Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects and Samples
2.2. Quantification of Serum Markers
2.3. Statistical Analysis
3. Results
3.1. Cohort Characteristics
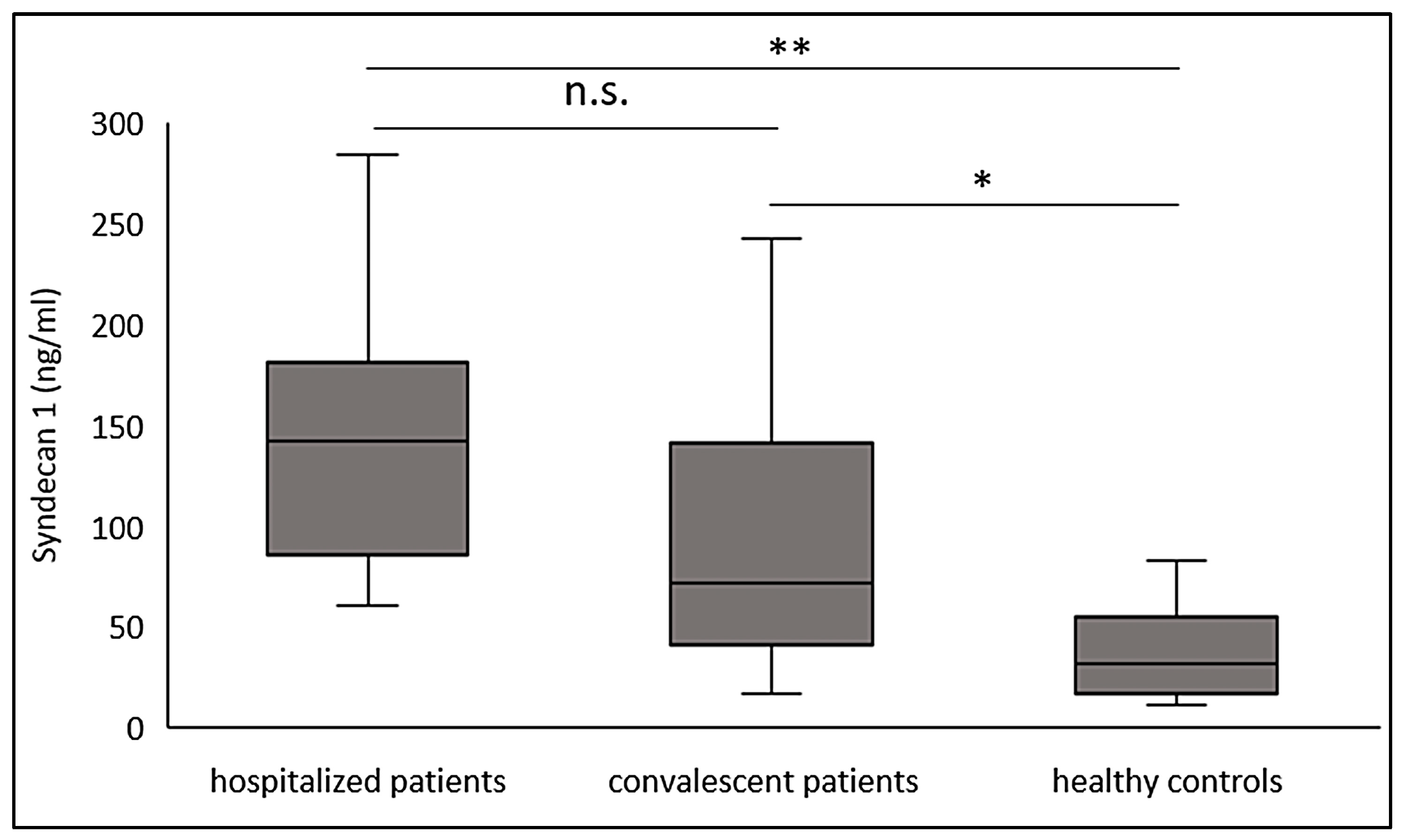
3.2. Significantly Increased Syndecan-1 Levels in Convalescent COVID-19 Patients
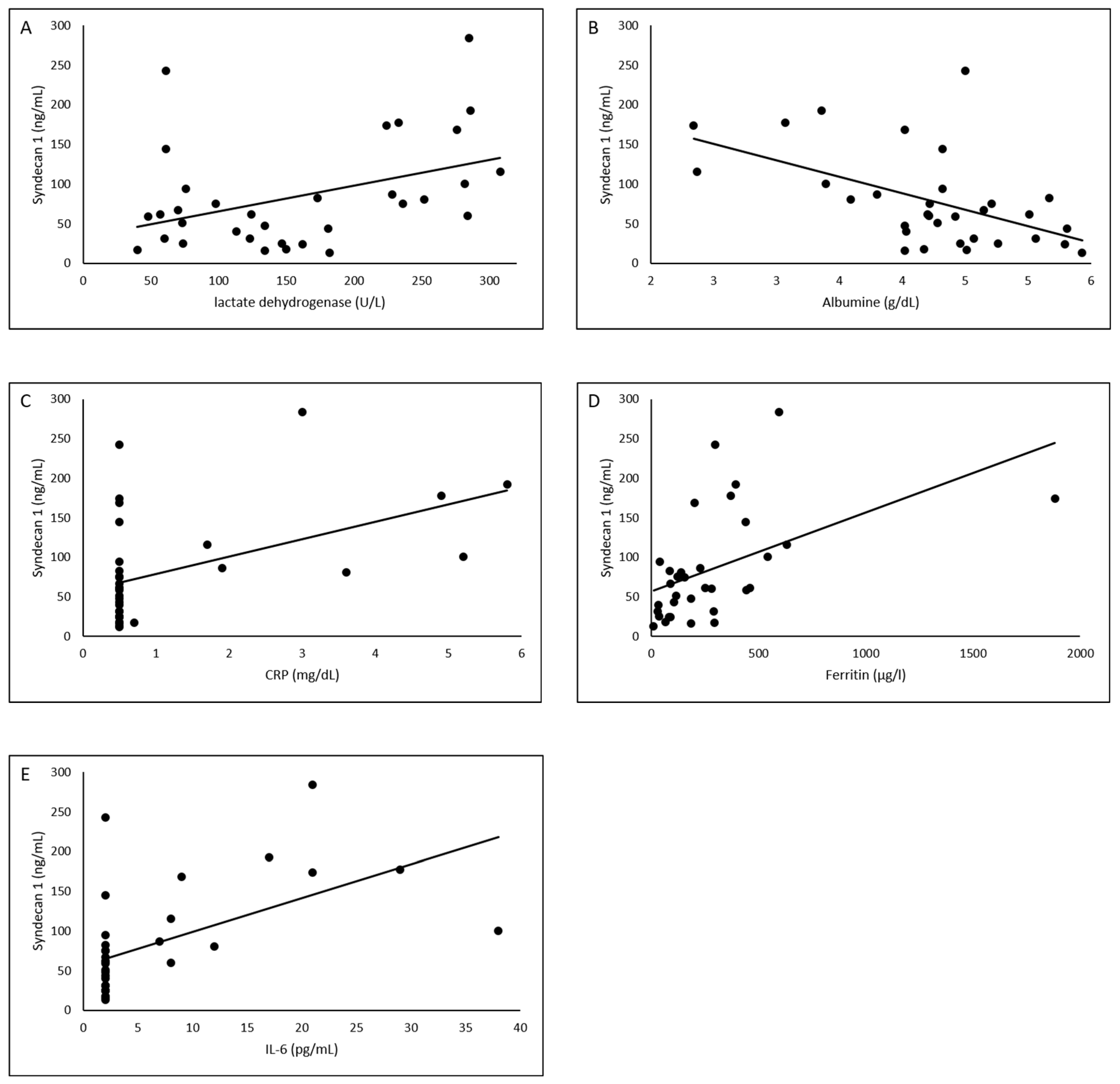
3.3. Association of Laboratory Values with Syndecan-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Yoshida, S.; Hara, A.; Ogura, S.; Tomita, H. Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection. Microcirculation 2021, 28, e12654. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; de Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [Green Version]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflüg. Arch. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodcock, T.E.; Woodcock, T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012, 108, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; Dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, R.; Ge, L.; Schlesinger, S.Y.; Birkland, T.P.; Huang, Y.; Parimon, T.; Lee, V.; McKinney, B.L.; McGuire, J.K.; Parks, W.C.; et al. Syndecan-1 Attenuates Lung Injury during Influenza Infection by Potentiating c-Met Signaling to Suppress Epithelial Apoptosis. Am. J. Respir. Crit. Care Med. 2016, 194, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef] [PubMed]
- Tepasse, P.-R.; Vollenberg, R.; Fobker, M.; Kabar, I.; Schmidt, H.; Meier, J.A.; Nowacki, T.; Hüsing-Kabar, A. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients 2021, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Zahednasab, H.; Farahmand, M.; Mirzaei, R.; Zamani, F.; Tabibzadeh, A.; Bouzari, B.; Ajdarkosh, H.; Nikkhah, M.; Hashemi, M.R.; et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, T.D.; Bang-Christensen, S.R.; Jørgensen, A.M.; Løppke, C.; Spliid, C.B.; Sand, N.T.; Clausen, T.M.; Salanti, A.; Agerbæk, M.Ø. The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis. Front. Cell Dev. Biol. 2020, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Berkestedt, I.; Bodelsson, M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol. Scand. 2014, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Kautzky-Willer, A.; Gremmel, T.; Koppensteiner, R.; Wolf, P.; Ertl, S.; Weikert, C.; Schörgenhofer, C.; Jilma, B. Sublingual microvasculature in diabetic patients. Microvasc. Res. 2020, 129, 103971. [Google Scholar] [CrossRef]
- Ambrosino, P.; Calcaterra, I.; Molino, A.; Moretta, P.; Lupoli, R.; Spedicato, G.A.; Papa, A.; Motta, A.; Maniscalco, M.; Di Minno, M.N.D. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines 2021, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Molino, A.; Calcaterra, I.; Formisano, R.; Stufano, S.; Spedicato, G.A.; Motta, A.; Papa, A.; Di Minno, M.N.D.; Maniscalco, M. Clinical Assessment of Endothelial Function in Convalescent COVID-19 Patients Undergoing Multidisciplinary Pulmonary Rehabilitation. Biomedicines 2021, 9, 614. [Google Scholar] [CrossRef]
- Chioh, F.W.; Fong, S.-W.; Young, B.E.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L.L.; Gao, F.; et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife 2021, 10, e64909. [Google Scholar] [CrossRef]
| Patients (Samples) | Hospitalized Patients (n = 10) | Convalescent Patients (n = 24) | Control Collective (n = 13) | p-Value | |
|---|---|---|---|---|---|
| Patient Characteristics | Age, years median (IQR) | 56.5 (54–63) | 56 (55–58) | 54 (51–57) | 0.08 |
| Gender, male (%) | 10 (100) | 22 (91.6) | 4 (31) | 0.046 | |
| BMI (IQR) | 23.8 (22–25.9) | 26.1 (22.8–28.1) | 22.3 (20.2–25.7) | 0.161 | |
| Invasive Vent. (%) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Oxygen therapy (%) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Death (abs.) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Interval 1. symptom to blood sample, days (IQR) | 6 (2–17) | 88 (70–135) | <0.001 | ||
| SARS CoV-2 therapy | Remdesivir (5 days) | 1 (10) | 0 (0) | 0 (0) | 0.151 |
| Dexamethasone (6 mg, 10 days) | 2 (20) | 0 (0) | 0 (0) | 0.021 | |
| Pre-existing conditions | Chronic inflammatory disease (%) | 4 (40) | 0 (0) | 0 (0) | <0.001 |
| Respiratory disease (%) | 3 (30) | 0 (0) | 0 (0) | 0.003 | |
| Kidney insufficiency (%) | 1 (10) | 0 (0) | 0 (0) | 0.151 | |
| Metastatic neoplasm (%) | 1 (10) | 0 (0) | 0 (0) | 0.151 | |
| Diabetes (%) | 1 (10) | 0 (0) | 0 (0) | 0.151 | |
| Arterial hypertension (%) | 4 (40) | 0 (0) | 0 (0) | <0.001 | |
| Coronary heart disease (%) | 2 (20) | 0 (0) | 0 (0) | 0.021 | |
| Medication | Angiotensin-1 receptor antagonist (%) | 1 (10) | 0 (0) | 0 (0) | 0.151 |
| Angiotensin converting enzyme inhibitor (%) | 2 (10) | 0 (0) | 0 (0) | 0.021 |
| Hospitalized Patients (n = 10) | Convalescent Patients (n = 24) | Healthy Controls (n = 14) | p-Value | |
|---|---|---|---|---|
| Syndecan-1 (ng/mL), median (IQR) | 142 (85.2–181.3) | 71.2 (40.1–141.5) | 31.6 (17.1–54.7) | <0.001 |
| Lymphocytes (rel., %), median (IQR) | 22.2(16.5–33.2) | 28.5 (27.2–34.4) | n.d. | 0.23 |
| Creatinine (mg/dL), median (IQR) | 0.95 (0.88–1.3) | 0.9 (0.8–01.0) | 1 (0.8–1.2) | 0.77 |
| Bilirubin (mg/dL), median (IQR) | 0.4 (0.3–0.7) | 0.5 (0.4–1.0) | 0.5 (0.2–0.7) | 0.43 |
| AST (U/L), median (IQR) | 36 (26–52) | 28 (25–34) | 29 (22–35) | 0.63 |
| Gamma-GT (U/L), median (IQR) | 82 (40–146) | 27 (22–46) | 22 (12–24) | 0.003 |
| LDH (U/L), median (IQR) | 279 (232–285) | 61 (57–74) | 149 (127–179) | <0.001 |
| CRP (mg/dL), median (IQR) | 2.5 (0.5–5) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | <0.001 |
| Albumin (g/dL), median (IQR) | 3.4 (2.7–3.9) | 4.4 (4.3–4.5) | 4.9 (4.1–5.3) | <0.001 |
| Ferritin (µg/L), median (IQR) | 383 (220–606) | 252 (89–298) | 87 (42–178) | 0.002 |
| Interleukin-6 (pg/mL), median (IQR) | 14.5 (8–23) | 2 (2–2) | 2 (2–2) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollenberg, R.; Tepasse, P.-R.; Ochs, K.; Floer, M.; Strauss, M.; Rennebaum, F.; Kabar, I.; Rovas, A.; Nowacki, T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses 2021, 13, 2324. https://doi.org/10.3390/v13112324
Vollenberg R, Tepasse P-R, Ochs K, Floer M, Strauss M, Rennebaum F, Kabar I, Rovas A, Nowacki T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses. 2021; 13(11):2324. https://doi.org/10.3390/v13112324
Chicago/Turabian StyleVollenberg, Richard, Phil-Robin Tepasse, Kevin Ochs, Martin Floer, Markus Strauss, Florian Rennebaum, Iyad Kabar, Alexandros Rovas, and Tobias Nowacki. 2021. "Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis" Viruses 13, no. 11: 2324. https://doi.org/10.3390/v13112324
APA StyleVollenberg, R., Tepasse, P.-R., Ochs, K., Floer, M., Strauss, M., Rennebaum, F., Kabar, I., Rovas, A., & Nowacki, T. (2021). Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses, 13(11), 2324. https://doi.org/10.3390/v13112324






