Abstract
Edible insects are expected to become an important nutrient source for animals and humans in the Western world in the near future. Only a few studies on viruses in edible insects with potential for industrial rearing have been published and concern only some edible insect species. Viral pathogens that can infect insects could be non-pathogenic, or pathogenic to the insects themselves, or to humans and animals. The objective of this systematic review is to provide an overview of the viruses detected in edible insects currently considered for use in food and/or feed in the European Union or appropriate for mass rearing, and to collect information on clinical symptoms in insects and on the vector role of insects themselves. Many different virus species have been detected in edible insect species showing promise for mass production systems. These viruses could be a risk for mass insect rearing systems causing acute high mortality, a drastic decline in growth in juvenile stages and in the reproductive performance of adults. Furthermore, some viruses could pose a risk to human and animal health where insects are used for food and feed.
Keywords:
edible insects; mass rearing; production system; controlled environment; virus; regulation 1. Introduction
Edible insects are expected to become an important nutrient source for animals and humans in the Western world in the near future and traditionally hold this status in many tropical countries [1,2]. The reasons why insects are regarded as an alternative source of animal protein are environmental, nutritional and economic [3,4,5,6]. More than 2000 edible insect species are consumed worldwide [7]. The insects most widely consumed by humans belong to the Coleoptera (31%), Lepidoptera (18%), Hymenoptera (14%), Orthoptera (13%) and Hemiptera (10%) [8] orders and can be consumed at different life stages—eggs, larvae, pupae, or adults [4,8,9]. Only a few of the edible species reported in the literature [7] meet the demands of mass rearing systems and industrial activities. Twelve insect species have been reported by EFSA to have the greatest potential to be used as food and feed in the European Union [10]. Edible insects could be harvested seasonally in the wild or reared in controlled environments in most European countries [11]. The different production systems (i.e., industrialized rearing, insect farming, or wild harvesting) by which the edible insects are bred can contribute to differences in their safety [2,11,12,13]. In recent years, due to the novelty and microbial complexity of industrial insect rearing for human consumption, many articles and reviews have explored possible food safety hazards to human and animal health associated with the use of insects for food and feed, including chemical, microbiological and allergenic agents, and prions [10,11,14,15,16,17,18,19,20,21]. In addition to ‘general food hygiene requirements’, the production and marketing of insects as food in the EU is governed by ‘Novel Foods’ legislation—i.e., Regulation (EU) No 2015/2283 [22]; Regulation (EU) 2017/2469 [23]; Regulation (EU) 2017/625 [24]; Regulation (EU) 2019/1381 [25]; Regulation (EU) No 142/2011 [26]; Regulation (EU) 2017/893 [27]. EFSA Scientific Opinion (2015) and adoptions and discussions of the EFSA NDA Panel on Nutrition (2016) are also taken into consideration [10,28].
Both insects collected in Nature and those raised on farms may be infected with pathogenic microorganisms, including bacteria, viruses, fungi, protozoa and other organisms that can affect their safety as food [16,29]. Insects represent the largest group of animals on earth in terms of biodiversity, with an estimated 5.5 million different species [30,31]. This diversity is reflected in a matching range of infecting viruses, which not only positively or negatively affect insect populations, but can also have a major impact on human well-being [32,33]. Insects have been shown to contain a wide variety of viruses up to indicate insects as major reservoirs and vectors of viruses [34,35,36,37,38] and recent papers reveal an enormous diversity in RNA viruses detected in insect viruses [36].
There is an abundant literature on the presence of viruses in insects of economic value or importance to public health, as in the case of silkworms and mosquitoes [39,40,41]. Few studies have been conducted on viruses in edible insects with potential for industrial rearing and concern mainly pathogenic insect viruses, including only some edible insect species [10,16,42]. Insects could harbor a plethora of viruses: (i) microbiota viruses; (ii) viruses pathogenic to insects themselves; (iii) viruses pathogenic to vertebrates, both animal and human. Viruses are part of the normal microbiota of an insect, i.e., its virome, and thus intrinsically associated with insect metabolism, behavior and survival. Albeit under situations of stress, the virome could become pathogenic for the insect [31,43]. Viruses pathogenic to insects can cause a decline in growth and reproductive performance, in addition to disease and mortality. For these reasons, said viruses pose a major concern in insect mass rearing systems where insects are raised at high densities [16,44]. The fact that invertebrate viruses can be transmitted to vertebrates further increases the importance of screening measures for commercially bred prey insects [45]. Viruses pathogenic to vertebrate hosts could be found in insects. While these viruses do not replicate in insects and are not actively transmitted to vertebrates by insect vectors, they could be transmitted passively by insects acting as mechanical vectors [10,46]. This vector capacity highlights the potential of insects produced for food and feed to transmit viral diseases to vertebrates [10]. In some cases, however, insects are replicative vectors of viruses infecting vertebrates. Arboviruses can replicate in their vector, infect vertebrates, and cause severe human (i.e., dengue fever, West Nile disease, Rift Valley fever, hemorrhagic fever, chickungunya fever) and animal (Schmallenberg) diseases [47,48]. To date there is no evidence of these viruses in edible insects.
The focus of this study was on virus species detected to date in edible insect species currently considered for use in food and/or feed in the EU or with characteristics suited to mass rearing systems.
2. Materials and Methods
2.1. Data Collection Process (Information Sources, Search Strategy, Eligibility Criteria)
For this systematic review, we performed a literature search to identify scientific articles reporting viruses in diverse edible insect species. The information retrieved from the literature conforms to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement [49].
The insect species included in this review are those considered by EFSA, with the exception of Bombyx mori: Musca domestica, Hermetia illucens, Tenebrio molitor, Zophobas morio, Alphitobus diaperinus, Galleria mellonella, Achroia grisella, Acheta domesticus, Gryllodes sigillatus, Locusta migratora and Schistocerca americana (Table 1). Since the taxonomy and classification of Zophobas morio is still unclear and is currently identified as conspecific with Zophobas atratus [50], in this review, as in the one by Rumbos and Athanassiou [51], we consider Z. morio and Z. atratus as one species, referred to as Z. morio. However, in order to collect all possible information, both scientific names were used in the string terms.

Table 1.
List of edible insect species considered in this review.
In addition to the species considered promising by EFSA, we decided to also include: Tribolium castaneum, Gryllus assimilis, Gryllus bimaculatus, and Schistocerca gregaria in this review. These species have good potential as edible sources due to their breeding characteristics and/or resistance to viruses. Two of them (G. assimilis and S. gregaria) have already been listed by Mleck et al. [52] as suitable food species in Europe and other developed countries. Furthermore, G. assimilis and G. bimaculatus are listed by Weissmann et al. [44] as two of the five most commercially important cricket species worldwide.
Silk and honey are the primary products of the Bombyx mori (Silkworm) and Apis mellifera (Western honeybee), respectively. Although the edible part of these insects (silkworm pupae and bee brood) is already consumed in many parts of the world and constitutes a promising edible resource, it is not specifically covered in this review because it is only a marginal part of the production system of these insect species [53,54,55].
Relevant studies were searched through two online database repositories, PubMed and Web of Science, using the keywords: “virus*” AND “scientific name of edible insect species” OR “common name of edible insect species” (see Table 1 for the name details). The research was limited to studies published in English up to February 2021. Subsequently, after removing duplicates, only full-text articles were screened, including the grey literature (i.e., materials and research produced by organizations outside traditional commercial or academic publishing and distribution channels); only papers providing original research and data were selected and considered for this review (eligibility assessment). Relevant papers and reviews were also manually cross checked to identify further references, which have been identified as “other sources” in Table S1 The following data were extracted from selected articles and listed in Table S1: bibliographic details (database source, authors, title, source, year of publication, DOI or any PMID) and name of the edible insect species considered. Information regarding viruses detected in each insect species, grouped by order, is discussed in the text and summarized in table form.
2.2. Summary of Extracted Data
The four-phase study selection process in the present review is reported in Figure 1. In the initial research a total of 783 studies were identified (PubMed = 248, Web of Science = 490, citations from retrieved articles = 45). Of these, 608 papers were retrieved after removal of duplicates (161) and documents without full text availability (14). Of these, a final 176 articles, fulfilling the inclusion criteria, were identified for this review. The other publications (n = 432) were discarded due to: no virus detected in the insect species considered; no monitoring activity; virus detected in insect species not considered in this review; lack of original data; studies regarding other topics; unclear presentation of data.
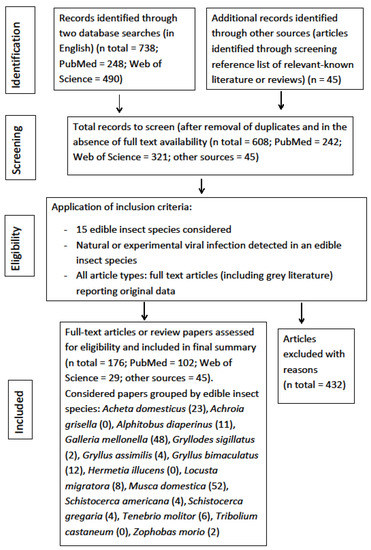
Figure 1.
PRISMA flowchart diagram of the selection of eligible studies (literature research up to February 2021).
3. Results
The edible insect species considered in this review are grouped below by order (Coleoptera, Diptera, Lepidoptera and Orthoptera). A specific paragraph has been created for each insect species, describing the main nutritional and productive characteristics, potential for mass rearing systems, and associated viruses. Information on virus characteristics (i.e., family, genus, species, and genomic aspects), insect stage involved during viral infection, type of viral infection (i.e., natural if virus was detected in the wild or experimental if a species of insect was tested for susceptibility to a virus), any detected symptoms or mortality, have been summarized in the tables (Note 1: in this review, chilo iridescent virus (CIV) is used as synonym of invertebrate iridescent virus 6 (IIV-6), the type species of the genus Iridovirus within the family Iridoviridae [56]; Note 2: within the Iridoviridae family, different or novel strains of invertebrate iridescent virus 6 (i.e., invertebrate iridescent virus 29, cricket iridescent virus, Gryllus bimaculatus iridescent virus, and lizard-cricket iridescent virus) have been considered separately [57,58,59,60]).
3.1. Coleoptera
3.1.1. Alphitobius diaperinus
The darkling beetle, Alphitobius diaperinus, is one of the most abundant insect pests in commercial poultry production facilities, with cosmopolitan occurrence [61,62,63]. Since 1 July 2017, A. diaperinus has been listed as one of the seven insect species authorized to date for use in the large-scale production of processed animal protein (PAP) for aquaculture feeds, in accordance with EU Regulation (EC) No. 2017/893 [27]. For this reason, several papers describing nutritional aspects, breeding facilities, and microbial dynamics during the industrial production cycle [64,65,66,67,68,69] are present in the literature. Although A. diaperinus belongs to the species with the greatest potential to be used as food and feed in the EU [10], it could serve as a reservoir and vector for a plethora of pathogenic microorganisms, as bacteria, fungi, coccidia, worms, and tapeworms, and of viruses that cause serious diseases [51,70,71]. It is a mechanical vector of avian viruses belonging to different genuses: Marek’s disease, avian leucosis virus, fowl pox virus (FWPV), infectious bursal disease virus (IBDV), turkey coronavirus (TCV), Newcastle disease viruses, infectious laryngotracheitis virus (ILTV), and reovirus 24 [70,72,73,74,75,76,77,78,79]. These viruses could survive, from a few days to several weeks, inside and on the external surfaces of both adult beetles and larvae [70,75,76,77,79] and could also survive the metamorphosis of this beetle [74].
Only two insect viruses have been found in the lesser mealworm that parasitize honeycombs: black queen cell virus (BQCV) and Israeli acute paralysis virus (IAPV) [80]. These viruses cause severe disease in honeybees, but no signs or symptoms of disease have been noted in A. diaperinus, which appears to act only as a vector and reservoir for honeybee viruses [80]. At the present time, neither human foodborne pathogens nor insect viruses pathogenic for this species have been identified [81] but since the analyses involved only a limited number of samples, more studies are needed.
3.1.2. Tenebrio molitor
The yellow mealworm Tenebrio molitor is one of the largest stored-product beetles, which are widespread across the world [82]. This beetle is increasingly recognized as an optimal alternative and sustainable nutrient source for animal feed and human food due to its protein-rich content and low ecological footprint [65,66,83]. Furthermore, recent studies have shown the ability of mealworm larvae to efficiently degrade polystyrene and plastic waste [84]. Besides being authorized to be used for the production of processed animal protein (PAP) for aquaculture feeds (EU Regulation No. 2017/893) [27], T. molitor, in the form of dried larvae, has recently been declared a safe novel food pursuant to Regulation (EU) No. 2015/2283 [22]. T. molitor can be infected by or harbor parasites, entomopathogenic fungi, and viruses which reduce mealworm survival or reproductive success [82]. To date, only three insect viruses, belonging to the Iridovirus and Densovirus genera, were isolated from or tested against T. molitor and in one paper this insect was used as an animal model. To date, no foodborne viruses have been detected in industrially reared mealworm larvae [81].
Two species of Iridovirus can cause disease leading to death in T. molitor: invertebrate iridescent virus 6 (IIV-6) and invertebrate iridescent virus 29 (IIV-29). IIV-6 causes paralysis in T. molitor larvae 3 days after virus infection [85] and then death; the color of infected larvae appeared to be darkened only after death. IIV-29, a tentative species of the Iridovirus genus [59], rarely encountered in mealworm larvae, produces a particular bluish iridescence in pupae and adults, causing important mortality in T. molitor pupae [86,87] within a few days. The third species of virus detected in healthy T. molitor larvae is Acheta domesticus densovirus (AdDNV) [88]. Mealworms are not considered to be densovirus hosts but when associated with cricket colonies in the same insect farm, they could mechanically transmit AdDNV via their body surface or in the gut [22,88]. T. molitor was used as a bioassay animal for Penaeus merguiensis densovirus (PmergDNV), which is particularly relevant for aquaculture since it causes disease in crustaceans [89], but it did not appear to be an optimal model for studying this virus.
3.1.3. Zophobas morio
Zophobas morio, also known as the super worm, is a large beetle originating in South and Central America and introduced into other regions of Europe and Asia in recent years [50,90,91]. Z. morio has been used as a protein source for small pets such as birds, reptiles, and small mammals [92], but has the potential to become a promising nutrient source for food and feed [51]. Over the last decade the literature on Z. morio as a nutrient source for food, livestock animal feed, and aquaculture has increased considerably [51,93,94]. To date, Z. morio is not listed in Regulation (EU) No 2017/893 and is therefore not officially authorized for inclusion in aquafeeds in the EU [27]. The main strengths of this species are: large size compared to T. molitor, density independent cannibalism [95], increasing size and weight, formation of supernumerary larval instars that do not pupate until death in crowded conditions [96], high dependence on isolation for metamorphosis onset [97,98].
Little information is available on viral diseases in the super mealworm, with only two reports available to date on virus infection in Z. morio larvae [99,100]. The first refers to the collapse of a Z. morio colony in Hungary, where sudden large losses (80–90%) of Z. morio larvae were observed by a breeder in Budapest. Larvae displayed less activity, unusual behavior, and failed to pupate. Diagnostic methods detected densovirus in colony larvae but no further identification was performed [99]. Tokarev and colleagues [100] reported isolation of a densovirus in Z. morio larvae cultured in Russia; in one month, middle-aged larvae developed symptoms of acute disease affecting the locomotion system, inducing swirling, rolling and chaotic wandering of larvae, and within several days, up to 90–100% of the colony had died. Prior to death the larvae showed no morphological changes but after death the cadavers blackened quickly, were more intensively stained, and the inner content was partially liquefied. Through biomolecular techniques the virus was attributed to Densoviridae, with maximal similarity to two Blatella germanica densovirus-like isolates from the bat [100]. One of these latter viruses could develop within vertebrate tissue [101]. Thus, the hazard of vertebrate host infection with insect densoviruses cannot be excluded [100].
Viruses detected in coleopteran edible species are listed in Table 2.

Table 2.
Viruses detected in coleopteran edible species.
3.2. Orthoptera
3.2.1. Acheta domesticus
The European house cricket, Acheta domesticus, is currently one of the most widely farmed insects, particularly in North America and Europe, and constitutes a thriving pet/reptile feeder insect market worldwide. This cricket also represents an emerging, vibrant insect-based food industry due to its high protein content (about 70% by dry weight), its short life cycle (around 5 weeks), and its prolificacy (females lay more than 1500 eggs) [2,102]. Nutritional and food safety aspects as well as the risk profile of this cricket were extensively studied in recent years [35,103,104,105,106]. At the present time, A. domesticus has been authorized for use as processed animal protein (PAP) for aquafeeds (EU Regulation 2017/893) [27].
Viruses affecting this species of cricket have been reported in several papers [16,35,42]. In a recent investigation, five different virus species were detected in commercially reared house crickets from different Swedish retailers [107]. At the present time, the reported viruses consist of insect viruses but no human foodborne viruses have been identified in this specimen [81]. Today, the main virus affecting A. domesticus industry is Acheta domesticus densovirus, which is able to decimate commercial mass rearings in just a few days, leading to a fall in production and even extinction of local cricket populations [44,88,108,109,110]. This virus was first isolated from diseased A. domesticus from a Swiss commercial mass rearing facility in 1977 [111]. In North America, the first report of a densovirus disease in crickets was in 1991 [108] in a small epidemic outbreak. Almost twenty years later, severe outbreaks were observed in commercial facilities in Canada and the United States, causing an acute crisis in the pet food industry [88]. Symptoms of infection include a loss of consistency, smaller dimensions, malnutrition (i.e., absence of contraction and completely empty digestive caeca), inhibited growth, reduced fecundity, increasing sluggishness, less activity, and lower jumping [88,108,109]. Crickets have been observed to lie on their backs paralyzed for several days prior to succumbing to viremia. The highest mortality, of up to 100%, is observed in the last larval stage and in young adults [88,109]. AdDNV positive tissues included the fat body, midgut, hypodermis, and Malpighian tubules [88].
Other viruses observed in cricket facilities are Acheta domesticus mini ambidensovirus (AdMADV), a new ambisense densovirus, and Acheta domesticus volvovirus (AdVVV), a single-stranded, circular DNA virus; but no further information is currently available on these viruses [112,113,114]. A new iflavirus, Acheta domesticus iflavirus (AdIV), has recently been isolated from both wild and commercially reared A. domesticus [107,115]. A. domesticus is highly susceptible to both IIV-6 and its novel strain, cricket iridovirus (CrIV) [58]. CrIV was isolated in 1996 from A. domesticus nymphs and adults from colonies of a commercial cricket producer in Europe. This virus, transmitted orally, led to unusual mortalities as well as greatly reduced fecundity and life span [116]. The fat body was strikingly hypertrophied and, on dissection, displayed a bluish iridescence, which is a typical sign of an iridovirus infection [116].
Cricket paralysis virus (CrPV) was isolated from A. domesticus from dead crickets of a commercial cricket farm in the United States of America [117,118]. A. domesticus is an omnivorous scavenger which could ingest virus-killed insects, thereby simply becoming a mechanical vector of other insect viruses, as Autographa californica multiple nucleopolyhedrovirus (AcMNPV) and two recombinant strains (AcMNPV.AaIT and AcJHE.SG AcMNPV), bait-cricket virus (BCV), and Solenopsis invicta virus 3 (SINV-3) [119,120,121,122]. The house cricket has been used as an alternative bioassay model (i.e., in aquaculture) for viral pathogens, with promising results [89,123,124].
3.2.2. Gryllodes sigillatus
The banded cricket (or tropical or Indian house cricket), Gryllodes sigillatus, is a cosmopolitan cricket that lives in close association with human dwellings [44]. Little information is available on its biology, ecology, rearing, and processing requirements [125,126] but it is one of the species authorized for use for the production of PAP in aquaculture feed (EU Regulation No. 2017/893) [27]. G. sigillatus is sold in both US and European pet food stores [44] and its nutritional aspects, functional properties, and microbiological characteristics have recently been studied [125,127,128]. To date only Acheta domesticus densovirus (AdDNV) has been detected in G. sigillatus but it is less susceptible to AdDNV compared to other orthopteran species [44,81].
3.2.3. Gryllus assimilis
The Jamaican field cricket, Gryllus assimilis, was first described from Jamaica and is widespread in the West Indies, Brazil, Central America, Mexico, and in five of the southernmost U.S. States [44]. This cricket is used for animal feed in Brazil, is the third orthopteran species authorized for PAP for aquafeeds (EU Regulation 2017/893) [27], but limited literature exists on its application in food [94]. To date, three different insect viruses have been detected in G. assimilis: cricket iridovirus (CrIV), Acheta domesticus densovirus (AdDNV), and a new volvolovirus named Acheta domesticus volvolovirus (AdVVV). G. assimilis, especially its first instars, is highly susceptible to CrIV [58], but usually seems to be infected with AdDNV to a much lower degree [44]. Furthermore, AdDNV-exposed G. assimilis, reared to adulthood, were reported to produce large numbers of eggs that hatched and developed without displaying signs of virus infection [88]. The novel volvovirus responsible for mass house cricket die-offs in America [112] has been isolated also in G. assimilis in Japan but little is known about it [113].
3.2.4. Gryllus bimaculatus
Commonly called the two spotted cricket, Gryllus bimaculatus is apparently the most widely distributed Gryllus species and is found at the tip of South Africa, in northern Europe, and as far east as Thailand [129]. G. bimaculatus is commonly consumed as food in different parts of the world [130,131]. Numerous studies have investigated its dietary requirements for rearing procedures [132,133], and its utilization as a biowaste consumer [134] and food and feed resource [130,135,136,137].
To date seven different viral pathogens have been described in reared G. bimaculatus [34,35,42] belonging to Iridovirus, Densovirus, Nudivirus and Cripavirus. G. bimaculatus seems to be highly susceptible to iridovirus but resistant to densovirus [58,88]. Within the Iridoviridae family, invertebrate iridescent virus (IIV-6) and two variants or novel strains of IIV-6—Gryllus bimaculatus iridescent virus (GbIV) and cricket iridovirus (CrIV) could seriously damage G. bimaculatus mass production, causing colony collapse in two weeks [58,116]. Invertebrate iridescent virus type 6 (IIV-6) is reported to be responsible for bluish iridescence in the fat body, malformations i.e., distorted development of the wings in patently infected crickets, and inability to complete ecdysis [60]. Cricket iridovirus (CrIV), instead, caused fatal infections in this cricket species with mortality potentially exceeding 90% [116]; third instars had overt signs and symptoms of virus infection, as swollen abdomens and a striking sluggishness. Gryllus bimaculatus iridescent virus (GbIV) caused the death of all individuals of a colony by 14 days, showing clinically apparent behavior as apathy, ataxia, and disorientation [57]. The fact that neither CrIV nor GbIV have been observed in the ovarian cells of the insect host, suggests that transovarial transmission of these viral infections is unlikely [57] and could help in viral disease management in an epidemic spot. An invertebrate iridovirus was isolated from several tissues of a high-casqued chameleon (Cham_IIV). The pathogenicity of this isolate for G. bimaculatus was tested, revealing mortality rates of between 20% and 35%, and the virus was then re-isolated from several fat-body samples of the cricket [45]. These findings support the hypothesis that IIV from insects can infect reptiles and amphibians via the insects on which they feed [45,60,138,139]. Specifically, GbIV have been detected in three different species of reptiles [138], while lizard-cricket iridovirus (Liz_CrIV), a new strain of CrIV, has been isolated from crickets, reptiles and amphibians [60].
Gryllus bimaculatus nudivirus (GbNV) [140,141,142] can cause disease and mortality in G. bimaculatus nymphs but has a chronic course in adult specimens. Infections are reported to occur primarily during nymphal development, especially by cannibalistic feeding on moribund or dead specimens [140]. Affected crickets are smaller and sometimes get crippled. They may molt repeatedly while becoming progressively uncoordinated and show lethargic behavior till they finally die, within weeks, often only in the final instar stage. In the advanced stage of disease, crickets are often strikingly swollen, and harbor an enormous amount of viscous, milky opalescent hemolymph, giving them a sticky consistency [140]. As regards Acheta dometicus densovirus (AdDNV), while testing positive at the lowest dilution, G. bimaculatus so far appears resistant [44,88]. Cricket paralysis virus (CrPV), multiplying in the two spotted crickets, causes paralysis of the hind legs, especially in young instars, and death in 8 to 9 days with a very high mortality [117,118,143].
3.2.5. Locusta migratoria
The African migratory locust Locusta migratoria is one of the insects responsible for crop devastation in certain developing countries and among the most economically important locusts [58,144]. Viruses detected in L. migratoria belong to four families, i.e., Poxviridae, Baculoviridae, Iridoviridae, and Reoviridae [58,116,144,145,146,147,148,149,150]. L. migratoria is susceptible, to varying degrees, to entomopoxviruses (EPVs), isolated from different orthopteran species [144,145,146], and capable of causing disease and mass mortality in its natural population [144]. Usually the immature specimens (1st to 4th instars) are the most affected and the disease leads to heavy mortalities, despite following a chronic course [145,146]. For example, the mortality of L. migratoria nymphs fed with entomopoxvirus occlusion bodies isolated from Melanoplus sanguinipes reached 90% in the 60 days after virus inoculation [146] or caused entire colony death before maturation [145]. Arphia conspersa entomopoxvirus displays lower pathogenicity, causing a mortality level of approximately 68% of the colony population within 60 days after virus inoculation [147].
L. migratoria can be infected with nucleolyhedrovirus of Spodoptera littoralis (SINPV-type B), a Lepidopteran species [148,149]. This virus was reported to be involved in a disease outbreak in immature L. migratoria, showing a disease pattern termed ‘dark cheeks’. However, the viral amounts in the infected locusts were very low and the role of the virus in generating the observed disease remained ambiguous [148]. A subsequent experiment revealed the slow, gradual disappearance over time of viral DNA post infection while no signs of disease were observed in the infected locusts, suggesting that SINPV does not multiply in locusts and is not therefore pathogenic to L. migratoria instars [149]. A significant increase in mortality in L. migratoria was recorded during cytoplasmic polyhedrosis virus (CPV) infection [150]; to date, there is no further information about this virus. Young nymphs of L. migratoria were heavily infected by the two virus isolates, invertebrate iridescent virus (IIV-6) and cricket iridovirus (CrIV), with mortality as high as 100% [58]. Iridoviruses can be transmitted perorally to L. migratoria, causing characteristic symptoms and fatal disease [116].
3.2.6. Schistocerca gregaria
The desert locust Schistocerca gregaria, due its high reproductive potential, represents another major pest in agriculture and, as other locust species, has been extensively studied in previous years [58,144]. In 1968, a virus called “Schistocerca virus” was detected in 5th instars of S. gregaria, showing signs of inactivity and a high death rate [151]. Spodoptera littoralis nucleopolyhedrovirus (SINPV) was reported to be involved in a disease outbreak in immature S. gregaria [149]. Entomopoxvirus in wild specimens of S. gregaria was detected by Purrini and Rohde [152]. Younger instars of S. gregaria (L1–L3) could also be heavily infected by the two Iridovirus: invertebrate iridescent virus (IIV-6) and cricket iridovirus (CrIV) [58].
3.2.7. Schistocerca americana
Limited information exists on Schistocerca americana, commonly known as the American grasshopper [153]. In 1966, Henry and Jutila [154] isolated a polyhedrosis virus from a grasshopper, M. sanguinipes, and infected S. americana. The latter species proved only slightly susceptible: after a latent period of about 12 days, infected specimens exhibited general torpor, a decreased rate of development, and eventual death. The fat body—the only tissue invaded—became generally hypertrophic, changing from its normal glossy yellow to a fluffy gray. Examination of sectioned tissues revealed that most of the fat-body cells were filled with large numbers of polyhedral bodies [154]. Field-collected S. americana specimens, showing symptoms and signs of disease, were found to be infected with Melanoplus sunguinipes entomopoxvirus. Placed in a rearing facility, these specimens spread the virus to other grasshopper species through horizontal transmission, apparently by consumption of infected cadavers [147,155]. The crystalline-array virus (CAV), a small RNA virus belonging to the picornavirus group, causes death or morbidity of 5th instars of S. americana 6 days after intrathoracic inoculation [156,157].
Viruses detected in orthopteran edible species are listed in Table 3.

Table 3.
Viruses detected in orthopteran edible species.
3.3. Diptera
3.3.1. Hermetia illucens
The Black Soldier Fly (BSF), Hermetia illucens, is a saprophytic insect, which currently has a cosmopolitan distribution in tropical and temperate areas [159,160]. H. illucens is one of the most promising insect species for food and feed production, efficient bioconversion of food waste, and biodiesel and fertilizer production [161,162,163,164,165]. The main aspects that make the BSF easy to rear and a suitable tool to valorize waste and sustainable animal feed or human food sources are: (i) the diversity of the substrates they can process and the efficiency with which they do so may be highest among the flies [166]; (ii) their feed conversion ratios are known to be superior to both crickets and mealworms [3]; (iii) prepupae instinctively leave the substrate and move to a high, clean place, a behavior called “self-harvesting” which removes an otherwise labor-intensive step from their farming [167].
In recent years, economic interest in this species has grown yielding abundant scientific literature on its behavior, rearing, nutritional value, and industrial applications [164,168,169,170,171,172,173]. According to current knowledge, no viruses have been reported in this species, either at the larval stage or in the adult, despite it being a scavenger and its life cycle being associated with polluted environments [174]. In one paper, BSF larvae proved capable of efficient microbial load reduction in the substrate contaminated with different pathogen microrganisms (Salmonella spp., Orthoreovirus, Mastadenovirus, Teschovirus), but the analyses were performed only on the substrate and not on the larvae [175].
3.3.2. Musca domestica
The housefly, Musca domestica, distributed worldwide, is the most common species of fly, living in close association with humans and domestic animals, making it one of the most highly studied insect pests [176,177]. Besides being a source of irritation and spoiling food, the house fly acts as a vector for many medical and veterinary pathogens [178]. The ability of M. domestica to reproduce quickly could be exploited to produce larvae as a source of protein for animal feed [179,180,181,182]. Moreover, like H. illucens, larvae could grow in different substrates making M. domestica a promising insect for organic waste degradation [183,184,185,186]. An abundant literature already exists in relation to mass rearing systems for M. domestica [180,187].
The housefly is known to carry more than 130 pathogens, including bacteria, viruses, fungi and parasites, some of which can cause serious, life-threatening diseases in humans and animals, or diseases in flies themselves [176,177]. M. domestica can harbor several viruses pathogenic for both humans and livestock but, to date, only two insect viruses that are pathogenic for the fly itself have been detected [188,189,190,191]. Flies have been shown to mechanically transmit pathogens via their mouthparts, vomit, faeces, and whole body surface [176,190]. Contrary to H. illucens, M. domestica adults are synanthropic and therefore any pathogens being carried could easily be transmitted to humans, animals, and other flies. Adult flies act only as mechanical carriers of human viruses after contamination by infected human fecal material [191,192,193]. These potential passive contaminators are capable of carrying and depositing the virus at a considerable distance from the point of original contamination. Human viral pathogens mechanically transmitted by adult flies include: coxsackieviruses, enteroviruses, rotaviruses, and poliomyelitis virus [190,191,192,193,194,195,196,197]. In addition, one study demonstrated the ability of the housefly to carry the Ebola virus in laboratory experiments but the role of the common fly in transmission of the virus remains to be confirmed [198].
The main virus affecting adult M. domestica is Musca domestica salivary gland hypertrophy virus (MdSGHV). It was isolated in 1993 [199] and then extensively studied. MdSGHV has been detected in housefly samples from North America, Europe, Asia, the Caribbean, and the southwestern Pacific [199]. Populations of M. domestica (only adults) are naturally infected with MdSGHV but its incidence varies widely among farms and at different times of the year [200], with the highest prevalence in summertime [200,201]. The virus causes symptomatic salivary gland hypertrophy (both nuclear and cellular), with a characteristic white-blue color, in both genders of M. domestica flies (although males seem more affected), in addition to suppressing ovarian development in infected females, inhibiting egg production and resulting in female sterility [189,202,203,204,205]. Male reproductive performance is also affected by virus infection [203]. Since sexual and vertical transmission have been ruled out, this virus only spreads horizontally [201,202,203,204]. MdSGHV, produced in the gland cells, is continuously shed during feeding, resulting in contamination of food material; the deposition of oral secretions and excreta onto a shared food substrate is the main route of natural MdSGHV transmission among adult house flies [199,206]. Typically, in natural populations, this virus has not been observed to cause the widespread epizootics characteristic of other insect viruses [200] and the introduction of MdSGHV-infected flies into confined populations does not produce epizootics but results in a persistent, albeit declining, prevalence of viral infection [207]. The virus was able to infect > 50% of newly eclosed adults whereas older adults were highly resistant to infection (0–5%) [199]. Infected male and female flies consumed significantly lower quantities of protein and sucrose than control flies; this suggests that MdSGHV has a negative consumption effect (e.g., hunger, starvation) on its host [203,208,209,210].
The second virus causing disease and mortality in M. domestica adults is a reovirus, detected by Moussa in 1978 and now named idnoreovirus 3 (Idno-3) [188]. This virus, multiplying in the hemocytes of infected flies, produced morphological alterations (i.e., swollen abdomen, enlarged, brownish midgut) and motor dysfunctions such as trembling of wings and legs and total paralysis. Mortality began within the first 24 h after emergence of adults, causing colony collapse in 10 days [188,211]. No mortality was observed in early larvae, but a few dead final instar larvae were found.
M. domestica has also been reported to mechanically transmit several types of viral pathogens to livestock including: avian influenza virus (AIV), both high and low pathogenic strains [46,212,213,214,215,216], turkey coronavirus (TCV) [217], Newcastle disease virus (NDV) [218,219,220,221,222], reticuloendotheliosis virus (REV) [223], porcine reproductive and respiratory syndrome virus (PRRSV) [224,225,226,227,228], porcine circovirus genotype 2 (PCV2b) [229], porcine epidemic diarrhea virus (PEDV) [230], African swine fever virus (ASF) [231,232], Aujeszky’s virus (PRV-1) [233], senecavirus A (SVA) [234], Rift Valley fever virus (RVFV) [235], Aleutian mink disease virus (AMDV) [236,237], and lumpy skin disease (LSDV) [238,239].Viruses detected in dipteran edible species are listed in Table 4.

Table 4.
Viruses detected in dipteran edible species.
3.4. Lepidoptera
3.4.1. Achroia grisella
The lesser wax moth, Achroia grisella, is a species closely related to Galleria mellonella [241]. As compared to the greater wax moth, the lesser wax moth is less destructive and less common [242,243]. To date in the literature no virus has been detected in this species.
3.4.2. Galleria mellonella
The greater wax moth (GWM), G. mellonella, is a ubiquitous pest of field-based honeybee colonies and stored combs due to the destructive feeding habit of its larvae [241,244]. Recently, this pest has garnered greater attention as a promising food and feed resource and as an infection model organism. G. mellonella has been available as pet food and bait for many years in several European countries and in the USA [42]. This moth can be easily reared, standardized protocols already exist for its breeding and diets [245,246,247,248,249], and it has high nutritional value [19]. G. mellonella is a reliable model organism to assay pathogenicity of human, animal and insect pathogens (i.e., bacteria, fungi and viruses) as well as to test the effectiveness and toxicity of antimicrobial compounds [250,251,252,253]. A few studies have collected information on viral diseases involving both insect and mammal pathogenic viruses in G. mellonella [42,250], but viral infections have been detected or tested in larval stages only. Regarding animal pathogens, only one paper has to date studied the immune response of G. mellonella infected with bovine herpes simplex virus-1 (BHSV-1) [254]. In the wax moth larvae, BHSV-1 stimulates both cellular and humoral immune response in a dose-dependent manner in G. mellonella larvae, but no mortality was detected [254].
Nodamura virus, an insect picornavirus that can also infect vertebrates, is able to infect and kill greater wax moth larvae [255,256]. The infected larvae manifest paralysis of the last 5 or 6 segments four to six days after inoculation of the virus. The paralysis progressively spreads to the other segments, leading to death 15 to 20 days after infection [256]. Replication of Nodamura virus takes place in the interfibrillar spaces of the sarcoplasm in close association with the mitochondria in the infected muscles, causing aggregation and shape modification of numerous mitochondria (elongation, interdigitation, and vesiculation) [257]. At a later stage, degenerated, dilated mitochondria show clear assembling of virus particles on their outer membrane and occasionally on some inner membranes [257].
Many insect viruses have been detected in G. mellonella larvae. They belong to Densovirus, Iridovirus, Baculoviruses, Cripavirus and Triatovirus genera and can lead to patent and asymptomatic infections or severe symptoms and mass mortality. For example, Galleria mellonella densovirus (GmDV) can cause the death of the entire colony while Acheta domesticus densovirus (AdDNV) cannot even infect the colony [90,258]. Galleria mellonella densovirus (GmDV) is the most highly studied and described densovirus in this moth species [42,259]. GmDV is highly virulent for young larvae, the most susceptible being third instar larvae, where it seems to replicate more successfully compared to older instars [258]. The mortality rate in infected larvae can reach up to 100% in an average period of 10 days. Infection of prepupae also causes abnormal or absent pupation with no adults emerging [258]. Nucleopolyhedrovirus has been isolated from both G. mellonella specimens and other insect species (i.e., B. mori, H. virescens, M. franconicum). All said viral isolates have been shown to be pathogenic especially for G. mellonella larvae [260,261,262,263,264,265].
Galleria mellonella nucleopolyhedrovirus (GmNPV) is highly pathogenic for G. mellonella, particularly in the preimaginal stages (third instars) [262,265,266,267]. This virus multiplies in insect cells causing hypertrophy of the nuclei with the formation of virus-containing inclusion bodies (polyhedra), while non-occluded virus (NOV) particles can be seen in diseased tissues. After 8–10 days, cell destruction causes the release of polyhedra in the infected tissue [268]. During viral infection, the amount of potassium dramatically drops causing severe acidosis, and higher sulfur levels are present compared to healty larvae [261]. G. mellonella larvae infected with Bombyx mori nucleopolyhedrovirus (BmNPV) ceased to feed and began to wilt after 17 days. Mortality occurred during between 19 and 27 days. Some infected insects did not die until entering the pupal stage [263]. The Malacosoma franconicum nucleopolyhedrovirus (ManeNPV-T3) caused 25% mortality in third instars of G. mellonella [264].
G. mellonelIa is highly permissive, with different levels of magnitude, to many Iridoviruses (IVs) isolated from insect species belonging to different orders [50,61,88,159,269,270,271,272,273]. However, in some cases, IIVs causing patent disease in certain insect species do not replicate in G. mellonella larvae [274]. Invertebrate iridescent viruses cause pupal malformation and patches of translucent cuticle in the area between the abdomen and ventral thorax through which the iridescent color of the insect can be viewed [270,271]. The viruses cause patent infection with a prevalence of up to 75%; in the case of high-level infection, all insects became patently infected; mortality started 10 days after infection and larvae failed to reach adulthood [271,275]. Cricket iridovirus (CrIV) caused fatal infections in larvae of the greater wax moth [61,116]. Tipula iridescent virus (TIV) can infect and multiply in hemocytes of G. mellonella larvae [269,276,277,278,279,280]. Tipula iridescent virus was consistently infectious and caused complete mortality among G. mellonella, with the average period to death occurring at about 14 days after injection [269]. Wax-moth larvae inoculated with TIV and reared at 23–25 °C died from virus infection, but at 30 °C and higher temperatures, most of them survived to become adults [281]. Apis cerana iridescent virus (AIV) failed to multiply in G. mellonella larvae [282]. Seriscethis iridescent virus (SIV), isolated from Sericesthis pruinosa, develops in G. mellonella plasmatocytes and adipohemocytes leading to visible alteration (hypertrophy) in these cells within 24 h of virus inoculation [283,284,285]. Cricket paralysis virus caused mortality in G. mellonella larvae [286]; specifically, mortality occurred within 5 days in infected penultimate instar larvae [287]. Honeybee viruses, Israeli acute paralysis virus (IAPV), and black queen cell virus (BQCV), have been detected in wax moth larvae but no data are available on their possible pathogenicity in this species [288].
Mycoviruses are a specific group of viruses that naturally infect and replicate in fungi and are able to alter fungal growth. Accordingly, a lower initial concentration of spores can still become lethal post infection. Infection of A. fumigatus with A78 mycovirus caused a significant increase in radial growth and virulence in a moth model [289]. Other reports of viruses detected in G. mellonella concern a small spherical virus, Galleria free virus (GFV), isolated from G. mellonella [290]. The Galleria mellonella cell line virus (GmclV), apparently persistently infects the G. mellonella cell line (GmclV) and can be efficiently induced to replicate by the introduction of other insect viruses, causing complete death of the colony in 5 days [51]. The last two viruses reported in G. mellonella—Pariacoto virus and Heteronychus arator virus—show striking similarities to Nodamura virus [291]. Pariaocto virus, isolated in Peru from the Southern armyworm (Spodoptera eridania), is able to multiply in G. mellonella larvae [292,293]. Fourteen days post infection, Heteronychus arator virus renders G. mellonella larvae inactive, flaccid, unresponsive to touch, and paralyzed. Mortality reaches 50% in 20 days after infection [294] and the midgut seems to be the primary target tissue.
Viruses detected in lepidopteran edible species are listed in Table 5.

Table 5.
Viruses detected in lepidopteran edible species.
4. Prevention, Control and Management of Viruses in Edible Insect Mass-Rearing Facilities
At present there is no treatment for viral infections in edible insects and therefore prevention and control measures are pivotal for insect mass rearing systems. Mass rearing strategies must focus on defining and standardizing Good Farming Practices (GFP) [35]. First of all, avoiding the entry of insect viruses into highly intensive rearing facilities must be considered paramount. For this purpose, regular analyses must be performed on products entering the rearing systems, including both feeding substrates and new individuals for inbreeding avoidance. Since horizontal (oral) transmission is the main route for the spread of insect viruses, attention should be paid when new breeders (from the same or different facilities), are added to older ones in the reproduction sector, or when eggs and deposition substrates are introduced into the production sector. High attention should be paid also to operator handling hygiene. In addition, stressful conditions (i.e., feeding imbalances or high density) must be avoided to reduce the spread of infection associated with the cannibalistic behavior of certain insect species. It is crucially important in commercial large-scale production to avoid high breeding densities because they increase cannibalism, which in turn enhances the transmission of microbial agents [296]. It is equally necessary to develop efficient protocols that permit early detection of viruses [158]. For example, at the present moment it is possible to perform a qualitative PCR-based detection of AdDV in different substrates, i.e., whole body, body parts or fecal material [44,88,158].
5. Discussion
The retrieved literature reveals that many viruses, belonging to 22 different families, have been observed in edible insect species (Table 2, Table 3, Table 4 and Table 5). However, there are no reports at present on virus detection for two species (A. grisella and H. illucens), while reports on others are sometimes very limited in size or old. Among the insect orders considered, orthopteran seems to be the one most affected by viral pathogens belonging to seven families (i.e., Dicistoviridae, Parvoviridae, Nudiviridae, Iridoviridae, Baculoviridae, Poxviridae, and Picornaviridae); the most concerning viruses affecting Orthoptera are species belonging to Iridoviridae and Densoviridae families. The Lepidoptera order (represented by G. mellonella) seems heavily affected by virus species belonging to Baculoviridae and Iridoviridae families, while Densoviruses are rarely reported; other virus species reported to affect G. mellonella are member of Dicistoviridae, Parvoviridae and Picornaviridae families. Two iridoviruses and one densovirus are reported to cause mortality in the coleopteran order while only two insect viruses have been described as pathogenic for Diptera.
In the retrieved literature, viruses detected in edible insects can be divided into three major categories: (i) viruses that neither multiply nor cause disease in edible insect species; (ii) viruses that multiply and cause disease and mortality in edible insect species; (iii) viruses that multiply but do not cause disease or decrease performance in edible insect species (asymptomatic). In the first case, those viruses are casually associated with edible insects that act only as a mechanical vector without affecting their productivity or prolificacy (for example: viruses reported from wild caught M. domestica). In the other two cases (viruses that multiplied in edible insect species), edible insects act as a biological vector. In the investigated edible insect species, different patterns of viral infection can occur ranging from asymptomatic to highly pathogenic, or even lethal [31]. These distinct outcomes exist in a variable range and can be classified into three main groups namely: acute, persistent, and latent. Acute infections, characterized by high levels of viral replication, are limited in time due to the death of the host or clearance of the virus by the host immune system. Persistent infections, characterized by low viral replication, do not affect host fitness even when they last for longer periods. Abundant covert infections have also been reported from several host insect species [297]. Covertly infected insects appear healthy and the infection is not lethal. Latent infections consist of the presence of a viral genome in the host, without viral particle production, but virus reactivation is possible [31,298].
Viruses that pose the highest risk for the collapse of mass insect rearing systems are those causing acute and high mortality because they can decimate commercial mass farms or entire colonies within a few days [44,88,100,258]. Another threat for edible insect producers are viruses that do not cause mortality but bring about a drastic decline in growth in the juvenile stages and in adult reproductive performance, with total collapse of the colony taking longer [190,204]. Finally, attention should be paid to latent viruses which can reactivate when insects are subject to stressful conditions (i.e., a change in diet or environmental conditions) or concomitant infections, starting with viral particle production and visible effects on reared insects [31,299]. Some viruses affect only the adult stage (MdSGHV in adults of M. domestica or Invertebrate iridovirus 29 in T. molitor pupae and adults) with no consequences for larvae, representing the valuable edible part, while other viruses affect only larvae (Galleria mellonella densovirus in third instar G. mellonella). Greater attention must therefore be paid to these viruses in the production sector of insect facilities.
In heterometabolic insects, all stages can be infected by viruses but the juvenile stages are usually the most affected (i.e., cricket iridovirus in nymphs and adults of A. domesticus) [58,116]. The same virus could affect different insect species with different degrees of severity. A. domesticus is highly susceptible to Acheta domesticus densovirus while G. assimilis usually seems to be infected with AdDNV to a much lower degree; G. sigillatus is less susceptible to AdDNV compared to other orthopteran species and G. bimaculatus so far appears resistant [44,47,90]. For this reason, G. assimilis, G. sigillatus, and G. bimaculatus have been proposed as the best replacement crickets to avoid heavy losses in commercial production [44,81]. Besides causing losses in insect mass rearing systems, insect viruses can infect vertebrate hosts [45,63] and the fact that invertebrate viruses may be transmitted to vertebrates further increases the importance of screening measures for commercially produced prey insects [45].
To date, only one article has investigated the presence of foodborne viruses in three species of industrially reared insects for food [81], yielding negative results for the presence of detectable quantities of hepatitis A virus, hepatitis E virus, and norovirus genogroup II. The possibility that human viruses could infect and multiply within edible insects is unclear, but it seems unlikely [35,298]. At the present day, the risk of transmitting foodborne viruses to humans via edible insects (T. molitor, A. diaperinus and G. sigillatus) is considered low. Foodborne viruses could be introduced through rearing substrate or operator handling and transferred beyond primary production. This prompts the need to carry out more studies and experimental infections to produce more evidence of the low safety risk from foodborne viruses.
6. Conclusions
The retrieved literature revealed that the number of viruses detected in edible insects is high, with more than 70 species listed and 36 able to cause disease and mortality. Viruses could be more or less species-specific and could infect edible insects at different life stages. Only insect-specific viruses could be a matter of concern in mass-rearing systems as they actively replicate and persist on the target species. Viral infection could have different consequences on mass rearing systems ranging from asymptomatic infection to the entire collapse of the colony. Since to date there is no cure for viral infections in edible insects, preventative measures are the only affordable strategy available. Thus, biosecurity is pivotal for insect mass rearing systems.
To enable edible insects to become a safe nutrient source for animals and humans in the Western world, more investigations are warranted to better understand the effective impact of both insect and vertebrate viruses in industrialized rearing systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13112280/s1, Table S1: Elected articles list: bibliographical details (authors, title, source, publication year, and DOI or PMID) and edible insect species investigated.
Author Contributions
Conceptualization, M.B. and F.M.; methodology, M.B.; validation, M.B.; formal analysis, M.B.; investigation, M.B.; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, M.B. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for food and feed security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; N. 171; pp. 1–201. Available online: https://www.fao.org/3/i3253e/i3253e.pdf (accessed on 29 October 2021).
- Van Huis, A. Potential of insects as food and feed in assuring food security. Ann. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect rearing: Potential, challenges, and circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insect Species of the World; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2017; Available online: http://www.entwurnl/UK/edible+insects/worldwide+species+list/ (accessed on 29 October 2021).
- Sun-Waterhouse, D.; Waterhouse, G.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Scientific Opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Murefu, T.R.; Macheka, L.; Musundire, R.; Manditsera, F.A. Safety of wild harvested and reared edible insects: A review. Food Control 2019, 101, 209–224. [Google Scholar] [CrossRef]
- Eilenberg, J.; van Oers, M.M.; Jensen, A.B.; Lecocq, A.; Maciel-Vergara, G.; Santacoloma, L.P.A.; van Loon, J.J.A.; Hesketh, H. Towards a coordination of European activities to diagnose and manage insect diseases in production facilities. J. Insects Food Feed 2018, 4, 157–166. [Google Scholar] [CrossRef]
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2020, 15, 123013. [Google Scholar] [CrossRef]
- Wales, A.D.; Carrique-Mas, J.J.; Rankin, M.; Bell, B.; Thind, B.B.; Davies, R.H. Review of the carriage of zoonotic bacteria by arthropods, with special reference to Salmonella in mites, flies and litter beetles. Zoonoses Public Health 2010, 57, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a food safety and nutritional perspective: A critical review. Com. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.M.; Nielsen-LeRoux, C.; Cappellozza, S.; Jensen, A.B. Diseases in insects produced for food and feed. J. Insects Food Feed 2015, 1, 87–102. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food Safety Issues Related to Uses of Insects for Feeds and Foods. Com. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Com. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Dibusz, K.; Vejvodova, P. Systematic literature search to assist EFSA in the preparatory work for the safety assessment of Novel Food applications and Traditional Food notifications. EFSA Supp. Publ. 2020, 17, 1774E. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. OJL 2015, 327, 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:JOL_2015_327_R_0001 (accessed on 28 October 2021).
- European Union. European Union Commission implementing regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 64–71. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2469 (accessed on 29 October 2021).
- European Union. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). Off. J. Eur. Union 2017, L95, 1–142. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0625 (accessed on 28 October 2021).
- European Union. Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. Off. J. Eur. Union 2019, L231, 1–28. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32019R1381 (accessed on 28 October 2021).
- European Union. Regulation (EC) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. Off. J. Eur. Union 2011, L54, 1–254. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2011.054.01.0001.01.ENG (accessed on 28 October 2021).
- Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017, L 138, 92–115. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0893 (accessed on 29 October 2021).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens; Turck, D.; Castenmiller, J.; De Henauw, S.; Ildico Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Vega, F.E. Scope and basic principles of insect pathology. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–12. [Google Scholar]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.W.; Broeck, J.V. RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Front. Physiol. 2019, 11, 1912. [Google Scholar] [CrossRef]
- The Insect Viruses; Miller, L.K., Ball, L.A., Eds.; Kluwer Academic Publishers Group: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef]
- Williams, T.; Bergoin, M.; van Oers, M.M. Diversity of large DNA viruses of invertebrates. J. Invertebr. Pathol. 2017, 147, 4–22. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Vaga, M.; Jansson, A.; Boqvist, S.; Vagsholm, I. The house cricket (Acheta domesticus) as a novel food: A risk profile. J. Insects Food Feed 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Wu, H.; Pang, R.; Cheng, T.; Xue, L.; Zeng, H.; Lei, T.; Chen, M.; Wu, S.; Ding, Y.; Zhang, J.; et al. Abundant and Diverse RNA Viruses in Insects Revealed by RNA-Seq Analysis: Ecological and Evolutionary Implications. Msystems 2020, 5, e00039-20. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.-H.; Chen, X.; Liu, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Mettel, K.A.; Benner, B.E.; Johnson, R.; Scott, C.; Yusseff-Vanegas, S.Z.; Baker, C.C.M.; Cassill, D.L.; Storer, C.; Varsani, A.; et al. Virus discovery in all three major lineages of terrestrial arthropods highlights the diversity of single-stranded DNA viruses associated with invertebrates. PeerJ 2018, 6, e5761. [Google Scholar] [CrossRef]
- Lü, P.; Pan, Y.; Yang, Y.; Zhu, F.; Li, C.; Guo, Z.; Yao, Q.; Chen, K. Discovery of anti-viral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 2018, 154, 12–18. [Google Scholar] [CrossRef]
- Martinet, J.-P.; Ferté, H.; Failloux, A.-B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef]
- Öhlund, P.; Lundén, H.; Blomström, A.-L. Insect-specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Ros, V.I.D. Viruses of insects reared for food and feed. J. Invertebr. Pathol. 2017, 147, 60–75. [Google Scholar] [CrossRef]
- Bonning, B.C. The Insect Virome: Opportunities and Challenges. Curr. Issues Mol. Biol. 2020, 34, 1–12. [Google Scholar] [CrossRef]
- Weissman, D.B.; Gray, D.A.; Pham, H.T.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88. [Google Scholar] [CrossRef]
- Weinmann, N.; Papp, T.; de Matos, A.P.A.; Teifke, J.P.; Marschang, R.E. Experimental Infection of Crickets (Gryllus Bimaculatus) with an Invertebrate Iridovirus Isolated from a High-Casqued Chameleon (Chamaeleo Hoehnelii). J. Vet. Diagn. Investig. 2007, 19, 674–679. [Google Scholar] [CrossRef]
- Nielsen, A.A.; Skovgård, H.; Stockmarr, A.; Handberg, K.J.; Jørgensen, P.H. Persistence of Low-Pathogenic Avian Influenza H5N7 and H7N1 Subtypes in House Flies (Diptera: Muscidae). J. Med. Entomol. 2011, 48, 608–614. [Google Scholar] [CrossRef][Green Version]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasit. Vectors 2018, 11, 77. [Google Scholar] [CrossRef]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges-Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 339, b2700. [Google Scholar] [CrossRef]
- Bousquet, Y.; Thomas, D.B.; Bouchard, P.; Smith, A.D.; Aalbu, R.L.; Johnston, M.A.; Steiner, W.E., Jr. Catalogue of Tenebrionidae (Coleoptera) of North America. ZooKeys 2018, 728, 1–455. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. The Superworm, Zophobas morio (Coleoptera: Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M.A. Comprehensive Look at the Possibilities of Edible Insects as Food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Finke, M.D. Nutrient Composition of Bee Brood and its Potential as Human Food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm pupae (Bombyx mori) are new sources of high quality protein and lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Bruun Jensen, A.; Evans, J.; Jonas-Levi, A.; Benjamin, O.; Martinez, I.; Dahle, B.; Roos, N.; Lecocq, A.; Foley, K. Standard methods for Apis mellifera brood as human food. J. Apicult. Res. 2019, 58, 1–28. [Google Scholar] [CrossRef]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A Decade of Advances in Iridovirus Research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [CrossRef]
- Just, F.T.; Essbauer, S.S. Characterization of an Iridescent Virus Isolated from Gryllus bimaculatus (Orthoptera: Gryllidae). J. Invertebr. Pathol. 2001, 77, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Jakob, N.J.; Kleespies, R.G.; Tidona, C.A.; Müller, K.; Gelderblom, H.R.; Darai, G. Comparative analysis of the genome and host range characteristics of two insect iridoviruses: Chilo iridescent virus and a cricket iridovirus isolate. J. Gen. Virol. 2002, 83, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Natural invertebrate hosts of iridoviruses (Iridoviridae). Neotrop. Entomol. 2008, 37, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Papp, T.; Marschang, R.E. Detection and Characterization of Invertebrate Iridoviruses Found in Reptiles and Prey Insects in Europe over the Past Two Decades. Viruses 2019, 11, 600. [Google Scholar] [CrossRef]
- Dunford, J.C.; Kaufman, P.E. Lesser Mealworm, Litter Beetle, Alphitobius diaperinus (Panzer) (Insecta: Coleoptera: Tenebrionidae); IFAS Extension, University of Florida: Gainesville, FL, USA, 2006; Available online: https://edis.ifas.ufl.edu/pdf/IN/IN662/IN662-D7vxrv2lkq.pdf (accessed on 28 October 2021).
- Esquivel, J.F.; Crippen, T.L.; Ward, L.A. Improved Visualization of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae)—Part I: Morphological Features for Sex Determination of Multiple Stadia. Psyche J. Entomol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Dinev, I. The darkling beetle (Alphibotbius diaperinus)—A health hazard for broiler chicken production. Trakia J. Sci. 2013, 1, 1–4. Available online: http://www.uni-sz.bg/tsj/vol11N1_2013/Iv.Dinev.pdf (accessed on 5 November 2021).
- Wynants, E.; Crauwels, S.; Verreth, C.; Gianotten, N.; Lievens, B.; Claes, J.; Van Campenhout, L. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiol. 2018, 70, 181–191. [Google Scholar] [CrossRef]
- Jensen, L.D.; Miklos, R.; Dalsgaard, T.K.; Heckmann, L.H.; Nørgaard, J.V. Nutritional evaluation of common (Tenebrio molitor) and lesser (Alphitobius diaperinus) mealworms in rats and processing effect on the lesser mealworm. J. Insects Food Feed 2019, 5, 257–266. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J. Insects Food Feed. 2020, 6, 393–404. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-Food Side-Stream Inclusion in The Diet of Alphitobius Diaperinus. Part 2: Impact on Larvae Composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef]
- McAllister, J.C.; Steelman, C.D.; Newberry, L.A.; Skeeles, J.K. Isolation of Infectious Bursal Disease Virus from the Lesser Mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 1995, 74, 45–49. [Google Scholar] [CrossRef]
- Retamales, J.; Vivallo, F.; Robeson, J. Insects associated with chicken manure in a breeder poultry farm of Central Chile. Arch. Med. Vet. 2011, 43, 79–83. [Google Scholar] [CrossRef]
- Eidson, C.S.; Schmittle, S.C.; Goode, R.B.; Lal, J.B. Induction of leukosis tumors with the beetle Alphitobius diaperinus. Am. J. Vet. Res. 1966, 27, 1053–1057. [Google Scholar] [PubMed]
- Snedeker, C.; Wills, F.K.; Moulthrop, I.M. Some Studies on the Infectious Bursal Agent. Avian Dis. 1967, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- De las Casas, E.R.; Harein, P.K.; Deshmukh, D.R.; Pomeroy, B.S. The relationship between the lesser mealworm and avian viruses. I. Reovirus 24. Environ. Entomol. 1973, 2, 1043–1047. [Google Scholar] [CrossRef]
- De Las Casas, E.; Harein, P.K.; Deshmukh, D.R.; Pomeroy, B.S. Relationship Between the Lesser Mealworm, Fowl Pox, and Newcastle Disease Virus in Poultry. J. Econ. Entomol. 1976, 69, 775–779. [Google Scholar] [CrossRef]
- Despins, J.L.; Axtell, R.C.; Rives, D.V.; Guy, J.S.; Ficken, M.D. Transmission of Enteric Pathogens of Turkeys by Darkling Beetle Larva (Alphitobius diaperinus). J. Appl. Poult. Res. 1994, 3, 61–65. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Waltman, W.D. Transmission of Eimeria, Viruses, and Bacteria to Chicks: Darkling Beetles (Alphitobius diaperinus) as Vectors of Pathogens. J. Appl. Poult. Res. 1996, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.W.; Guy, J.S.; Stringham, S.M. Limited Transmission of Turkey Coronavirus in Young Turkeys by Adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 2000, 37, 480–483. [Google Scholar] [CrossRef][Green Version]
- Ou, S.-C.; Giambrone, J.J.; Macklin, K.S. Detection of infectious laryngotracheitis virus from darkling beetles and their immature stage (lesser mealworms) by quantitative polymerase chain reaction and virus isolation. J. Appl. Poult. Res. 2012, 21, 33–38. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Huang, W.; Geng, H.; Zhao, Y.; Li, M.; Chen, Y.; Su, S. A scientific note on detection of honeybee viruses in the darkling beetle (Alphitobius diaperinus, Coleoptera: Tenebrionidae), a new pest in Apis cerana cerana colonies. Apidologie 2016, 47, 759–761. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lievens, B.; Van Campenhout, L. Identification of bacterial endospores and targeted detection of foodborne viruses in industrially reared insects for food. Nat. Food 2020, 1, 511–516. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune Defenses of a Beneficial Pest: The Mealworm Beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Nat. C J Biosci. 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.M.; Gao, S.H.; Tian, R.M.; Ning, D.L.; Yang, S.S.; Zhou, J.Z.; Wu, W.-M.; Criddle, C.S. Affiliations expand Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Gençer, D.; Yeşilyurt, A.; Güllü, M.; Demir, I.; Nalçacioğlu, R. Insecticidal activities of wild type and recombinant invertebrate iridescent viruses on five common pests. Türk. Entomol. Derg. 2020, 44, 365–373. [Google Scholar] [CrossRef]
- Kelly, D.C.; Ayres, M.D.; Lescott, T.; Robertson, J.S.; Happ, G.M. A Small Iridescent Virus (Type 29) Isolated from Tenebrio molitor: A Comparison of its Proteins and Antigens with Six Other Iridescent Viruses. J. Gen. Virol. 1979, 42, 95–105. [Google Scholar] [CrossRef]
- Black, P.N.; Blair, C.D.; Butcher, A.; Capinera, J.L.; Happ, G.M. Biochemistry and ultrastructure of iridescent virus type 29. J. Invertebr. Pathol. 1981, 38, 12–21. [Google Scholar] [CrossRef]
- Szelei, J.; Woodring, J.; Goettel, M.S.; Duke, G.; Jousset, F.-X.; Liu, K.Y.; Zadori, Z.; Li, Y.; Styer, E.; Boucias, D.G.; et al. Susceptibility of North-American and European crickets to Acheta domesticus densovirus (AdDNV) and associated epizootics. J. Invertebr. Pathol. 2011, 106, 394–399. [Google Scholar] [CrossRef]
- La Fauce, K.A.; Owens, L. The use of insects as a bioassay for Penaeus merguiensis densovirus (PmergDNV). J. Invertebr. Pathol. 2008, 98, 1–6. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Song, S.H.; Kim, N.J. Developmental characteristics of Zophobas atratus (Coleoptera: Tenebrionidae) larvae in different instars. Int. J. Indust. Entomol. 2015, 30, 45–49. [Google Scholar] [CrossRef]
- Fursov, V.N.; Cherney, L.S. Zophobas atratus (Fabricius, 1775)—New genus and species of darkling beetles (Coleoptera, Tenebrionidae) for the fauna of Ukraine. Ukr. Entomol. J. 2018, 1, 10–24. [Google Scholar] [CrossRef]
- Park, H.C.; Jung, B.H.; Han, T.; Lee, Y.B.; Kim, S.-H.; Kim, N.J. Taxonomy of introduced commercial insect, Zophobas atratus (Coleoptera; Tenebrionidae) and a comparison of DNA barcoding with similar tenebrionids, Promethis valgipes and Tenebrio molitor in Korea. J. Sericult. Entomol. Sci. 2013, 51, 185–190. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Santos, E.M. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Zaelor, J.; Kitthawee, S. Growth response to population density in larval stage of darkling beetles (Coleoptera; Tenebrionidae) Tenebrio molitor and Zophobas atratus. Agric. Nat. Res. 2018, 52, 603–606. [Google Scholar] [CrossRef]
- Tschinkel, W.R.; Willson, C.D. Inhibition of pupation due to crowding in some tenebrionid beetles. J. Exp. Zool. 1971, 176, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Quennedey, A.; Aribi, N.; Everaerts, C.; Delbecque, J.-P. Postembryonic development of Zophobas atratus Fab. (Coleoptera: Tenebrionidae) under crowded or isolated conditions and effects of juvenile hormone analogue applications. J. Insect Physiol. 1995, 41, 143–152. [Google Scholar] [CrossRef]
- Delbecque, J.-P.; Pitoizet, N.; Quennedey, A.; Aribi, N. Ecdysteroid titres in a tenebrionid beetle, Zophobas atratus: Effects of grouping and isolation. J. Insect Physiol. 1997, 43, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Forgach, P.; Marton, S.; Bercic, R.L.; Vida, L.; Rusvai, M. Identification of a novel densovirus in the darkling beetle Zophobas morio. Acta Microbiol. Immunol. Hungar. 2015, 62, 130–131. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Malysh, S.M.; Volodartseva, Y.V.; Gerus, A.V.; Berezin, M.V. Molecular Identification of a Densovirus in Healthy and Diseased Zophobas morio (Coleoptera, Tenebrionidae). Intervirology 2019, 62, 222–226. [Google Scholar] [CrossRef]
- Yang, W.T.; Shi, S.H.; Jiang, Y.L.; Zhao, L.; Chen, H.L.; Huang, K.Y.; Yang, G.-L.; Wang, C.-F. Genetic characterization of a densovirus isolated from great tit (Parus major) in China. Infect. Genet. Evol. 2016, 41, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Perkin, L.C.; Lorenzen, M.; Dossey, A.T. Transcriptome analysis of life stages of the house cricket, Acheta domesticus, to improve insect crop production. Sci. Rep. 2020, 10, 3471. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Granberg, F.; Low, M.; Onorati, P.; Semberg, E.; Jansson, A.; Berggren, Å. Virus Diversity and Loads in Crickets Reared for Feed: Implications for Husbandry. Front. Vet. Sci. 2021, 8, 642085. [Google Scholar] [CrossRef] [PubMed]
- Styer, E.L.; Hamm, J.J. Report of a densovirus in a commercial cricket operation in the southeastern United States. J. Invertebr. Pathol. 1991, 58, 283–285. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Jousset, F.-X.; Zadori, Z.; Szelei, J.; Yu, Q.; Tijssen, P. The Acheta domesticus Densovirus, Isolated from the European House Cricket, Has Evolved an Expression Strategy Unique among Parvoviruses. J. Virol. 2011, 85, 10069–10078. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Iwao, H.; Szelei, J.; Li, Y.; Liu, K.; Bergoin, M.; Tijssen, P. Comparative Genomic Analysis of Acheta domesticus Densovirus Isolates from Different Outbreaks in Europe, North America, and Japan. Genome Announc. 2013, 1, e00629-13. [Google Scholar] [CrossRef]
- Meynadier, G.; Matz, G.; Veyrunes, J.-C.; Bres, N. Virus de type densonucléose chez les orthoptères. Ann. Soc. Entomol. Fr. 1977, 13, 487–493. [Google Scholar]
- Pham, H.T.; Bergoin, M.; Tijssen, P. Acheta domesticus volvovirus, a novel single-stranded circular DNA virus of the house cricket. Genome Announc. 2013, 1, e00079-13. [Google Scholar] [CrossRef]
- Pham, H.T.; Iwao, H.; Bergoin, M.; Tijssen, P. New Volvovirus Isolates from Acheta domesticus (Japan) and Gryllus assimilis (United States). Genome Announc. 2013, 1, e00328-13. [Google Scholar] [CrossRef]
- Pham, H.T.; Yu, Q.; Bergoin, M.; Tijssen, P. A Novel Ambisense Densovirus, Acheta domesticus Mini Ambidensovirus, from Crickets. Genome Announc. 2013, 1, e00914-13. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Granberg, F.; Onorati, P.; Jansson, A.; Berggren, Å. Virus Prospecting in Crickets—Discovery and Strain Divergence of a Novel Iflavirus in Wild and Cultivated Acheta domesticus. Viruses 2021, 13, 364. [Google Scholar] [CrossRef]
- Kleespies, R.; Tidona, C.; Darai, G. Characterization of a New Iridovirus Isolated from Crickets and Investigations on the Host Range. J. Invert. Pathol. 1999, 73, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Plus, N.; Scotti, P.D. The biological properties of eight different isolates of cricket paralysis virus. Ann. Inst. Pasteur/Virol. 1984, 135, 257–268. [Google Scholar] [CrossRef]
- Christian, P.D.; Scotti, P. A Suggested Taxonomy and Nomenclature for the Cricket Paralysis and Drosophila C Virus Complex. J. Invert. Pathol. 1994, 63, 157–162. [Google Scholar] [CrossRef]
- Lee, Y.; Fuxa, J.R. Transport of Wild-Type and Recombinant Nucleopolyhedroviruses by Scavenging and Predatory Arthropods. Microb. Ecol. 2000, 39, 301–313. [Google Scholar] [CrossRef]
- Lee, Y.; Fuxa, J.R. Ingestion and Defecation of Recombinant and Wild-Type Nucleopolyhedroviruses by Scavenging and Predatory Arthropods. Environ. Entomol. 2000, 29, 950–957. [Google Scholar] [CrossRef][Green Version]
- Valles, S.M.; Chen, Y. Serendipitous discovery of an RNA virus from the cricket, Acheta domesticus. Fla. Entomol. 2006, 89, 282–283. [Google Scholar] [CrossRef]
- Porter, S.D.; Valles, S.M.; Pereira, R.M. Scavenging crickets (Orthoptera: Gryllidae) transmit Solenopsis invicta virus 3 to red imported fire ant (Hymenoptera: Formicidae) colonies. Fla. Entomol. 2016, 99, 811–881. [Google Scholar] [CrossRef][Green Version]
- La Fauce, K.A.; Owens, L. RNA interference reduces PmergDNV expression and replication in an in vivo cricket model. J. Invert. Pathol. 2009, 100, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Sakuna, K.; Elliman, J.; Owens, L. Assessment of a cricket, Acheta domesticus, bioassay for Chequa Iflavirus and bunya-like virus from redclaw crayfish Cherax quadricarinatus. J. Invert. Pathol. 2017, 150, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Dynamics during Industrial Rearing, Processing, and Storage of Tropical House Crickets (Gryllodes sigillatus) for Human Consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Otte, D.; Cade, W. African Crickets (Gryllidae). 6. The Genus Gryllus and Some Related Genera (Gryllinae, Gryllini). Proc. Nat. Acad. Sci. Phila. 1984, 136, 98–122. [Google Scholar]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Chae, K.-S.; Shin, C.-S.; Shin, W.-S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci. Biotechnol. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Merkel, G. The effects of temperature and food quality on the larval development of Gryllus bimaculatus (Orthoptera, Gryllidae). Oecologia 1977, 30, 129–140. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Roininen, H. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, D.; Michaelson, L.; Field, L.M. The effect of an initial high-quality feeding regime on the survival of Gryllus bimaculatus (black cricket) on bio-waste. J. Insects Food Feed. 2019, 5, 117–123. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Sci. Technol. Int. 2016, 23, 17–23. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, J.H. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, B.-J.; You, S.-G.; Lee, S.-M. 2021. Nutritional evaluation of cricket, Gryllus bimaculatus, meal as fish meal substitute for olive flounder, Paralichthys olivaceus, juveniles. J. World Aquac. Soc. 2021, 52, 859–880. [Google Scholar] [CrossRef]
- Just, F.; Essbauer, S.; Ahne, W.; Blahak, S. Occurrence of an Invertebrate Iridescent-Like Virus (Iridoviridae) in Reptiles. J. Vet. Med. B 2001, 48, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Papp, T.; Spann, D.; Marschang, R.E. Development and use of a real-time polymerase chain reaction for the detection of Group II Invertebrate Iridoviruses in pet lizards and prey insects. J. Zoo Wildl. Med. 2014, 45, 219–227. [Google Scholar] [CrossRef]
- Huger, A.M. A new virus disease of crickets (Orthoptera: Gryllidae) causing macronucleosis of fatbody. J. Invertebr. Pathol. 1985, 45, 108–111. [Google Scholar] [CrossRef]
- Wang, Y.; Kleespies, R.G.; Huger, A.M.; Jehle, J.A. The Genome of Gryllus bimaculatus Nudivirus Indicates an Ancient Diversification of Baculovirus-Related Nonoccluded Nudiviruses of Insects. J. Virol. 2007, 81, 5395–5406. [Google Scholar] [CrossRef]
- Wang, Y.; Jehle, J.A. Nudiviruses and other large, double-stranded circular DNA viruses of invertebrates: New insights on an old topic. J. Invertebr. Pathol. 2009, 101, 187–193. [Google Scholar] [CrossRef]
- Scotti, P.D.; Longworth, J.F.; Plus, N.; Croizier, G.; Reinganum, C. The Biology and Ecology of Strains of an Insect Small RNA Virus Complex. Adv. Vir. Res. 1981, 26, 117–143. [Google Scholar] [CrossRef]
- Purrini, K.; Kohring, G.-W.; Seguni, Z. Studies on a new disease in a natural population of migratory locusts, Locusta migratoria, caused by an entomopoxvirus. J. Invertebr. Pathol. 1988, 51, 284–286. [Google Scholar] [CrossRef]
- Levin, D.B.; Adachi, D.; Williams, L.L.; Myles, T.G. Host Specificity and Molecular Characterization of the Entomopoxvirus of the Lesser Migratory Grasshopper, Melanoplus sanguinipes. J. Invertebr. Pathol. 1993, 62, 241–247. [Google Scholar] [CrossRef]
- Jaeger, B.; Langridge, W.H.R. Infection of Locusta migratoria with entomopoxviruses from Arphia conspersa and Melanoplus sanguinipes grasshoppers. J. Invertebr. Pathol. 1984, 43, 374–382. [Google Scholar] [CrossRef]
- Streett, D.; Woods, S.; Erlandson, M. Entomopoxviruses of grasshoppers and locusts: Biology and biological control potential. Mem. Ent. Soc. Can. 1997, 129, 115–130. [Google Scholar] [CrossRef]
- Faktor, O.; Raviv, D. A polymerase chain reaction for the detection of nucleopolyhedroviruses in infected insects: The fate of the Spodoptera littoralis virus in Locusta migratoria. J. Virol. Methods 1996, 61, 95–101. [Google Scholar] [CrossRef]
- Bensimon, A.; Zinger, S.; Gerassi, E.; Hauschner, A.; Harpaz, I.; Sela, I. “Dark cheeks,” a lethal disease of locusts provoked by a lepidopterous baculovirus. J. Invertebr. Pathol. 1987, 50, 254–260. [Google Scholar] [CrossRef]
- Cologan, D.J. Studies of the mortality of Locusta migratoria (L.) treated with a polyhedrosis virus from the grasshopper Caledia captiva (F.) (Orthoptera: Acrididae). Bull. Entomol. Res. 1986, 76, 539. [Google Scholar] [CrossRef]
- Hawkes, F. A virus-like structure in the desert locust. Naturwissenschaften 1968, 55, 547. [Google Scholar] [CrossRef]
- Purrini, K.; Rohde, M. Light and electron microscope studies on two new diseases in natural populations of the desert locust, Schistocerca gregaria, and the grassland locust, Chortipes sp., caused by two entomopoxviruses. J. Invertebr. Pathol. 1988, 51, 281–283. [Google Scholar] [CrossRef]
- Capinera, J.L.; Squitier, J.M. American Grasshopper, Schistocerca americana (Drury) (Insecta: Orthoptera: Acrididae); IFAS Extension, University of Florida: Gainesville, FL, USA, 2017. [Google Scholar] [CrossRef]
- Henry, J.E.; Jutila, J.W. The isolation of a polyhedrosis virus from a grasshopper. J. Invert. Pathol. 1966, 8, 417–418. [Google Scholar] [CrossRef]
- Streett, D.A.; Oma, E.A.; Henry, J.E. Cross infection of three grasshopper species with the Melanoplus sanguinipes entomopoxvirus. J. Invertebr. Pathol. 1990, 56, 419–421. [Google Scholar] [CrossRef]
- Henry, J.E. Development of the crystalline-array virus (CAV) in cultures of dorsal vessels from Schistocerca americana (Orthoptera: Acrididae). J. Invertebr. Pathol. 1972, 19, 325–330. [Google Scholar] [CrossRef]
- Henry, J.E.; Oma, E.A. Ultrastructure of the replication of the grasshopper crystalline-array virus in Schistocerca americana compared with other picornaviruses. J. Invertebr. Pathol. 1973, 21, 273–281. [Google Scholar] [CrossRef]
- Semberg, E.; de Miranda, R.J.; Low, M.; Jansson, A.; Forsgren, E.; Berggren, Å. Diagnostic protocols for the detection of Acheta domesticus densovirus (AdDV) in cricket frass. J. Virol. Methods 2019, 264, 61–64. [Google Scholar] [CrossRef]
- Martínez, G.; Christian, P.; Marina, C.; Williams, T. Sensitivity of Invertebrate iridescent virus 6 to organic solvents, detergents, enzymes and temperature treatment. Virus Res. 2003, 91, 249–254. [Google Scholar] [CrossRef]
- Spranghers, T.; Noyez, A.; Schildermans, K.; De Clercq, P. Cold Hardiness of the Black Soldier Fly (Diptera: Stratiomyidae). J. Econom. Entomol. 2017, 110, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energ. 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Da Silva, G.D.P.; Hesselberg, T. A Review of the Use of Black Soldier Fly Larvae, Hermetia illucens (Diptera: Stratiomyidae), to Compost Organic Waste in Tropical Regions. Neotrop. Entomol. 2019, 49, 151–162. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-Sagan, A.A.; Alkhateeb, M.; et al. Black Soldier Fly (Hermetia illucens) Meal as A Promising Feed Ingredient for Poultry: A Comprehensive Review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- English, G.; Wanger, G.; Colombo, S.M. A review of advancements in black soldier fly (Hermetia illucens) production for dietary inclusion in salmonid feeds. J. Agr. Food Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Gougbedji, A.; Agbohessou, P.; Lalèyè, P.A.; Francis, F.; Megido, R.C. Technical basis for the small-scale production of black soldier fly, Hermetia illucens (L. 1758), meal as fish feed in Benin. J. Agr. Food Res. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agr. 2016, 97, 2594–2600. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—a review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on Different Organic By-Products: Influence on Growth, Waste Reduction, and Environmental Impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Ewusie, E.A.; Kwapong, P.K.; Ofosu-Budu, G.; Sandrock, C.; Stamer, A.; Akumah, A.; Adamtey, N. The black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Trapping and culturing of wild colonies in Ghana. Sci. Afr. 2019, 5, e00134. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Gligorescu, A.; Christian, H.F.; Larsen, P.F.; Nørgaard, J.V.; Lars-Henrik, L.H. Production and Optimization of Hermetia illucens (L.) Larvae Reared on Food Waste and Utilized as Feed Ingredient. Sustainability 2020, 12, 9864. [Google Scholar] [CrossRef]
- Joosten, L.; Lecocq, A.; Jensen, A.B.; Haenen, O.; Schmitt, E.; Eilenberg, J. Review of insect pathogen risks for the black soldier fly (Hermetia illucens) and guidelines for reliable production. Entomol. Exp. Appl. 2020, 168, 432–447. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2014, 35, 261–271. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef] [PubMed]
- Stoffolano, J.G. Fly foregut and transmission of microbes. Adv. Insect Phys. 2019, 57, 27–95. [Google Scholar] [CrossRef]
- Förster, M.; Klimpel, S.; Sievert, K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet. Parasitol. 2009, 160, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.; Molnar, C.; Morley, F.; Wray, T.; Robinson, F.; Khan, B.; Goonewardene, L. Nutritive value of house fly (Musca domestica) larvae as a feed supplement for turkey poults. Anim. Feed Sci. Technol. 2003, 105, 225–230. [Google Scholar] [CrossRef]
- Koné, N.; Sylla, M.; Nacambo, S.; Kenis, M. Production of house fly larvae for animal feed through natural oviposition. J. Insects Food Feed. 2017, 3, 177–186. [Google Scholar] [CrossRef]
- Fitches, E.C.; Dickinson, M.; De Marzo, D.; Wakefield, M.E.; Charlton, A.C.; Hall, H. Alternative protein production for animal feed: Musca domestica productivity on poultry litter and nutritional quality of processed larval meals. J. Insects Food Feed. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Ganda, H.; Abihona, H.A.; Zannou-Boukari, E.T.; Kenis, M.; Chrysostome, C.A.A.M.; Mensah, G.A. Influence of adult diet on biological parameters of the housefly, Musca domestica L. (Diptera: Muscidae). J. Basic Appl. Zool. 2020, 81, 46–53. [Google Scholar] [CrossRef]
- Čičková, H.; Pastor, B.; Kozánek, M.; Martínez-Sánchez, A.; Rojo, S.; Takáč, P. Biodegradation of pig manure by the housefly, Musca domestica: A viable ecological strategy for pig manure management. PLoS ONE 2012, 7, e32798. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. J. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Czapar, G.F.; Winkler, M.K.; Zheng, J. A full-scale house fly (Diptera: Muscidae) larvae bioconversion system for value-added swine manure reduction. Waste Manag. Res. 2013, 31, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yu, L.; Li, H.; Xu, X.; Yang, Z. Use of housefly (Musca domestica L.) larvae to bioconversion food waste for animal nutrition and organic fertilizer. Environ. Sci. Pollut. Res. 2021, 28, 48921–48928. [Google Scholar] [CrossRef] [PubMed]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Morales-Ramos, J.A., Thomas, M., Rojas, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar] [CrossRef]
- Moussa, A.Y. A new virus disease in the housefly, Musca domestica (Diptera). J. Invertebr. Pathol. 1978, 31, 204–216. [Google Scholar] [CrossRef]
- Coler, R.R.; Boucias, D.G.; Frank, J.H.; Maruniak, J.E.; Garcia-Canedo, A.; Pendland, J.C. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 1993, 7, 275–282. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Knight, R.; Gilman, R.H.; Cranfield, M.R. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes Infect. 2001, 3, 231–235. [Google Scholar] [CrossRef]
- Noguchi, H. The relation of mosquitoes and flies to the epidemiology of acute poliomyelitis. J. Exp. Med. 1917, 26, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir, M.G. Studies of the fate of type 1 polioviruses in flies. J. Exp. Med. 1961, 113, 159–176. [Google Scholar] [CrossRef]
- Hurlbut, H.S. The Recovery of Poliomyelitis Virus after Parenteral Introduction into Cockroaches and Houseflies. J. Inf. Dis. 1950, 86, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.W. Experiments on insect transmission of the virus of poliomyelitis. J. Exp. Med. 1912, 16, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Melnick, J.L. The survival of poliomyelitis and coxsackie viruses following their ingestion by flies. J. Exp. Med. 1952, 96, 255–271. [Google Scholar] [CrossRef]
- Gregorio, S.B.; Nakao, J.C.; Beran, G.W. Human enteroviruses in animals and arthropods in the central Philippines. Southeast Asian J. Trop. Med. Public Health 1972, 3, 45–51. [Google Scholar] [PubMed]
- Tan, S.W.; Yap, K.L.; Lee, H.L. Mechanical Transport of Rotavirus by the Legs and Wings of Musca domestica (Diptera: Muscidae). J. Med. Entomol. 1997, 34, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Nasar, F.; Schellhase, C.W.; Moon, R.D.; Padilla, S.L.; Zeng, X.; Trefry, J.C. Low potential for mechanical transmission of Ebola virus via house flies (Musca domestica). Parasit. Vectors. 2017, 10, 218. [Google Scholar] [CrossRef]
- Prompiboon, P.; Lietze, V.-U.; Denton, J.S.S.; Geden, C.J.; Steenberg, T.; Boucias, D.G. Musca domestica Salivary Gland Hypertrophy Virus, a Globally Distributed Insect Virus That Infects and Sterilizes Female Houseflies. Appl. Environ. Microbiol. 2009, 76, 994–998. [Google Scholar] [CrossRef]
- Geden, C.J.; Lietze, V.-U.; Boucias, D.G. Seasonal Prevalence and Transmission of Salivary Gland Hypertrophy Virus of House Flies (Diptera: Muscidae). J. Med. Entomol. 2008, 45, 42–51. [Google Scholar] [CrossRef]
- Geden, C.J.; Steenberg, T.; Lietze, V.-U.; Boucias, D.G. Salivary gland hypertrophy virus of house flies in Denmark: Prevalence, host range, and comparison with a Florida isolate. J. Vector Ecol. 2011, 36, 231–238. [Google Scholar] [CrossRef]
- Geden, C.; Garcia-Maruniak, A.; Lietze, V.U.; Maruniak, J.; Boucias, D.G. Impact of House Fly Salivary Gland Hypertrophy Virus (MdSGHV) on a Heterologous Host, Stomoxys calcitrans. J. Med. Entomol. 2011, 48, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Geden, C.J.; Blackburn, P.; Boucias, D.G. Effects of Salivary Gland Hypertrophy Virus on the Reproductive Behavior of the Housefly, Musca domestica. Appl. Environ. Microbiol. 2007, 73, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Abd-Alla, A.M.M.; Boucias, D.G. Two hytrosaviruses, MdSGHV and GpSGHV, induce distinct cytopathologies in their respective host insects. J. Invertebr. Pathol. 2011, 107, 161–163. [Google Scholar] [CrossRef]
- Lietze, V.-U.; Salem, T.Z.; Prompiboon, P.; Boucias, D.G. Tissue tropism of the Musca domestica salivary gland hypertrophy virus. Virus Res. 2011, 155, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Sims, K.R.; Salem, T.Z.; Geden, C.J.; Boucias, D.G. Transmission of MdSGHV among adult house flies, Musca domestica (Diptera: Muscidae), occurs via oral secretions and excreta. J. Invertebr. Pathol. 2009, 101, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Geden, C.J.; Doyle, M.A.; Boucias, D.G. Disease Dynamics and Persistence of Musca domestica Salivary Gland Hypertrophy Virus Infections in Laboratory House Fly (Musca domestica) Populations. Appl. Environ. Microbiol. 2011, 78, 311–317. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Yao, X.; Yu, F.; Teal, P.E.; Verhoeven, C.P.; Boucias, D.G. Responses of the Housefly, Musca domestica, to the Hytrosavirus Replication: Impacts on Host’s Vitellogenesis and Immunity. Front. Microbiol. 2017, 8, 583. [Google Scholar] [CrossRef]
- Schaler, J.; Stoffolano, J.; Fausto, A.M.; Gambellini, G.; Burand, J. Effect of diet on adult house fly (Diptera: Muscidae) injected with the Salivary Gland Hypertrophy Virus (MdSGHV). J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Rachimi, S.; Burand, J.P.; Geden, C.; Stoffolano, J.G. The Effect of the Musca domestica Salivary Gland Hypertrophy Virus on Food Consumption in Its Adult Host, the Common House Fly (Diptera: Muscidae). J. Med. Entomol. 2021, 58, 1398–1404. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Hawkes, R.A.; Dickson, M.R.; Shipp, E.; Woods, A. Serological relationships of the housefly virus and some members of the family Reoviridae. Aust. J. Biol. Sci. 1982, 35, 669–678. [Google Scholar] [CrossRef]
- Wanaratana, S.; Panyim, S.; Pakpinyo, S. The potential of house flies to act as a vector of avian influenza subtype H5N1 under experimental conditions. Med. Vet. Entomol. 2010, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wanaratana, S.; Amonsin, A.; Chaisingh, A.; Panyim, S.; Sasipreeyajan, J.; Pakpinyo, S. Experimental Assessment of Houseflies as Vectors in Avian Influenza Subtype H5N1 Transmission in Chickens. Avian Dis. 2013, 57, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wuryastuty, H.; Wasito, R. Molecular Identification of Avian Influenza A Virus in House Flies (Musca domestica Linnaeus) Collected from Different Poultry Farms in Indonesia. J. Sain Vet. 2013, 31, 1–7. [Google Scholar] [CrossRef]
- Tyasasmaya, T.; Wuryastuty, H.; Wasito, W.; Sievert, K. Avian Influenza Virus H5N1 Remained Exist in Gastrointestinal Tracts of House Flies 24 Hours Post-infection. Biology 2016, 17, 205–210. Available online: https://ojs.unud.ac.id/index.php/jvet/article/view/22119 (accessed on 29 October 2021).
- Salamatian, I.; Moshaverinia, A.; Razmyar, J.; Ghaemi, M. In vitro Acquisition and Retention of Low-Pathogenic Avian Influenza H9N2 by Musca domestica (Diptera: Muscidae). J. Med. Entomol. 2019, 57, 563–567. [Google Scholar] [CrossRef]
- Calibeo-Hayes, D.; Denning, S.S.; Stringham, S.M.; Guy, J.S.; Smith, L.G.; Watson, D.W. Mechanical Transmission of Turkey Coronavirus by Domestic Houseflies (Musca domestica Linnaeaus). Avian Dis. 2003, 47, 149–153. [Google Scholar] [CrossRef]
- Rogoff, W.M.; Carbrey, E.G.; Bram, R.A.; Clark, T.B.; Gretz, G.H. Transmission of Newcastle Disease Virus by Insects: Detection in Wild Fannia Spp. (Diptera: Muscidae). J. Med. Entomol. 1975, 12, 225–227. [Google Scholar] [CrossRef]
- Watson, D.W.; Niño, E.L.; Rochon, K.; Denning, S.; Smith, L.; Guy, J.S. Experimental Evaluation of Musca domestica (Diptera: Muscidae) as a Vector of Newcastle Disease Virus. J. Med. Entomol. 2007, 44, 666–671. [Google Scholar] [CrossRef]
- Chakrabarti, S.; King, D.J.; Afonso, C.; Swayne, D.; Cardona, C.J.; Kuney, D.R.; Gerry, A.C. Detection and Isolation of Exotic Newcastle Disease Virus from Field-Collected Flies. J. Med. Entomol. 2007, 44, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; King, D.J.; Cardona, C.J.; Gerry, A.C. Persistence of Exotic Newcastle Disease Virus (ENDV) in Laboratory Infected Musca domestica and Fannia canicularis. Avian Dis. 2008, 52, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Barin, A.; Arabkhazaeli, F.; Rahbari, S.; Madani, S.A. The housefly, Musca domestica, as a possible mechanical vector of Newcastle disease virus in the laboratory and field. Med. Vet. Entomol. 2010, 24, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Braverman, Y. Insect Contribution to Horizontal Transmission of Reticuloendotheliosis virus. J. Med. Entomol. 2005, 42, 128–133. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Farnham, M.; Pijoan, C. Survival of porcine reproductive and respiratory syndrome virus in houseflies. Can. J. Vet. Res. 2003, 67, 198–203. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC227053/ (accessed on 28 October 2021).
- Otake, S.; Dee, S.A.; Rossow, K.D.; Moon, R.D.; Trincado, C.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet. Rec. 2003, 152, 73–76. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Pijoan, C. Studies on the carriage and transmission of porcine reproductive and respiratory syndrome virus by individual houseflies (Musca domestica). Vet. Rec. 2004, 154, 80–85. [Google Scholar] [CrossRef]
- Schurrer, J.A.; Dee, S.A.; Moon, R.D.; Murtaugh, M.P.; Finnegan, C.P.; Deen, J.; Pijoan, C.B.J. Retention of ingested porcine reproductive and respiratory syndrome virus in houseflies. Am. J. Vet. Res. 2005, 66, 1517–1525. [Google Scholar] [CrossRef]
- Pitkin, A.; Deen, J.; Otake, S.; Moon, R.; Dee, S. Further assessment of houseflies (Musca domestica) as vectors for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus under field conditions. Can. J. Vet. Res. 2009, 73, 91–96. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2666325/ (accessed on 28 October 2021).
- Blunt, R.; McOrist, S.; McKillen, J.; McNair, I.; Jiang, T.; Mellits, K. House fly vector for porcine circovirus 2b on commercial pig farms. Vet. Microbiol. 2011, 149, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Masiuk, D.N.; Nedzvetsky, V.S.; Sosnitskiy, A.I.; Kokarev, A.V.; Koliada, S.G. The characteristics, emergent properties and manner of spread in Ukraine of the Porcine Epidemic Diarrhea Virus. Reg. Mech. Biosyst. 2018, 9, 401–408. [Google Scholar] [CrossRef]
- Herm, R.; Tummeleht, L.; Jürison, M.; Vilem, A.; Viltrop, A. Trace amounts of African swine fever virus DNA detected in insects collected from an infected pig farm in Estonia. Vet. Med. Sci. 2019, 6, 100–104. [Google Scholar] [CrossRef]
- Turčinavičienė, J.; Petrašiūnas, A.; Bernotienė, R.; Masiulis, M.; Jonušaitis, V. The contribution of insects to African swine fever virus dispersal: Data from domestic pig farms in Lithuania. Med. Vet. Entomol. 2020, 35, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Medveczky, I.; Kovács, L.; Kovács Sz, F.; Papp, L. The role of the housefly, Musca domestica, in the spread of Aujeszky’s disease (pseudorabies). Med. Vet. Entomol. 1988, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Mohr, K.A.; Clement, T.; Hain, K.S.; Myers, B.; Yaros, J.; Diel, D.G. Detection of the Emerging Picornavirus Senecavirus A in Pigs, Mice, and Houseflies. J. Clin. Microbiol. 2016, 54, 1536–1545. [Google Scholar] [CrossRef]
- Turell, M.J.; Dohm, D.J.; Geden, C.J.; Hogsette, J.A.; Linthicum, K.J. Potential for Stable Flies and House Flies (Diptera: Muscidae) to Transmit Rift Valley Fever Virus1. J. Am. Mosq. Control Assoc. 2010, 26, 445–448. [Google Scholar] [CrossRef]
- Bouillant, A.; Lee, V.H.; Hanson, R.P. Epizootiology on mink enteritis. II. Musca domestica L. as a possible vector of virus. Can. J. Comp. Med. Vet. Sci. 1965, 29, 148–152. [Google Scholar]
- Prieto, A.; Díaz-Cao, J.M.; Fernández-Antonio, R.; Panadero, R.; López-Lorenzo, G.; Díaz, P.; Fernández, G. Lesser housefly (Fannia canicularis) as possible mechanical vector for Aleutian mink disease virus. Vet. Microbiol. 2018, 221, 90–93. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Prutnikov, P.; Kononov, A. Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transbound. Emerg. Dis. 2018, 65, 1137–1144. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Boucias, D.; Baniszewski, J.; Prompiboon, P.; Lietze, V.; Geden, C. Enhancement of the Musca domestica hytrosavirus infection with orally delivered reducing agents. J. Invertebr. Pathol. 2015, 124, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 2013, 52, 1–17. [Google Scholar] [CrossRef]
- Anderson, D.L. Pests and Pathogens of the Honeybee (Apis Mellifera L.) in Fiji. J. Apic. Res. 1990, 29, 53–59. [Google Scholar] [CrossRef]
- Malfroy, S.F.; Roberts, J.M.K.; Perrone, S.; Maynard, G.; Chapman, N. A pest and disease survey of the isolated Norfolk Island honey bee (Apis mellifera) population. J. Apic. Res. 2016, 55, 202–211. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ongamo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ansari, J.M.; Al Ghamdi, A.; Mohamed, O.M.; Kaur, M. Effect of larval nutrition on the development and mortality of Galleria mellonella (Lepidoptera: Pyralidae). Rev. Colomb. Entomol. 2014, 40, 49–54. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-04882014000100009 (accessed on 28 October 2021).
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef]
- Kumar, G.; Khan, M.S. Study of the life cycle of greater wax moth (Galleria mellonella) under storage conditions in relation to different weather conditions. J. Entomol. Zool. Stud. 2018, 6, 444–447. Available online: https://www.entomoljournal.com/archives/2018/vol6issue3/PartG/6-2-76-351.pdf (accessed on 28 October 2021).
- Sohail, M.; Aqueel, M.A.; Dai, P.; Ellis, J.D.; Johnson, R. The Larvicidal and Adulticidal Effects of Selected Plant Essential Oil Constituents on Greater Wax Moths. J. Econom. Entomol. 2021, 114, 397–402. [Google Scholar] [CrossRef]
- Hickin, M.; Nadel, H.; Schal, C.; Cohen, A.C. Optimization of a Diet for the Greater Wax Moth (Lepidoptera: Pyralidae) Using Full Factorial and Mixture Design. J. Econom. Entomol. 2021, 114, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.; Titball, R.; Bates, S. Standardization of G. mellonella Larvae to Provide Reliable and Reproducible Results in the Study of Fungal Pathogens. J. Fungi 2018, 4, 108. [Google Scholar] [CrossRef]
- Andrea, A.; Krogfelt, K.A.; Jenssen, H. Methods and Challenges of Using the Greater Wax Moth (Galleria mellonella) as a Model Organism in Antimicrobial Compound Discovery. Microorganisms 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; de Camargo Ribeiro, F.; Santana dos Santos, H.F.; dos Santos, J.D.; de Sousa Oliveira, N.; Theotonio dos Santos Dutra, M.T.; Biazzi de Lapena, S.A.; Junqueira, J.C. Galleria mellonella as an experimental model to study human oral pathogens. Arch. Oral Biol. 2019, 101, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Tunaz, H.; Stanley, D.; Büyükgüzel, K. Eicosanoids mediate Galleria mellonella cellular immune response to viral infection. J. Insect Physiol. 2007, 53, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Newman, J.F.E.; Porterfield, J.S. The Multiplication of Nodamura Virus in Insect and Mammalian Cell Cultures. J. Gen. Virol. 1975, 26, 15–20. [Google Scholar] [CrossRef]
- Garzon, S.; Charpentier, G.; Kurstak, E. Morphogenesis of the nodamura virus in the larbae of the lepidopteran Galleria mellonella (L.). Arch. Virol. 1978, 56, 61–76. [Google Scholar] [CrossRef]
- Garzon, S.; Strykowski, H.; Charpentier, G. Implication of mitochondria in the replication of Nodamura virus in larvae of the Lepidoptera, Galleria mellonella (L.) and in suckling mice. Arch. Virol. 1990, 113, 165–176. [Google Scholar] [CrossRef]
- 259 Tal, J.; Attathom, T. Insecticidal potential of the insect parvovirusGmDNV. Arch. Insect Biochem. Physiol. 1993, 22, 345–356. [Google Scholar] [CrossRef]
- Meynardier, G.; Vago, C.; Plantevin, G.; Atger, P.X. Virose d’un type habitue1 chez le Lepidoptere, Galleria mellonella. Rev. Zool. Agric. Appl. 1987, 63, 207. [Google Scholar]
- Jafri, R.H.; Khan, A.A. A study of the development of a nuclear-polyhedrosis virus of Heliothis in Galleria mellonella larvae exposed to gamma rays. J. Invertebr. Pathol. 1969, 14, 104. [Google Scholar] [CrossRef]
- Smirnoff, W.A. The amount of potassium and sulfur in healthy and nuclear polyhedrosis virus-infected larvae of Galleria mellonella. J. Invertebr. Pathol. 1971, 18, 295–296. [Google Scholar] [CrossRef]
- Komissarenko, S.V.; Zherebtsova, E.N.; Sutugina, L.P.; Primatchenko, L.V. Non-occluded particles of nuclear polyhedrosis virus infecting Galleria mellonella L.: Titration in vivo and in vitro. Brief report. Arch. Virol. 1979, 61, 347–349. [Google Scholar] [CrossRef]
- Stairs, G.R. Quantitative studies on the infection of Galleria mellonella with a nuclear polyhedrosis virus of Bombyx mori. J. Invertebr. Pathol. 1991, 57, 402–405. [Google Scholar] [CrossRef]
- Demir, İ.; Nalçacioğlu, R.; Mohammad Gholizad, L.; Demirbağ, Z. A highly effective nucleopolyhedrovirus against Malacosoma spp. (Lepidoptera: Lasiocampidae) from Turkey: Isolation, characterization, phylogeny, and virulence. Turk. J. Agric. For. 2014, 38, 462–470. [Google Scholar] [CrossRef]
- El Husseini, M.M. Pathogenicity of nuclear polyhedrosis virus to Galleria mellonella L. (Lepidoptera: Pyralidae) and its control on stored beeswax foundations. Egypt J. Biol. Pest Control 2020, 30, 101. [Google Scholar] [CrossRef]
- Fraser, M.J.; Hink, W.F. The isolation and characterization of the MP and FP plaque variants of Galleria mellonella nuclear polyhedrosis virus. Virology 1982, 117, 366–378. [Google Scholar] [CrossRef]
- Fraser, M.J.; Stairs, G.R. Susceptibility of Trichoplusia ni, Heliothis zea (Noctuidae), and Manduca sexta (Sphingidae) to a nuclear polyhedrosis virus from Galleria mellonella (Pyralidae). J. Invert. Pathol. 1982, 40, 255–259. [Google Scholar] [CrossRef]
- Miloserdova, V.D.; Sukhorada, E.M. In vitro cultivation of testicular cysts of the greater wax moth and multiplication of the associated nuclear polyhedrosis virus. J. Invert. Pathol. 1975, 26, 7–10. [Google Scholar] [CrossRef]
- Bird, F.T. The development of Tipula iridescent virus in the crane fly, Tipula paludosa Meig., and the wax moth, Galleria mellonella L. Can. J. Microbiol. 1961, 7, 827–830. [Google Scholar] [CrossRef]
- Marina, C.F.; Feliciano, J.M.; Valle, J.; Williams, T. Effect of Temperature, pH, Ion Concentration, and Chloroform Treatment on the Stability of Invertebrate Iridescent Virus 6. J. Invertebr. Pathol. 2000, 75, 91–94. [Google Scholar] [CrossRef]
- Constantino, M.; Christian, P.; Marina, C.F.; Williams, T. A comparison of techniques for detecting Invertebrate iridescent virus J. Virol. Methods 2001, 98, 109–118. [Google Scholar] [CrossRef]
- Rud, Y.; Prylutska, S.; Buchatskyy, L.; Prylutskyy, Y.; Ritter, U.; Scharff, P. Photodynamic inactivation of mosquito iridovirus (MIV) by C60 fullerenes. Mater. Sci. Technol. 2011, 42, 2. [Google Scholar] [CrossRef]
- Rud, Y.; Buchatskyy, L.; Prylutskyy, Y.; Marchenko, O.; Senenko, A.; Schütze, C.; Ritter, U. Using C60 fullerenes for photodynamic inactivation of mosquito iridescent viruses. J. Enzym. Inhib. Med. Chem. 2012, 27, 614–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández, O.; Maldonado, G.; Williams, T. An epizootic of patent iridescent virus disease in multiple species of blackflies in Chiapas, Mexico. Med. Vet. Entomol. 2000, 14, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Nalcacioglu, R.; Muratoglu, H.; Yesilyurt, A.; van Oers, M.M.; Vlak, J.M.; Demirbag, Z. Enhanced insecticidal activity of Chilo iridescent virus expressing an insect specific neurotoxin. J. Invertebr. Pathol. 2016, 138, 104–111. [Google Scholar] [CrossRef]
- Younghusband, H.B.; Lee, P.E. Virus-cell studies of tipula iridescent virus in Galleria mellonella (L.): I. Electron microscopy of infection and synthesis of tipula iridescent virus in hemocytes. Virology 1969, 38, 247–254. [Google Scholar] [CrossRef]
- Younghusband, H.B.; Lee, P.E. Cytochemistry and autoradiography of Tipula iridescent virus in Galleria mellonella. Virology 1970, 40, 757–760. [Google Scholar] [CrossRef]
- Jafri, R.H.; Chaudhry, M.B. Development of Tipula iridescent virus (TIV) in Galleria mellonella larvae exposed to gamma radiation. J. Invertebr. Pathol. 1971, 18, 46–50. [Google Scholar] [CrossRef]
- Yule, B.G.; Lee, P.E. A cytological and immunological study of Tipula iridescent virus-infected Galleria mellonella larval hemocytes. Virology 1973, 51, 409–423. [Google Scholar] [CrossRef]
- Krell, P.; Lee, P.E. Polypeptides in Tipula iridescent virus (TIV) and in TIV-infected hemocytes of Galleria mellonella (L.) larvae. Virology 1974, 60, 315–326. [Google Scholar] [CrossRef]
- Tanada, Y.; Tanabe, A.M. Resistance of Galleria mellonella (Linnaeus) to the Tipula iridescent virus at high temperatures. J. Invertebr. Pathol. 1965, 7, 184–188. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V.; Woods, R.D. An Iridovirus from Bees. J. Gen. Virol. 1976, 31, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.S. A Sericesthis iridescent virus infection of the hemocytes of the waxmoth, Galleria mellonella (Lepidoptera). J. Invertebr. Pathol. 1985, 46, 219–230. [Google Scholar] [CrossRef]
- Lea, M.S. A Sericesthis iridescent virus infection of the hemocytes of the wax moth Galleria mellonella: Effects on total and differential counts and hemocyte ontogeny. J. Invertebr. Pathol. 1986, 48, 42–51. [Google Scholar] [CrossRef]
- Leutenegger, R. Development of an icosahedral virus in hemocytes of Galleria mellonella (L.). Virology 1964, 24, 200–204. [Google Scholar] [CrossRef]
- Rohel, D.Z.; Chadwick, J.; Faulkner, P. Tests with Inactivated Cricket Paralysis Virus as a Possible Immunogen against a Virus Infection of Galleria mellonella Larvae. Intervirology 1980, 14, 61–68. [Google Scholar] [CrossRef]
- Moore, N.F.; Kearns, A.; Pullin, J.S. Characterization of cricket paralysis virus-induced polypeptides in Drosophila cells. J. Virol. 1980, 33, 1–9. [Google Scholar] [CrossRef]
- Traiyasut, P.; Mookhploy, W.; Kimura, K.; Yoshiyama, M.; Khongphinitbunjong, K.; Chantawannakul, P.; Buawangpong, N.; Saraithong, P.; Burgett, M.; Chukeatirote, E. First detection of honey bee viruses in wax moth. Chiang Mai J. Nat. Sci. 2016, 43, 695–698. [Google Scholar]
- Ozkan, S.; Coutts, R.H. Aspergillus fumigatus mycovirus causes mild hypervirulent effect on pathogenicity when tested on Galleria mellonella. Fungal Genet. Biol. 2015, 76, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Barwise, A.H.; Walker, I.O. Studies on the DNA of a virus from Galleria mellonella. FEBS Lett. 1970, 6, 13–16. [Google Scholar] [CrossRef]
- Longworth, J.F.; Carey, G.P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoperai Scarabaeidae]. J. Gen. Virol. 1976, 33, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.N.; Ball, L.A. Recovery of Infectious Pariacoto Virus from cDNA Clones and Identification of Susceptible Cell Lines. J. Virol. 2001, 75, 12220–12227. [Google Scholar] [CrossRef][Green Version]
- Johnson, K.N. Virions of Pariacoto virus contain a minor protein translated from the second AUG codon of the capsid protein open reading frame. J. Gen. Virol. 2003, 84, 2847–2852. [Google Scholar] [CrossRef]
- Longworth, J.F.; Archibald, R.D. A virus of black beetle, Heteronychus arator (F.) (Coleoptera: Scarabaeidae). N. Z. J. Zool. 1975, 2, 233–236. [Google Scholar] [CrossRef]
- Yesilyurt, A.; Muratoglu, H.; Demirbag, Z.; Nalcacioglu, R. Chilo iridescent virus encodes two functional metalloproteases. Arch. Virol. 2019, 164, 657–665. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Jensen, A.; Eilenberg, J. Cannibalism as a Possible Entry Route for Opportunistic Pathogenic Bacteria to Insect Hosts, Exemplified by Pseudomonas aeruginosa, a Pathogen of the Giant Mealworm Zophoba. Insects 2018, 9, 88. [Google Scholar] [CrossRef]
- Williams, T.; Thompson, I.P. Fatty acid profiles of invertebrate iridescent viruses. Arch. Virol. 1995, 140, 975–981. [Google Scholar] [CrossRef]
- Dicke, M.; Eilenberg, J.; Salles, J.F.; Jensen, A.B.; Lecocq, A.; Pijlman, G.P.; van Loon, J.J.A.; van Oers, M.M. Edible insects unlikely to contribute to transmission of coronavirus SARS-CoV-2. J. Insects Food Feed 2020, 6, 333–339. [Google Scholar] [CrossRef]
- Lery, X.; Fediere, G.; Taha, A.; Salah, M.; Giannotti, J. A new small RNA virus persistently infecting an established cell line of Galleria mellonella, induced by a heterologous infection. J. Invertebr. Pathol. 1997, 69, 7–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).