased risk of preterm delivery Treatment for HR-HPV-related cervical dysplasia also increased the risk of delivering prematurely |
| Wiik et al., 2021 [96] | Retrospective population-based register study (400,583 pregnant women) | Cervical samples | HPV DNA testing Cervical cytology Cervical histology | Not specified | Women previously treated for CIN were excluded from this study | HPV infection identified via DNA testing was associated with a higher risk of PPROM than HPV infection, certified through cytology, without DNA testing Both positive cytology and positive HPV DNA testing were associated with an increased risk of preterm delivery, PROM, PPROM, and neonatal mortality |
| Aldhous et al., 2019 [85] | Data-linkage study (5598 pregnant women) | Cervical samples | HPV DNA testing Cervical cytology Cervical histology | HR-HPV LR-HPV undefined | No data regarding previous treatments for HPV-associated cervical disease | High-grade HPV-related cervical disease was associated with an increased risk of preterm birt hLow-grade HPV-related cervical disease and HR-HPV infection with no disease did not increase the risk of preterm delivery |
| Ambühl et al., 2017 [97] | Prospective case–control study (271 pregnant women) | Placental tissue | HPV DNA detection via nested PCR, followed by HPV genotyping via CISH | HR-HPV LR-HPV | Patients with genital warts, cervical dysplasia/carcinoma in situ/cancer were included in this study | Placental HPV infection was more frequent among women with history of cervical cancer The prevalence of placental HPV was similar in both complicated and uncomplicated pregnancies |
| Subramaniam et al., 2016 [84] | Retrospective cohort study (2321 pregnant women) | Cervical samples | HPV DNA testing Cervical cytology | HR-HPV | Women previously treated for CIN were excluded from this study | HR-HPV infection did not increase the risk of developing pregnancy-induced hypertensive disorders (PIHDs) and/or delivering prematurely HR-HPV infection was associated with an increased risk of placental abruption and severe preeclampsia |
| Ambühl et al., 2016 [98] | Systematic literature search (42 studies) | Cervical samples Placental tissue | HPV DNA testing (PCR, DNA chip, hybrid capture, Southern blot) Pathologic examination of the placenta | HR-HPV LR-HPV | Studies either included or excluded women with HPV-related lesions One-third of studies did not specify this aspect | Overall, the authors concluded that HPV infection could increase the risk of spontaneous abortion or spontaneous preterm delivery |
| Conde-Ferráez et al., 2013 [83] | Case–control study (127 cases) | Cervical samples | HPV DNA testing (PCR) HPV genotyping (NMPCR) | HR-HPV LR-HPV | Not specified | No significant association between HPV infection and spontaneous abortion was found |
| Cho et al., [87] | Cross-sectional study (311 cases) | Cervical samples | HPV DNA testing (via RNA–DNA hybrids) | HR-HPV | Not specified | HR-HPV infection was associated with an increased risk of PROM at term HR-HPV infection was not linked to a higher risk of preeclampsia |
| Nimrodi et al., 2018 [99] | Retrospective cohort study (15,357 cases) | Cervical samples | Cervical cytology | Not specified | Not specified | HPV infection did not increase the risk of developing preeclampsia, cervical insufficiency, placental abruption, PROM, PPROM, or preterm delivery |
| McDonnold et al., 2013 [100] | Retrospective cohort study (942 cases) | Cervical samples | Cervical cytology HPV DNA testing | HR-HPV | Not specified | HR-HPV appeared to contribute an approximately two-fold increase in preeclampsia risk |
| Slatter et al., 2015 [65] | Cross-sectional study (339 cases) | Placental tissue | Pathologic examination of the placenta | HPV DNA testing (IHC, CISH, PCR) | History of cervical HPV infection was available for two=thirds of women | Placental HPV infection was linked to negative pregnancy outcomes and complications, such as preterm birth, fetal growth restriction, fetal demise, diabetes, and preeclampsia Previous cervical HPV infection was a risk factor for developing placental HPV infection |
| Ford et al., 2019 [89] | Data-linkage study (31,827 pregnant women) | Cervical samples | Cervical cytology | Not specified | Women with previous abnormal cervical cytology were included in the study and adjusted for | Abnormal Pap results were an independent risk factor for IUGR, and especially very low birthweight |
| Giambanco et al., 2020 [101] | Case series (20 cases) | Cervical samples | Cervical cytology HPV DNA testing (multiplex RT-PCR) | HR-HPV LR-HPV (but not specified) | Women with previous history of CIN and/or abnormal Pap smears were excluded from the study | HPV infection was not associated with adverse pregnancy outcomes such as miscarriage, PPROM, and preterm birth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condrat, C.E.; Filip, L.; Gherghe, M.; Cretoiu, D.; Suciu, N. Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses 2021, 13, 2455. https://doi.org/10.3390/v13122455
Condrat CE, Filip L, Gherghe M, Cretoiu D, Suciu N. Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses. 2021; 13(12):2455. https://doi.org/10.3390/v13122455
Chicago/Turabian StyleCondrat, Carmen Elena, Lidia Filip, Mirela Gherghe, Dragos Cretoiu, and Nicolae Suciu. 2021. "Maternal HPV Infection: Effects on Pregnancy Outcome" Viruses 13, no. 12: 2455. https://doi.org/10.3390/v13122455
APA StyleCondrat, C. E., Filip, L., Gherghe, M., Cretoiu, D., & Suciu, N. (2021). Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses, 13(12), 2455. https://doi.org/10.3390/v13122455







