Abstract
Partitiviruses are one of the most prevalent double-stranded RNA viruses that have been identified mostly in filamentous fungi and plants. Partitiviruses generally infect host fungi asymptomatically but infrequently exert significant effect(s) on morphology and virulence, thus being considered a potential source of biological control agents against pathogenic fungi. In this study, we performed a screening for mycoviruses of a collection of Thai isolates of rice fungal pathogen Rhizoctonia oryzae-sativae, a causal agent of rice aggregated sheath spot disease. As a result, 36% of tested isolates carried potentially viral double-stranded RNAs with sizes ranging from 2 to 3 kbp. By conventional cDNA library construction and RNA-seq, we determined six new alphapartitiviruses that infected three isolates: tentatively named Rhizoctonia oryzae-sativae partitivirus 1 to 6 (RosPV1-6). Furthermore, RT-PCR detection of each virus revealed their omnipresent nature in different R. oryzae-sativae isolates. Although virus-curing of basidiomycetous fungi is generally difficult, our repeated attempts successfully obtained virus-free (for RosPV1, RosPV2, and uncharacterized partitiviruses), isogenic strain of R. oryzae-sativae TSS190442. The virus-cured strain showed slightly faster colony growth on the synthetic media and severe symptom development on the rice sheath compared to its virus-infected counterpart. Overall, this study shed light on the distribution of partitiviruses in R. oryzae-sativae in a paddy environment and exemplified a virus-curing protocol that may be applicable for other basidiomycetous fungi.
1. Introduction
Rice (Oryza sativa L.) is a staple food crop in most Asian countries, and its production is threatened by many pathogenic microbes, such as Rhizoctonia solani (rice sheath blight), Pyricularia oryzae (leaf blast), and Xanthomonas oryzae pv. oryzae (bacterial blight) [1]. In addition, R. oryzae and R. oryzae-sativae (teleomorph: Waitea circinata, Ceratobasidium oryzae-sativae, respectively) are soil-borne basidiomycetous fungal pathogens known to cause aggregate sheath spots on rice plants worldwide [2,3]. Both pathogens withstand hostile environments. Aggregated sheath spots diseases are reported to cause yield loss up to 20.3% in Australia and 9% in Uruguay; also, such diseases were reported as a significant threat to rice production in California, the USA [2,4].
Mycoviruses, viruses that infect fungi, have attracted much attention from researchers with their potential as a biocontrol (virocontrol) agent against phytopathogenic fungi [5,6]. For example, the application of Cryphonectria hypovirus 1 to control the chestnut blight fungus Cryphonectria parasitica [7,8] has led to discoveries of several mycoviruses with biocontrol potentials, such as Sclerotinia sclerotiorum hypovirulent-associated DNA virus 1 and Sclerotinia sclerotiorum partitivirus 1 (SsPV1) of the white mold fungus, Rosellinia necatrix megabirnavirus 1 of the white root rot fungus, and others [9,10,11]. Likewise, the rice blast fungus P. oryzae (formerly Magnaporthe oryzae) is reported to carry several mycoviruses, and, of those, Magnaporthe oryzae chrysovirus 1-A and 1-B are known to carry cytotoxic protein genes [12,13]. For instance, of mycoviruses in rice-infecting Rhizoctonia species, a capsidless positive-sense (+) single-stranded RNA (ssRNA) mycovirus, Rhizoctonia solani endornavirus 1 (RsEV1), was previously reported to confer hypovirulence to R. solani AG-1-IA GD 118-P, a causal agent of rice sheath blight disease. In addition, RsEV1 infection significantly downregulated the synthesis of serine and tyrosine, amino acids (aa) that are involved in the biosynthesis of secondary metabolites in its host fungus [14].
To the best of our knowledge, only two mycoviruses infecting R. oryzae-sativae are reported to date. Rhizoctonia oryzae-sativae mitovirus 1 (RoMV1) was detected in R. oryzae-sativae strain 89-1 isolated from rice sheath showing typical symptoms of aggregate sheath spot in China [15]; Rhizoctonia oryzae-sativae partitivirus 1 (RosPV1) was characterized from a rice-infecting field isolate of R. oryzae-sativae (strain RS005) in India [16]. However, the influence of these mycoviruses on host pathogenicity has not been investigated.
Partitiviridae is an expanding double-stranded RNA (dsRNA) virus family that currently consists of five established genera (Alpha-, Beta-, Gamma-, Deltapartitivirus, and Cryspovirus) [17], and it may accommodate more diverse groups of related viruses, i.e., proposed genera tentatively named as “Zetapartitivirus” and “Epsilonpartitivirus” [10,18,19,20]. Members or potential members of Partitiviridae are infectious to plants, fungi, protozoa, and insects, in which fungal partitiviruses (approved members) are frequently detected in ascomycetous and basidiomycetous fungi and classified in the genera Alpha-, Beta-, and Gammapartitivirus. Limited numbers of partitiviruses are reported to confer hypovirulence to host fungi that include Rosellinia necatrix partitivirus 10 in association with Rosellinia necatrix virga-like virus, Botrytis cinerea partitivirus 2, SsPV1, and Rhizoctonia solani partitivirus 2 (RsPV2) [17].
This study attempted to isolate rice sheath bright fungus and aggregate sheath spot fungus from paddy fields in Thailand and detected several mycovirus-associated dsRNA elements in a total of 21.3% of isolates screened. Here, we report the genomic and phylogenetic characteristics of those mycoviruses and their distribution in the respective fungal population. Moreover, the biological impact of selected viruses is shown via a newly developed virus-curing protocol.
2. Materials and Methods
2.1. Fungal Collection, Culture, and Maintenance
A total of 80 fungal isolates were isolated from 200 symptomatic rice plants from paddy fields in Don Chedi and Song Phi Nong districts of Suphan Buri province, Thailand in March 2019. All the collected rice tissues were surface sterilized with 1.2% sodium hypochlorite (NaOCl), washed twice with ddH2O, then placed on 2% water agar (WA) containing 50 μg/mL streptomycin followed by 24 h incubation at 25 °C. The tip of newly elongated hyphae was cut and transferred to potato dextrose agar (PDA) plates for single specimen isolation and maintained at room temperature for regular use. For long-term or short-term storage, all isolates were cultured on sterilized glass fiber (Advantec, Tokyo, Japan), dried then stored at −30 °C, respectively. Based on the colony morphology of those isolates, 33 Bipolaris-like isolates and 47 Rhizoctonia-like isolates were distinguished.
2.2. Taxonomic Analysis of Fungal Species
Ten of approximately 25 mm2 mycelium plugs were used to inoculate Potato Dextrose Broth (PDB) and culture flasks were incubated at 25 °C in the dark for 5 days. The mycelial mats were harvested, and then homogenized into a fine powder in the presence of liquid nitrogen. The pulverized tissues were subjected to genomic DNA extraction using DNeasy Plant Mini-Kit following the manufacturer’s instructions (Qiagen, Venlo, Netherland). For taxonomic classification of fungal isolates, the internal transcribed spacer (ITS) regions were then amplified in a polymerase chain reaction (PCR) using the universal primer set, ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′). The PCR products were electrophoresed on 1% (w/v) agarose gel and visualized by ethidium bromide staining using a UV transilluminator. Subsequently, PCR products were purified using a Gel Clean-up Kit (Wizard SV Gel and PCR Clean-Up System, Promega, Madison, WI, USA) and quantified using a BioSpec-Nano (Shimadzu, Tokyo, Japan). PCR products were then subjected to Sanger Sequencing in both the forward and reverse directions using an Applied Biosystems 3130 Genetic Analyzer.
2.3. dsRNA Extraction
Total dsRNA fractions were extracted from 5 mg of mycelia by conventional cellulose affinity column chromatography, as previously described [21]. Briefly, fungal mycelial mats cultured in PDB were filtrated and homogenized using a mortar and pestle in the presence of liquid nitrogen. Total RNA fractions were obtained with a series of centrifugation using phenol-chloroform-isoamyl alcohol (PCI) 25:24:1 (v/v/v) and chloroform-isoamyl alcohol (CIA) 24:1 (v/v). dsRNA fractions were then purified by another round of centrifugation using a mixture of 1× Sodium/Tris/EDTA (100 mM NaCl, 10 mM Tris-HCl pH 8.0, and 1 mM EDTA pH 8.0) containing 16% ethanol and Cellulose Powder C (Advantech). The purified solution was further treated with S1 Nuclease and RQ1 RNase-Free DNase I (Promega) to eliminate genomic DNA and single-stranded RNA (ssRNA) contaminants. The presence of viral dsRNAs was confirmed by 1% agarose gel electrophoresis in 0.5× Tris/Borate/EDTA (40 mM Tris, 45 mM boric acid, and 1 mM EDTA).
2.4. cDNA Library Construction, RT-PCR, and RLM-RACE
The purified dsRNAs were used to construct complementary DNA (cDNA) libraries. dsRNA fragments were pre-denatured at 99 °C for 5 min, and then subjected to first-strand cDNA synthesis by reverse transcription using ReverTra Ace (TOYOBO, Tokyo, Japan) with an adapter-tagged random hexamer primer (5′-CCTGAATTCGGATCCTCCNNNNNN-3′). The second-strand synthesis was carried out using a single primer (5′-CCTGAATTCGGATCCTCC-3′), in which its sequence matches that of the adapter primer, and GoTaq Green Master Mix polymerase (Promega). DNA fragments were then separated by 1% (w/v) agarose gel electrophoresis (by running the gel at 100 V for 40 min), and DNA amplicons ranging between 1.0 and 2.0 kbp were exclusively purified using the Gel Clean-up Kit. The purified DNA fragments were subsequently ligated into pGEM T-easy vector (Promega) and the ligation products were used for transforming Escherichia coli DH5α competent cells. Recombinant plasmids were extracted and sequenced at both directions using universal M13F and M13R primers. The remaining first-strand cDNA products were used to fill the gaps between resulting contig sequences using gene-specific primers (respective primer sequences have been provided in Supplementary Table S1).
To obtain 5′ and 3′ terminal sequences, RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was performed. dsRNAs were initially denatured in 90% dimethyl sulphoxide (DMSO) at 65 °C for 20 min. Subsequently, 5′ phosphorylated DNA oligonucleotide adaptor (5′-PO4-CAATACCTTCTGACCATGCAGTGACAGTCAGCATG-3′) was ligated to 3′ termini of both dsRNA strands using T4 RNA ligase (TaKaRa, Kusatsu, Japan) at 16 °C for 16–18 h. Next, DMSO was added to the DNA adaptor-ligated dsRNA to a final concentration of 90% in the presence of oligonucleotide 3′-RACE-1st (5′-CATGCTGACTGTCACTGCAT-3′), which binds to the last 20 nt of the ligated adaptor sequence. The freshly denatured adaptor-ligated dsRNAs were used as templates for cDNA synthesis using M-MLV Reverse Transcriptase (Invitrogen, Thermo Fisher, Waltham, MA, USA). The synthesized cDNAs were amplified by PCR using 3′-RACE-2nd (5′-TGCATGGTCAGAAGGTATTG-3′) and respective gene-specific primers (Supplementary Table S1) targeting 5′ and 3′ termini. RACE amplicons were cloned and sequenced by the Sanger method. The sequence for each amplicon was determined by sequencing at least three independent clones.
2.5. Next-Generation Sequencing (NGS) Approach
Total RNA fraction was extracted using RNAiso Plus kit (TaKaRa) following the manufacturer’s protocol. The total RNA samples from R. oryzae-sativae TSD190123, Nigrospora oryzae TSD190144, and R. solani (Japanese isolate) were pooled together (3.3 µg/µL, RIN = 9.1) and characterized by NGS (RNA-seq) analysis. The ribosomal RNA (rRNA)-depleted samples were then employed as a template for the construction of cDNA libraries and pair-end sequencing using the Illumina HiSeq 2000 platform (Illumina, Hayward, CA, USA). The rRNA depletion, cDNA library construction, and deep sequencing analysis were performed by Macrogen Japan (Kyoto, Japan). Subsequently, adapter sequences were removed, and trimmed high-quality clean reads (62.5 M reads) were assembled into individual contigs (total 43998) using the CLC Genomic Workbench ver. 8 (CLC bio, Aarhus, Denmark). In this study, partitivirus-like contigs were squeezed and the presence of those sequences in TSD190123 were confirmed by RT-PCR. To obtain 5′ and 3′ terminal sequences, 3′ RLM-RACE was performed following the aforementioned methods (primer sequences are provided in Supplementary Table S1). Since we focused on partitiviruses in this study, the remaining viral sequences (contigs) from TSD190123 and the other fungal isolates will be reported elsewhere (Neang et al., unpublished data).
2.6. Bioinformatic Analysis
The vector contaminations and RACE adaptor sequences were removed from all the obtained sequences of cDNA clones using an online tool, VecScreen (https://www.ncbi.nlm.nih.gov/tools/vecscreen/, accessed on 2 November 2021). The resulting clean sequences were then assembled (with at least 80% identity) into contigs using ATSQ software (Genetyx Inc., Tokyo, Japan). The Open Reading Frames (ORF) Finder program (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 2 November 2021) was applied to determine the gene coding regions in viral contigs. Deduced aa sequences of coding proteins, RNA-dependent RNA polymerase (RdRp), and coat protein (CP), were predicted using Genetyx software (Genetyx Inc., Tokyo, Japan). The identification of virus-like sequences and their genetic relatedness to other viral sequences was analyzed with the online Basic Local Alignment Search Tool (BLAST). Subsequently, previously reported mycovirus sequences were downloaded from the National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/, accessed on 2 November 2021). The full-length aa sequences were subjected to phylogenetic analyses. These aa sequences were aligned using the online MAFFT program (https://mafft.cbrc.jp/alignment/server/index.html, accessed on 3 November 2021). Maximum-likelihood (ML) phylogenetic trees were constructed based on the aligned aa sequences using PhyML with the automatic model selection mode (http://www.atgc-montpellier.fr/phyml/, accessed on 3 November 2021). The ML trees were visualized and polished in FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 3 November 2021).
2.7. Virus Curing
To eliminate mycoviruses from R. oryzae-sativae TSS190442, protoplast purification, hyphal tip isolation, post-heat, and anti-viral drug treatments were applied. All incubation steps were carried out at 25 °C unless otherwise indicated.
Protoplasts were obtained by incubation of 16-hr-old mycelia in 0.6 M MgSO4, pH 5.2 containing Driselase (20 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA), Cellulase RS (20 mg/mL) (Yakult Pharmaceutical Ind. Co., Tokyo, Japan), Lysozyme (10 mg/mL) (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and Lysing enzyme (10 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA) for 3 h at 35 °C with 55 rpm aerial shaking. Protoplasts were purified by placing trapping buffer (0.3 M sorbitol in 100 mM Trish-HCl pH 7.0) on top of the enzymatic digestion solution in a ratio of 1:1. Subsequently, protoplasts were collected from the interphase. Healthy protoplasts were then recovered and grown on a regeneration medium (RM) (1 g/L yeast extract, 1 g/L casein hydrolysate, 1 M sucrose, and 1.5% agar). Hyphal tips from germinated mycelia were transferred onto new RM plates and screened for the virus infections by one-step colony RT-PCR with virus-specific primers (Supplementary Table S1).
The recovered fungal derivatives were further incubated in the dark at 37 °C for 5 days. Hyphal tips were later transferred onto new PDA plates and cultured in the dark at room temperature for another 5 days before being subjected to secondary mycovirus screening by one-step colony RT-PCR.
The mycelial plugs of isolate TSS190442 were cultured on the edge of PDA plates supplemented with ribavirin (0.2 mg/L) with or without cycloheximide (5 mg/L) for 3 days Subcultures were obtained from the tip of actively growing mycelium and transferred to fresh media. This step was repeated five times. Finally, sixth subcultures were placed and incubated on drug-free PDA. Newly germinated mycelia were subjected to one-step colony RT-PCR to screen for mycovirus infection. For further confirmation, total dsRNAs were extracted and profiled from detection-negative fungal colonies (grown in PDB) following the methods described previously.
2.8. Detection of Partitiviruses by RT-PCR
Detection of partitivirus infection among all the fungal isolates was conducted by RT-PCR using partitivirus-specific primers (Supplementary Table S1) that were designed from the RdRp-coding regions from partitivirus genome sequences identified in this study. Briefly, a portion of supernatant from the CIA step in dsRNA extraction (see above) was precipitated with lithium chloride (2 M final concentration) to enrich the ssRNA fraction. The obtained ssRNAs were further treated with RQ1 RNase-Free DNase I (Promega) before being subjected to cDNA synthesis. Subsequently, the cDNA was used as a template in PCR reaction along with gene-specific primers targeting individual partitiviruses. The presence of partitivirus was confirmed by 1.0% agarose gel electrophoresis of RT-PCR products.
2.9. Pathogenicity Test
To investigate the impact of mycoviruses on host fungal growth and virulence, the sizes of fungal colonies on PDA and symptoms on plants were elucidated. The mycelial growth of virus-infected R. oryzae-sativae TSS190442 and its virus-cured derivative on PDA were analyzed by measuring the radius of colonies for 7 days. Standard deviation and T-test were calculated on five replicates for each isolate.
The virulence of R. oryzae-sativae isolates was evaluated by an in vitro assay that is recently developed for R. solani pathogenicity (Arakawa et al., unpublished). Two-month-old rice (cv. Koshihikari) stems were detached from slightly above the soil, sterilized the surface with 1.2% NaOCl, then immediately transferred into a sterilized 14 cm test tube containing 3 mL distilled water. A piece of PDA block with actively growing mycelia of virus-free and virus-infected R. oryzae-sativae isolates were then placed on the node section between rice stem and first/second leaf. Excess rice tissues 3 cm above the inoculation site were chopped off. All tubes were sealed with sterilized silicon caps and maintained at 25 °C with 12 h light/dark condition. The degree of pathogenicity was evaluated by measuring the length of symptomatic area (spotted blight lesions) on the sheath from the inoculation site downwards. Standard deviation and T-test were calculated on three replicates of each isolate.
3. Results
3.1. Determination of Fungal Species
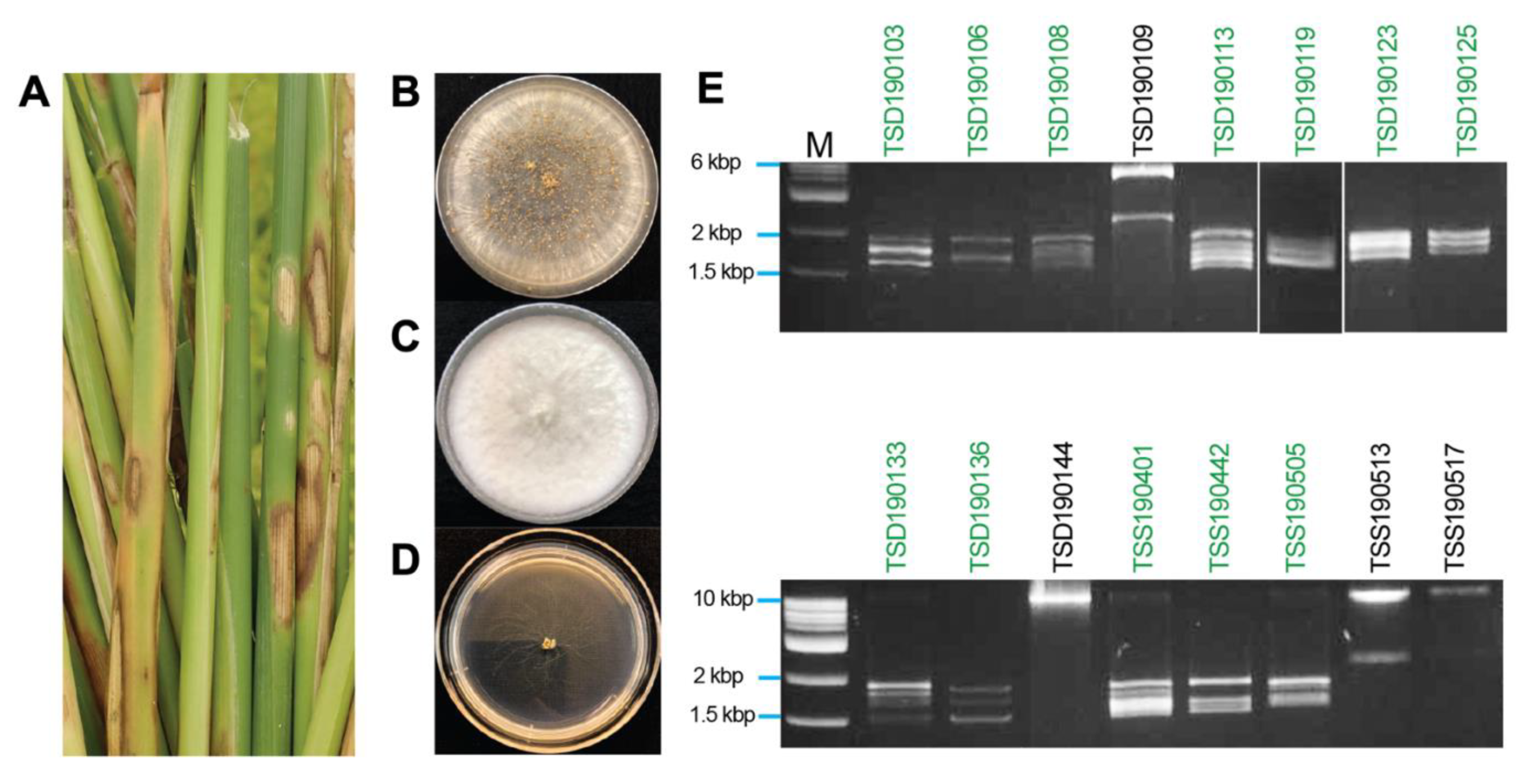
On water–agar plates, hyphal tip cultures of fungal mycelia that were originally developed from rice tissues showing blight-like symptoms (Figure 1A), exhibited Rhizoctonia-like and Bipolaris-like colony morphologies (Figure 1B,C). One of the isolates, TSD190117, showed strongly impaired growth (Figure 1D). Genomic DNA of 80 fungal isolates was extracted and used as a template for amplification of the ITS regions (540 to 849 bp) and sequencing. The BLASTn search with amplicon sequences revealed the best hit with ITS sequences of Rhizoctonia species (42 isolates), Bipolaris species (8 isolates), Fusarium species (7 isolates), Nigrospora species (6 isolates), Gaeumannomyces species (5 isolates), and others (12 isolates) (Table 1). A total of 13 out of 42 isolates related to the Rhizoctonia species showed 100% identity among each other. Detailed information about the fungal collection is summarized in Supplementary Table S2.
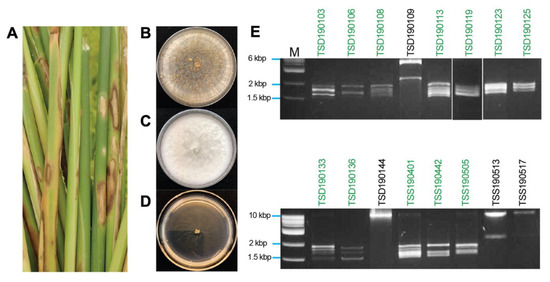
Figure 1.
Isolation of rice pathogenic fungi and mycovirus dsRNA profiles. (A) Rice sheaths with the blighted symptoms. Diseased leaf tissues were collected from paddies in Thailand and further subjected to single fungal species isolation. (B–D) Colony morphology of isolated fungi including Rhizoctonia oryzae-sativae (TSS190505 isolate) (B), Nigrospora oryzae (TSD190109 isolate) (C), and Rhizoctonia solani (TSD190117 isolate) (D). (E) Electrophoretic profile of dsRNAs purified from fungal isolates. The majority of the dsRNA elements appeared to be within the size range of approximately 1.5 to 2 kbp. Green letters above the panels indicate R. oryzae-sativae isolates, while those in black letters are others.

Table 1.
List of fungal species roughly predicted by BLASTn analysis of ITS sequences, and presence of dsRNA in each possible species.
3.2. Screening of Fungal Isolates for Viral dsRNAs
The dsRNA elements were isolated and profiled from 80 fungal isolates derived from rice sheaths showing blight-like symptoms. Of the 80 isolates, 17 isolates (21.3% of tested fungal isolates) were found to carry dsRNA elements, which was further confirmed based on their resistance to double digestion by S1 Nuclease and DNase I (Table 1 and Figure 1E). Agarose gel electrophoretic profiles showed commonly the presence of multiple dsRNA elements ranging from 1.5 to 2.0 kbp in length in all R. oryzae-sativae and R. oryzae isolates (Figure 1E). These dsRNAs could be the genomes of partitiviruses, curvulaviruses, and others based on the range of dsRNA sizes. R. solani TSD190117 with strongly impaired growth carried similar dsRNA elements (Supplementary Figure S1), but the fungal isolate was no longer viable in the successive culture. In addition, Nigrospora oryzae TSD190144 and N. sphaerica TSD190109 appeared to carry a ≥10 kbp dsRNA, and 2.5 and 6 kbp dsRNAs, respectively (Figure 1E). Likewise, R. oryzae TSS190517 and R. zeae TSS190513 harbored large dsRNA elements. Given that the dsRNA banding patterns from different R. oryzae-sativae isolates were similar to each other (Figure 1E, green letters), dsRNAs in isolates TSS190442 and TSS190505 were chosen for further molecular characterization.
3.3. Genomic Organization of Analyzed dsRNA Elements
Sanger sequencing of cDNA clones and RACE clones of dsRNAs in R. oryzae-sativae isolates TSS190442 and TSS190505 revealed the complete genomes of three partitiviruses (six dsRNA segments; see below). Following the nomenclature guidelines of ICTV, these fully-sequenced partitiviruses were named Rhizoctonia oryzae-sativae partitivirus 1 to 3 (RosPV1, 2, and 3). Note that one of the viruses in TSS190442, RosPV1, is handled as a different strain of RosPV1-RS001 [16]. Furthermore, NGS results detected three additional partitiviruses (total six dsRNA segments) infecting R. oryzae-sativae TSD190123, designated as Rhizoctonia oryzae-sativae partitivirus 4 to 6 (RosPV3, 4, and 6). The assignment of two genomic segments to one virus was based on their strictly conserved 5′-terminal end sequences (see below). The complete genome sequences of all the six viruses were submitted to GenBank/DDBJ/ENA with accession numbers: LC637850, LC637851, LC637852, LC637853, LC637854, LC637855, LC637856, LC637857, LC637858, LC637859, LC637860, and LC637861.
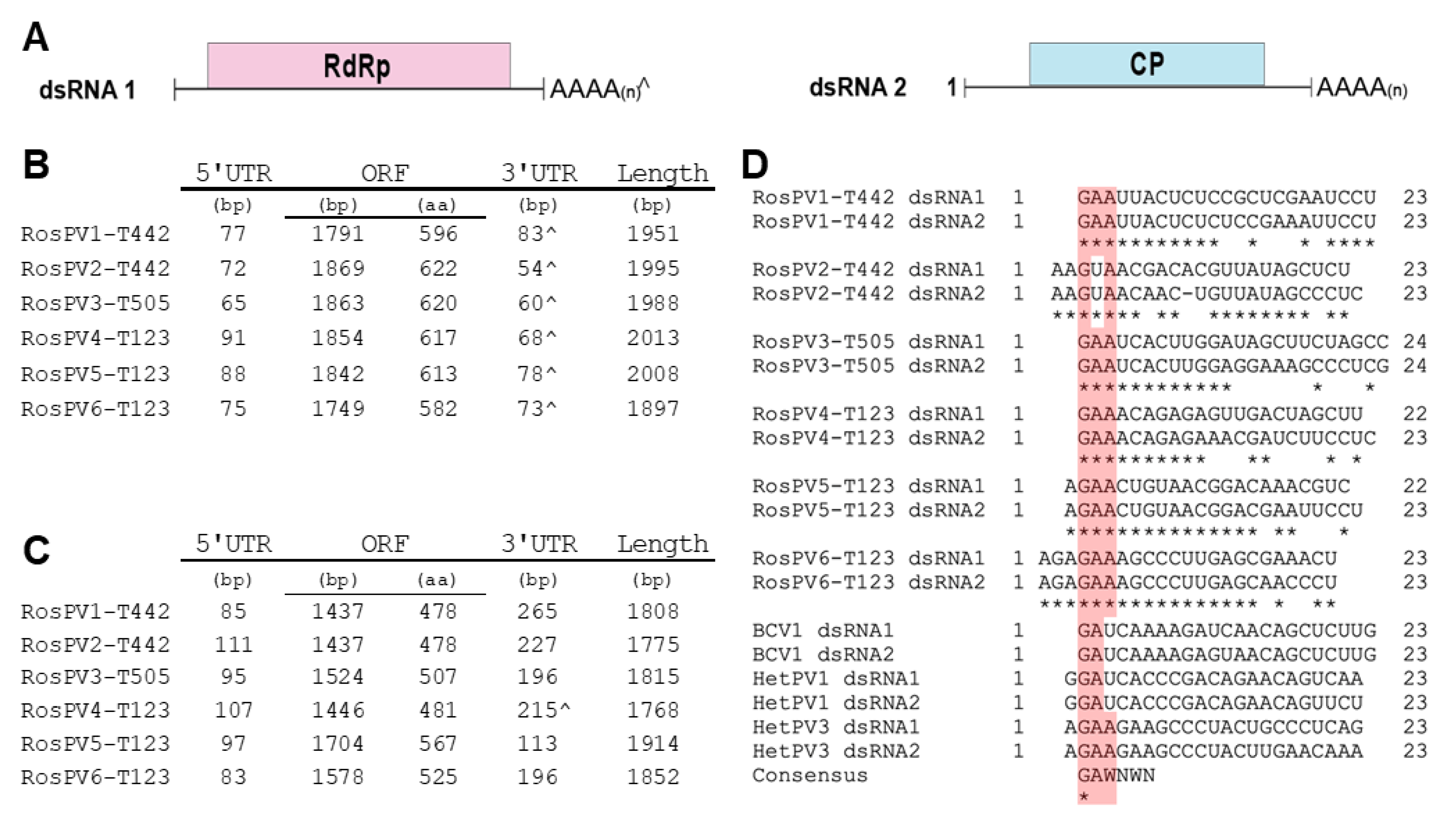
The 12 dsRNA segments were separated into two genomic groups. 1) larger dsRNAs (dsRNA1) of RosPV1 to RosPV6: these are 1951, 1995, 1988, 2004, 2008, and 1897 bp in length with GC content 43%, 44.1%, 46.2%, 47%, 43.4%, and 46.2%, respectively. Each dsRNA contains one ORF hypothetically encoding an RdRp with a predicted molecular weight of 69.5, 73.2, 72.7, 72.6, 72.5, and 68.7 kDa. 2) shorter dsRNAs (dsRNA2) of RosPV1 to RosPV6: these are 1808, 1775, 1815, 1768, 1914, and 1852 bp in length with 44.5, 48.3, 47.2, 47.6, 47.8, and 50.1% GC content, respectively. One ORF was predicted in each segment, encoding for a putative CP with an estimated molecular mass of 54.1, 53.6, 55.1, 54, 63.8, and 58.4 kDa (Figure 2A–C).
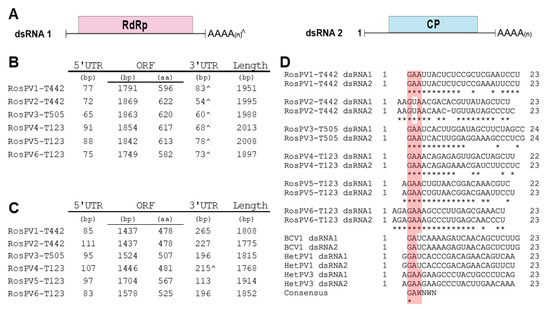
Figure 2.
Schematic representation of the genomic composition of the six partitiviruses discovered from three R. oryzae-sativae isolates. (A) Genomic organization of the two dsRNAs that constitute a single partitivirus. The red and blue rectangular boxes represent open reading frames (ORFs) in each segment encoding RdRp and CP. (B,C) The genomic composition of the dsRNA1 and dsRNA2 of each partitivirus are described in B, and C, respectively. ^ indicates interrupted poly(A) tails at 3′ UTR. (D) A portion of 5′ UTR sequences was aligned with previously-characterized alphapartitiviruses showing conserved residues at the terminal regions. The pink-highlighted box illustrates the conserved residues in the aligned sequences. Consensus was obtained from [22] to further support the conservation of the plus strand terminal regions of alphapartitiviruses.
Untranslated regions (UTRs) of these viral genome segments were generally short, as 5′-UTRs ranged from 65 to 111 bp, and those of 3′-UTRs were a little varied but their sizes ranging between 54 and 265 bp including poly(A) stretch (Figure 2C). The 5′ termini in the given dsRNA set of RdRp-coding dsRNA1 and CP-coding dsRNA2 segments were well conserved. Whereas, the 3′ terminal regions were moderately similar. Of note, five 3′-ends of CP-coding dsRNA2 segments, except for RosPV4, contained poly(A) tail, but all six of RdRp-coding segments appeared to have integrated poly(A). The numbers of A residues in these terminal sequences varied among RACE clones; thus, the final sequences presented here are based on the consensus (sequences that occurred most frequently). The 5′ termini (approximately 23–24 nt) of the 12 dsRNA segments were aligned, and the dsRNA pair, which constitutes the single viral individual, possessed identical nucleotides in the initial 7 to 17 nt (Figure 2D). In the alignment with other alphapartitiviruses, a conserved cluster at the 5′-ends (GAA) was identified (except for RosPV2, which has 5′-GUA), suggesting that these viruses may be members of the genus Alphapartitivirus [22,23] (Figure 2D, highlighted in pink).
3.4. BLAST Search with Deduced Amino Acid Sequences of Viral Proteins
BLASTp analyses based on deduced aa sequences revealed that the 12 dsRNAs were the six genomic segment sets of RosPV1 to RosPV6. The predicted aa sequences of RdRp and CP encoded by all six RosPVs had the highest hits with the following previously reported partitiviral RdRps and CPs:
RosPV1-T442, RosPV1-RS005 discovered in India (accession number MK015642 and MK015643) [16] (RdRp, E-value: 0.0; Query cover: 90%; Identity: 93.3%) (CP, E-value: 0.0; Query cover: 100%; Identity: 91.3%).
RosPV2-T442, Rhizoctonia solani partitivirus 4 (RsPV4) (accession number KX914902 and KX914903) [24] (RdRp, E-value: 0.0; Query cover: 90%; Identity: 67.3%) (CP, E-value: 1×10−110; Query cover: 88%; Identity: 42.8%).
RosPV3-T505, unreported virus sequences deposited in 2015 as Rhizoctonia fumigata partitivirus (RfPV) C314 strain (accession number KM668042 and KM668043) (RdRp, E-value: 0.0; Query cover: 91%; Identity: 99.4%) (CP, E-value: 0.0; Query cover: 100%; Identity: 94.5%).
RosPV4-T123, Rhizoctonia solani dsRNA 4 (accession number YP_009551520 and YP_009551521) (RdRp, E-value: 0.0; Query cover: 100%; Identity: 84.1%) (CP, E-value: 1×10−161; Query cover: 99%; Identity: 50.1%).
RosPV5-T123, Rhizoctonia solani partitivirus 7 (RsPV7) (QDW81313 and QDW81314) [25] (RdRp, E-value: 0.0; Query cover: 99%; Identity: 67.1%) (CP, E-value: 3×10−114; Query cover: 94%; Identity: 39.3%).
RosPV6-T123, Rhizoctonia solani partitivirus 5 (RsPV5) (AZQ25369 and AZQ25369) [26] (RdRp, E-value: 0.0; Query cover: 100%; Identity: 83.7%) (CP, E-value: 2×60−110; Query cover: 92%; Identity: 32.7%).
3.5. Phylogenetic Analysis of Partitiviruses
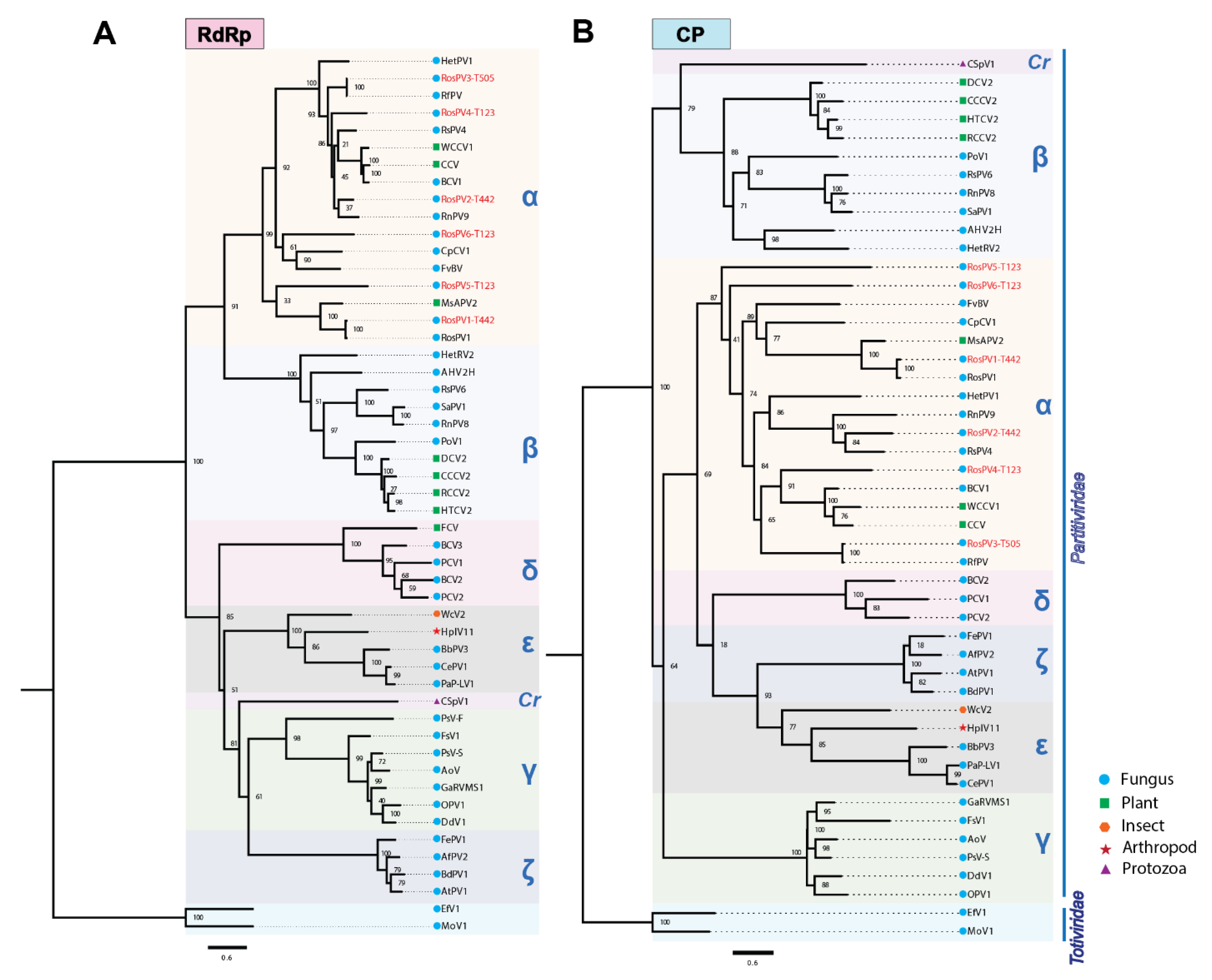
Complete aa sequences of RdRps and CPs were subjected to the construction of a multiple sequence alignment and the subsequent phylogenetic analysis (Figure 3). The six partitiviruses appeared to have a close relationship with the previously reported alphapartitiviruses (grouped in the same clade). As phylogenetic trees constructed based on the RdRp and CP aa sequences showed similar topology, these suggested that the pre-speculated combinations of RdRp/CP-coding segments as a virus genome were all reasonable (Figure 3A,B). As expected from BLAST analyses, RosPV1-T442 and RosPV3-T505 were phylogenetically very closely related to RosPV1-RS005 and RfPV, respectively. In contrast, RosPV2-T442, RosPV4-T123, RosPV5-T123, and RosPV6-T123 were substantially distant from reported partitiviruses (Figure 3A,B). Altogether, we propose that RosPV2-T442, RosPV4-T123, RosPV5-T123, and RosPV6-T123 represent novel alphapartitivirus species to be established, while RosPV1-T442 and RosPV3-T505 belong to the same species that accommodates RosPV1-RS005 and RfPV-C314, respectively.
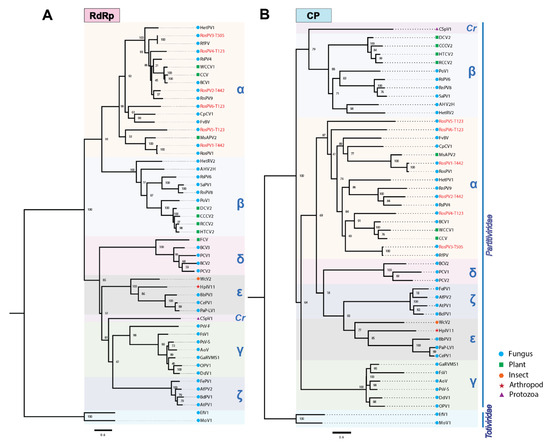
Figure 3.
Phylogenetic analyses based on the deduced aa sequences from the six partitiviruses. (A,B) Maximum-likelihood trees were constructed based on the alignment of complete aa sequences of RdRp (A) and CP (B) along with the previously reported members of the genera or proposed genera of the family Partitividae. Two members of the family Totiviridae were used as an outgroup for rooting. Tree construction parameters were chosen based on Smart Model Selection (SMS) function available in PhyML (http://www.atgc-montpellier.fr/sms/, accessed on 3 November 2021). Blanch support was obtained with the aLRT-SH-like method and indicated beside each node in percentage (1.00 = 100%). Red texts indicate partitiviruses discovered in this study. The scale bars represent genetic distance. α, Alphapartitivirus; β, Betapartitivirus; γ, Gammapartitivirus; δ, Deltapartitivirus; Cr, Cryspovirus; ε, “Epsilonpartitivirus”; and ζ, “Zetapartitivirus”. Accession number and the full name of viruses used in this phylogenetic tree constructions can be found in supplementary Table S3.
3.6. RT-PCR Based Detection of Partitiviruses from Fungal Isolates Used in This Study
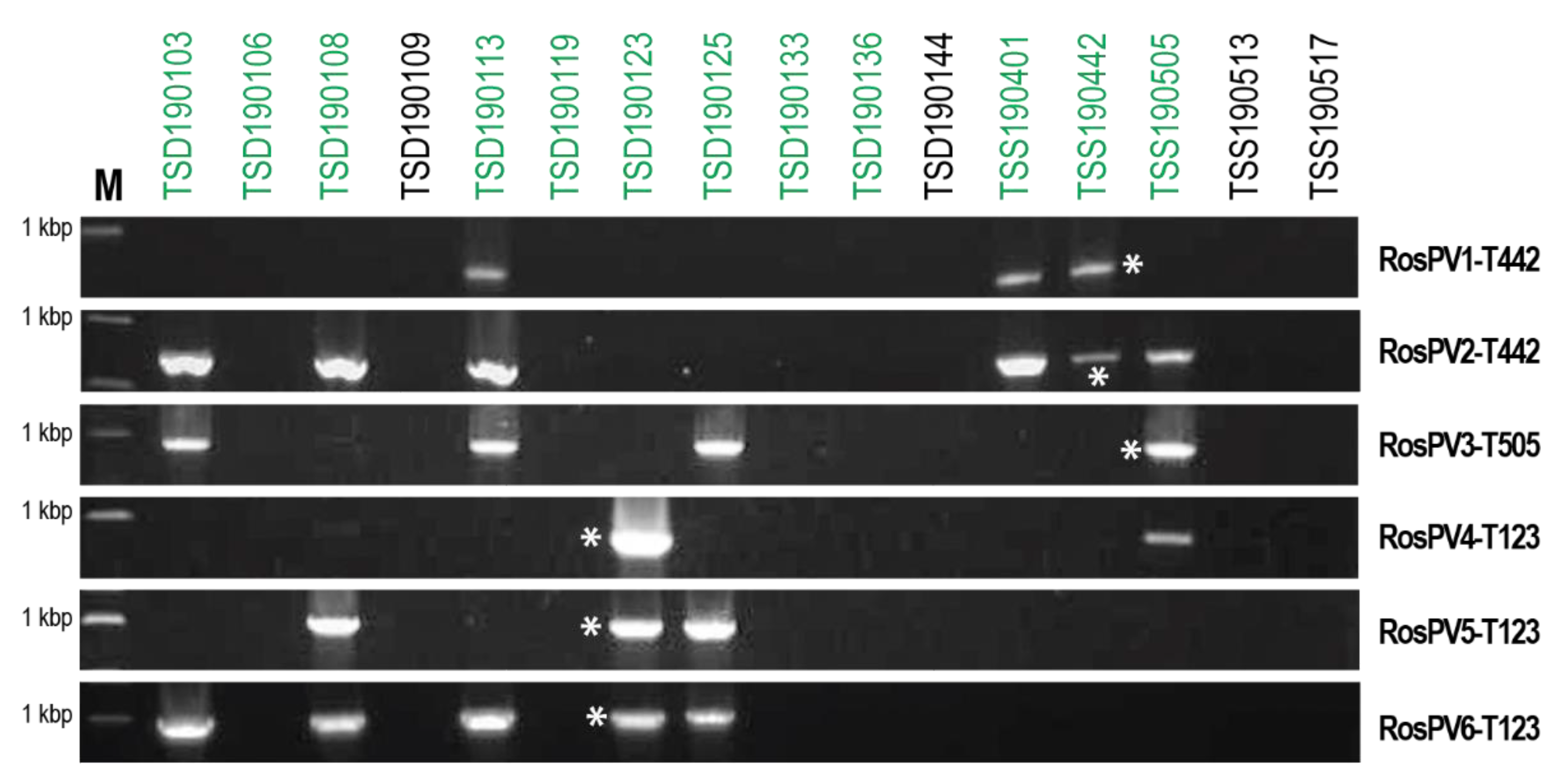
Rhizoctonia isolates collected in this study showed similar dsRNA profiles, and this made us speculate the multiple coinfections of the six characterized partitiviruses. Therefore, the detection of RosPV1 to RosPV6 in dsRNA-positive isolates was performed by RT-PCR with specific primers (Supplementary Table S1). In 16 fungal isolates tested, these partitiviruses were detected in eight isolates of R. oryzae-sativae (Figure 4). However, any of these partitiviruses were not found in N. oryzae TSD190144, N. sphaerica TSD190109, R. oryzae TSS190517, and R. zeae TSS190513 that accommodated dsRNA elements of over 3 kbp (Figure 1). The most prevalent RosPV2 was found in six R. oryzae-sativae isolates, and RosPV6 was similarly detected in five R. oryzae-sativae isolates. RosPV3 was detected from four R. oryzae-sativae isolates, while RosPV1 and RosPV5 were present in three R. oryzae-sativae isolates. RosPV4-T123 was detected only in two R. oryzae-sativae isolates (Figure 4). These results suggested that most of the six partitiviruses were widespread in R. oryzae-sativae population with the ability to coinfect a single isolate. For example, R. oryzae-sativae TSD190113 was coinfected by RosPV2-, RosPV3-, RosPV6-, and RosPV1-like viruses. Interestingly, none of the six partitiviruses were detected in the four R. oryzae-sativae isolates (TSD190106, TSD190119, TSD190136, and TSD190144) despite the fact that dsRNA profiles for these four isolates were similar to those of partitivirus-infected isolates (Figure 1 and Figure 4). It is possible that these isolates could harbor either partitiviruses unrelated to those of detected in this study or other small dsRNA or ssRNA viruses. From the different PCR band intensities (Figure 4), the accumulation levels of each mycovirus may vary among R. oryzae-sativae isolates, and/or genetic variation of partitiviruses in each viral strain might be rich, but these need further investigations to conclude.
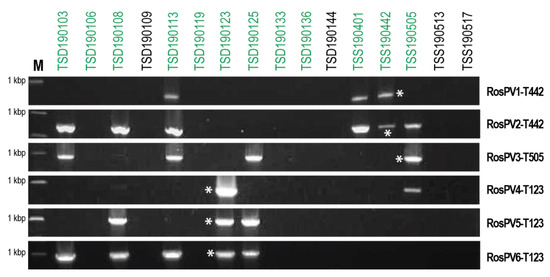
Figure 4.
Widespread infection of partitiviruses among R. oryzae-sativae isolates. cDNAs synthesized from total RNA fractions were used as templates to detect the presence of the six partitiviruses, RosPV1 to RosPV6 (indicated on the right side of panels). Coinfection of more than two partitiviruses in a single isolate was observed but no single infection. M indicates a DNA size marker in the length of 1 kbp, and green letters above the panel indicate R. oryzae-sativae isolates. Asterisks represent positive control by which each partitivirus was detected from their original host.
3.7. Other Partitiviruses Coinfecting R. oryzae and R. oryzae-sativae
During the characterization of RosPV1–3, we have also detected sequence contigs that closely related to but different from these viral sequences, indicating the presence of additional mycoviruses in R. oryzae-sativae TSS190442 and TSS190505. Briefly, the BLASTx analysis of these independent cDNA contigs suggested coinfection by distinct partitivirus(es) sharing aa sequence similarity (52–92%) to RosPV3, RsPV2, Rosellinia necatrix partitivirus 6 (RnPV6), and Brassica rapa cryptic virus 1 (BrCV1) in TSS190442; and those similar to RosPV2 (94%) and RsPV3 (15%) in TSS190505 (Table 2). In addition, partially characterized cDNA libraries from R. oryzae-sativae isolates TSD190103, TSD190108, and TSS190401 revealed sequence contigs sharing the deduced aa sequence identities 37–99% with a member of Alphapartitivirus. These results are reasonable if compared to the RT-PCR detection pattern in Figure 4, except the fact that RosPV3 in TSD190108 and TSS190401 could not be detectable by RT-PCR but the similar contigs of RosPV3 could be detected in sequencing. This could be due to mismatches between primers and targets. Taken together, this study, for the first time, revealed the omnipresence nature of diverse partitiviruses in R. oryzae and R. oryzae-sativae infecting rice in Thailand.

Table 2.
Sequence identities of cDNA contigs to newly or previously reported partitiviruses’ protein (RdRp or CP) by BLASTx.
3.8. Isogenic Virus-Cured Isolates
In general, virus-curing in basidiomycete fungi including Rhizoctonia spp. is difficult, and several attempts were reported to be unsuccessful (Table 3). However, it is reported that a hypovirus was successfully eliminated from the R. solani XN84 [27]. In this study, we tested previously reported virus-curing methods, such as heat treatment, protoplasting, and drug treatment in conjunction with hyphal tipping using the randomly selected R. oryzae-sativae TSS190442. After 5 days incubation at 37 ℃ (heat treatment), the fungal growth was severely hindered. However, its sub-cultures could reclaim a normal growth rate when incubated at room temperature. Unfortunately, no virus-free strain were obtained following this method.

Table 3.
An overview of mycovirus elimination in Rhizoctonia spp.
All the regenerated fungal colonies derived from TSS190442 protoplasts were found to be positive for the presence of RosPV1 and RosPV2 in an RT-PCR-based detection. Hyphal tipping in conjunction with the treatment of anti-viral drug ribavirin is often useful in virus-curing. However, this method also failed to eliminate viruses from TSS190442 in this study (Table 3).
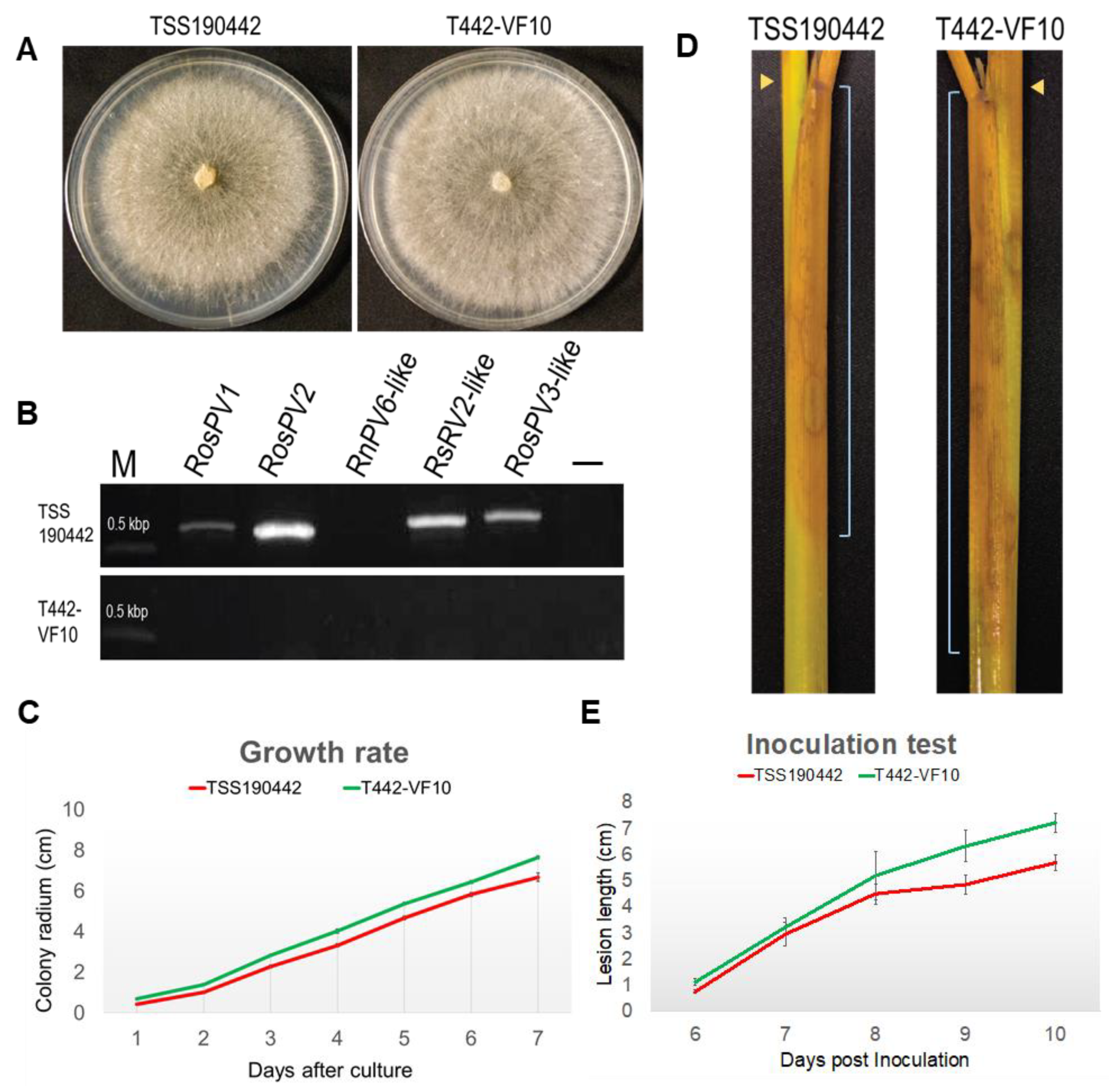
Finally, isogenic virus-free R. oryzae-sativae isolates were successfully obtained from TSS190442 through repetitive hyphal tipping from a culture growing on PDA supplemented with ribavirin (200 μm/L) combined with cycloheximide (5 mg/L) (Table 3). It is noteworthy that the use of cycloheximide coupled with ribavirin was essential to eliminate partitiviruses from R. oryzae-sativae TSS190442, especially when hyphal tipping from regenerated protoplasts and heat-treatment completely failed viral elimination. To examine the effect of partitivirus infection on the morphology and phenotype of R. oryzae-sativae, a representative virus-free sub-isolate T442-VF10 was selected for growth rate and colony morphology assays along with the virus-infected isolate (Figure 5A). The elimination of RosPV1 and RosPV2 in T442-VF10 was confirmed several times by one-step colony RT-PCR, conventional RT-PCR (using purified ssRNA fractions as template), and dsRNA extraction (Figure 5B; Table S4). In addition, no presence of partially characterized partitiviruses (RosPV3-like, RsPV2-like, and RnPV6-like viruses) in T442-VF10 was confirmed as well. With the absence of RosPV1, RosPV2, and other viruses, the growth rate of T442-VF10 on PDA was slightly improved, and it appeared to have developed denser mycelia and more aerial hyphae compared to its virus-infected counterpart (TSS190442) (Figure 5A,C). The pathogenicity of the two variants was tested on detached rice sheaths. The result showed that the virus-free isolate induced larger lesions (Figure 5D, see the lesion scale bars), albeit with no statistical significance (Figure 5E).
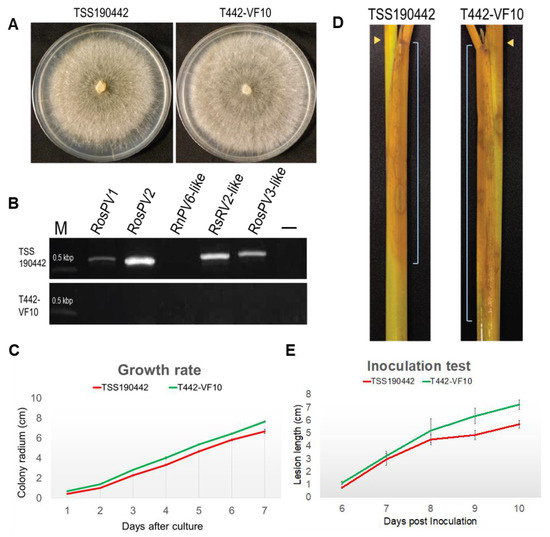
Figure 5.
Biological impact of partitivirus infections in R. oryzae-sativae TSS190442. Isogenic virus-free R. oryzae-sativae isolate T442-VF10 was successfully obtained from RosPV1- and RosPV2-infected TSS190442 by the successive treatments of ribavirin and cycloheximide. (A) Colony morphological comparison between virus-infected and virus-free isolates. (B) RT-PCR detection of two fully and three partially characterized partitiviruses in TSS190442 and T442-VF10. (C) Growth of fungi on the synthetic media. Mycelial plugs were inoculated at the edge of PDA plates and the radius of the colony were measured. (D,E) Symptom development on rice tissues. Mycelial plugs were inoculated at the node of rice sheath (D, yellow arrowheads) and lesions were observed daily-basis. The symptom appeared downward. The distance from the inoculation point to the edge of the symptom (scale bars in D) was measured (E). The virus-cured isolate T442-VF10 showed a slightly faster growth rate on both the plate and plant; however, this significance was not statistically supported (C,E).
4. Discussion
4.1. Multiple Infections of Partitiviruses in Rhizoctonia spp.
A total of 21% of all tested fungal isolates carry multiple dsRNA elements with a potential of viral origin (Figure 1, Table 2 and Table 3), although dsRNA-negative isolates might also carry mycoviruses that were below the detection limit. Six dsRNA sets among all were characterized, RosPV1- and RosPV2-T442 infecting R. oryzae-sativae TSS190442, RosPV3-T505 infecting TSS190505, and RosPV4-, RosPV5-, and RosPV6-T123 infecting TSD190123. The six mycoviruses were identified as alphapartitiviruses in the family Partitiviridae based on BLASTp search results and phylogenetic study (Figure 3). Although the ITS sequence alone is not sufficient to accurately confirm the fungal taxonomic placement, it showed that R. oryzae-sativae TSD190106, TSD190113, TSD190119, and TSD190133 were considered genetically close (100% identical ITS sequences) despite harboring marginally different dsRNA profiles (Figure 1E, Table S2). Likewise, R. oryzae-sativae TSD190103 and TSD190108 were indistinguishable based on their ITS sequences. We assumed that dsRNA elements detected in these fungi include partitiviruses since their dsRNA pattern is somewhat similar to partitiviruses identified in this study. In addition, these R. oryzae-sativae isolates are potentially infected by non-partitiviruses but similar-sized RNA viruses; for instance, the isolate TSD190123 subjected to RNA-seq was found to be infected at least eight RNA mycoviruses, excluding RosPV4, 5, and 6 (data not shown). Further, this study revealed that RosPV1-T442, RosPV2-T442, RosPV3-T505, and RosPV6-T123 together coinfect R. oryzae-sativae TSD190113 (Figure 4). The coinfection of a single fungal strain by multiple partitiviruses is interesting but not unprecedented as coinfection by multiple partitiviruses of the same host fungus has been reported in Rhizoctonia spp. For instance, two alphapartitiviruses, RsPV3 and RsPV4, coinfected R. solani strain HG81 isolated in Hubei province, China [24], three betapartitiviruses, RsPV6–8, were discovered from R. solani isolate YNBB-111 [31], and a Brazilian isolate of R. solani (strain IBRS23) was also reported to harbor one betapartitivirus and two alphapartitiviruses, RsPV6–8 [25]. It is worth noting that RsPV6 characterized in China is 100% identical to RsPV6 from Brazil, while the RsPV7 and eight discovered from both regions were distinct partitiviruses. This phenomenon is more common than it was previously thought, and found to occur not only in Rhizoctonia spp., but also in Rosellinia necatrix [23]. The possibility of interactions between the coinfecting partitiviruses even from different genera (Alphapartitivirus and Betapartitivirus) is being explored.
4.2. Widespread Nature of the Partitiviruses Found in This Study
Electrophoretic dsRNA profiles and sequencing analysis indicated the widespread nature of partitivirus infection. Out of 17 dsRNA-positive R. oryzae-sativae isolates, 7 were infected by multiple partitiviruses (Figure 1E and Figure 5). According to previous studies, viruses in the family Partitiviridae can be efficiently transmitted by horizontal (via hyphal anastomosis) and vertical transmission (via conidiospore or basidiospore) [10,22,32,33]. Indeed, Rhizoctonia spp. is widespread globally by the relocation of sclerotia and fragments of mycelium through infected plant samples, rarely by dispersion of basidiospore, and by a natural force such as wind and rain [34,35]. This nature of Rhizoctonia spp. and the high transmission rate of partitivirus may contribute to the prevalence of partitiviruses as reported in this study. For instance, RosPV3-T505 infecting R. oryzae-sativae TSD190505 isolated from a paddy field in Thailand was almost identical in genomic sequence to RfPV detected in China; similarly, RosPV1-T442 discovered from R. oryzae-sativae TSD190442 showed >90% sequence identity to RosPV1-RS005 discovered in India (Figure 3) [16]. These partitiviruses appeared to be nearly identical to one another despite the vast geographical distance. It is possibly due to the migration of host fungus via international translocation of infected plant materials vice versa, therefore, suggesting their same origin.
4.3. Possible Viral Transmission through Hyphal Contact
RosPV2-T442 and RosPV3-T505 from R. oryzae-sativae were nearly identical to the partitiviruses of R. solani AG-1 IA and R. fumigata. This finding leads to the speculation that RosPV2-T442 and RosPV3-T505 could have originated from other basidiomycetous or Rhizoctonia host species. Though it has already been proven that R. solani can undergo hyphal anastomosis strictly in an intraspecies manner, the same is true for R. oryzae-sativae [3,35]. Yet, hyphal contact and cytoplasmic exchange between the two species could have possibly taken place under the field conditions. It is noteworthy that sympatric R. solani and R. oryzae-sativae were isolated from rice tissues with blighted symptoms in India [36] as well as in this study. Transmission of partitiviruses from donors to taxonomically different recipients is definitely feasible via fungal protoplast transfection with purified virions, which was thoroughly elucidated via in vitro [10,33,37,38,39]. However, there is no evidence that virion introduction could occur under natural environmental conditions. Thus, partitiviruses were presumably migrated across taxonomically incompatible hosts via hyphal fusion. As demonstrated in a laboratory setting, Heterobasidion abietinum and H. parviporum (of H. annosum species complex), which harbored Heterobasidion partitivirus 1 (HQ541323 and HQ541324) and an unassigned partitivirus, Heterobasidion RNA virus 5 strain pa1 (HQ541326), were able to pass on the partitiviruses to H. ecrustosum of H. insulare species complex via hyphal anastomosis [40]. In the same study, another unassigned partitivirus, Heterobasidion RNA virus 1 strain au1 (HQ541328), infecting H. austral of H. insulare species complex was also able to infect four different H. annosum species. R. solani, R. oryzae-sativae, and R. fumigata belong to the same R. solani species complex [41]. RfPV (detected in R. fumigata) and RosPV3-T505 (detected in R. oryzae-sativae) shared extremely high aa sequence identities (RdRp: 99.4%, CP: 94.5%) despite lingering inside different hosts (Figure 3). Therefore, it is interesting to speculate the possible horizontal transmission of partitiviruses through hyphal contact among these fungi.
4.4. Mycovirus-Curing in Rhizoctonia spp.
Many isogenic virus-free variants were obtained from their originally virus-infected hosts by simple techniques such as single spore isolation, hyphal tipping, and protoplasting [42,43]. Obtaining virus-cured stain is a crucial step toward the etiological study of the mycoviral impact on fungal host’s transcriptome, small RNAome, proteome, metabolome, lipidome, and epigenome [44]. However, this is often not the case for basidiomycetous fungi, especially in Rhizoctonia spp. Many attempts to eliminate mycoviruses from Rhizoctonia spp. have been made in the past, but no isogenic sub-isolate could be obtained excepting one case of RsHV2 [27]. Due to such difficulty, possible effects of the virus have been unexplored in many viruses/Rhizoctonia spp. combinations (Table 3) [14,15,16,20,26,27,28,29,30,31]. These species do not produce spores, thus limiting the options only to hyphal tipping and protoplasting [20]. Hyphal tips retrieved from the edge of the actively growing mycelia of R. oryzae-sativae TSS190442 cultured on PDA with/without ribavirin retained RosPV1 and RosPV2. Likewise, all regenerated protoplasts retained both the partitiviruses, which was confirmed by one-step colony RT-PCR (data not shown). When cycloheximide was applied along with ribavirin, we could eliminate the two partitiviruses. Cycloheximide is a known growth inhibitor that interrupts protein elongation by the ribosomes in the eukaryotic cells, and ribavirin is a nucleoside analog that is integrated into the viral RNA genome and affects the replication of the viral genome [45,46,47]. It has been reported that partitivirus particles contain one or two copies of RdRp molecule to synthesize plus-strand RNA (mRNA) for further translation in the cytoplasm, but its proliferation mainly depends upon the translation by the fungal host ribosome [22]. Thus, it is very tempting to speculate that cycloheximide may have synergistically hampered the process of partitivirus translation and indirectly affected replication. When coupled with the anti-viral drug ribavirin, the transmission of partitiviruses to the newly developed fungal cells might occur inefficiently, producing some fungal cells without the virus. Nonetheless, further study is needed to confirm this hypothesis. The degree of mycovirus adaptation to its host fungus may influence the rate of mycovirus elimination, but this study has proven that hyphal tipping in conjunction with cycloheximide and ribavirin treatment is effective when curing partitiviruses of R. oryzae-sativae. This method may open up more opportunities to investigate fungus–mycovirus interaction in other pathosystems as well.
When viruses were eliminated, the virus-free R. oryzae-sativae isolate, T442-VF10, showed a slightly improved growth rate and formed a more severe symptom on the rice sheath. This implies that the coinfecting partitiviruses, to some extent, may influence the pathogenicity of their host (Figure 5). The partitivirus infections, in most cases, do not alter the phenotypes (growth, virulence, sporulation, and pigmentation) of their hosts. For example, phenotype and colony morphology of Trichoderma harzianum strain NCFC319 remained unaltered when Trichoderma harzianum partitivirus 1 was eliminated from this strain by hyphal tipping [48]. However, instances wherein partitivirus conferred hypervirulent traits or helped to induce hypovirulence in host fungi were also reported [19,33,49]. Nonetheless, further studies are needed to investigate the complex interaction between the two partitiviruses, RosPV1 and RosPV2, and their R. oryzae-sativae host.
In addition, R. solani TSD190117, possessing dsRNA elements that are similar in size with partitiviruses, exhibited extremely impaired growth on synthetic media (Supplementary Figure S1). However, it is unknown whether the phenotype is associated with mycoviral infection. Nonetheless, it would be of great interest to apply the developed virus-curing protocol for understanding the phenomenon.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13112269/s1, Figure S1: dsRNA profile and colony morphology of Rhizoctonia solani TSD190117, Table S1: List of primers used in this study, Table S2: Blastn analysis of the fungal ITS fragments and the presence of dsRNAs, Table S3: Full name and acronym of viruses used in phylogenetic analysis, Table S4: dsRNA detection during the partitivirus-curing experiment.
Author Contributions
Conceptualization, S.C., C.R. and R.P.; methodology, L.S., I.S., D.T., M.A. and S.C.; software, S.N. and H.K.; validation, M.A. and S.C.; formal analysis, N.S. and S.D.; investigation, S.N., S.B., S.R. (Sansern Rangsuwan), P.K. and S.R. (Soriya Rin); resources, L.S., C.R. and R.P.; data curation, S.N. and S.C.; writing—original draft preparation, S.N.; writing—review and editing, N.S., S.D. and S.C.; visualization, S.N. and S.C.; supervision, I.S., D.T., H.K., S.C. and N.S.; project administration, R.P. and S.C.; funding acquisition, R.P., N.S. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) as part of the Joint Research Program implemented at the Institute of Plant Science and Resources, Okayama University in Japan (to N.S. and S.C.); Japan Society for the Promotion of Science (JSPS) and National Research Council of Thailand (NRCT) under the NRCT-JSPS joint research program (to S.C. and R.P.); JSPS Grants-In-Aid for Scientific Research (B) (KAKENHI 17H03950 to S.C.).
Data Availability Statement
The complete viral cDNA sequences were deposited under the GenBank accession number LC637850–LC637861. Since the RNA-seq data (DRA) includes unpublished items, it will be publicly opened once the remaining data being reported elsewhere.
Acknowledgments
We thank Chiaki Narukawa, Rika Miura, Ayane Ota, Hironori Kubo, Kanoko Murata, Hiroyuki Morimura, Yukiyoshi Mizutani, and Yuri Mizuno for their technical assistance. S.N. is a scholar of the MEXT scholarship who was recommended through the Japanese embassy in the kingdom of Cambodia.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Savary, S.; Willocquet, L.; Elazegui, F.A.; Castilla, N.P.; Teng, P.S. Rice pest constraints in tropical Asia: Quantification of yield losses due to rice pests in a range of production situations. Plant Dis. 2000, 84, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Chaijuckam, P.; Davis, R.M. Efficacy of natural plant products on the control of aggregate sheath spot of rice. Plant Dis. 2010, 94, 986–992. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunnell, P.S.; Webster, R.K. Ceratobasidium oryzae-sativae sp. nov., the teleomorph of rhizoctonia oryzae-sativae and Ceratobasidium setariae comb. nov., the probable teleomorph of Rhizoctonia fumigata comb. nov. Mycologia 1987, 79, 731. [Google Scholar] [CrossRef]
- Lanoiselet, V.L.; Cother, E.J.; Ash, G.J.; Harper, J.D.I. Yield loss in rice caused by Rhizoctonia oryzae and R. oryzae-sativae in Australia. Australas. Plant Pathol. 2005, 34, 175–179. [Google Scholar] [CrossRef]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Chen, B.; Nuss, D.L. Infectious cDNA clone of hypovirus CHV1-Euro7: A comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J. Virol. 1999, 73, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Nuss, D.L. Biological control of chestnut blight: An example of virus-mediated attenuation of fungal pathogenesis. Microbiol. Rev. 1992, 56, 561–576. [Google Scholar] [CrossRef]
- Chiba, S.; Salaipeth, L.; Lin, Y.-H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: Molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, J.; Tang, J.; Fu, Y.; Jiang, D.; Baker, T.S.; Ghabrial, S.A.; Xie, J. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J. Virol. 2014, 88, 10120–10133. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Urayama, S.; Le, M.T.; Katoh, Y.; Higashiura, T.; Fukuhara, T.; Arie, T.; Teraoka, T.; Komatsu, K.; Moriyama, H. Infection by Magnaporthe oryzae chrysovirus 1 strain A triggers reduced virulence and pathogenic race conversion of its host fungus, Magnaporthe oryzae. J. Gen. Plant Pathol. 2018, 84, 92–103. [Google Scholar] [CrossRef]
- Urayama, S.; Sakoda, H.; Takai, R.; Katoh, Y.; Minh Le, T.; Fukuhara, T.; Arie, T.; Teraoka, T.; Moriyama, H. A dsRNA mycovirus, Magnaporthe oryzae chrysovirus 1-B, suppresses vegetative growth and development of the rice blast fungus. Virology 2014, 448, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular characterization of a novel endornavirus conferring hypovirulence in rice sheath blight fungus Rhizoctonia solani AG-1 IA strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Abdoulaye, A.H.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. Complete genome sequence of a novel mitovirus from the phytopathogenic fungus Rhizoctonia oryzae-sativae. Arch. Virol. 2017, 162, 1409–1412. [Google Scholar] [CrossRef]
- Das, S.; Das, S. First report of a novel alphapartitivirus in the basidiomycete Rhizoctonia oryzae-sativae. Arch. Virol. 2019, 164, 889–892. [Google Scholar] [CrossRef]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M. ICTV virus taxonomy profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef]
- Arjona-López, J.M.; Telengech, P.; Suzuki, N.; López-Herrera, C.J. Coinfection of Rosellinia necatrix by a partitivirus and a virga-like virus is associated with hypovirulence. Eur. J. Plant Pathol. 2020, 158, 111–119. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; He, G.; Wu, M.; Zhang, J.; Yang, L.; Chen, W.; Li, G. A novel partitivirus in the hypovirulent isolate QT5-19 of the plant pathogenic fungus Botrytis cinerea. Viruses 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, M.; Chen, Q.; Zhu, M.; Zhou, E. A novel mycovirus closely related to viruses in the genus Alphapartitivirus confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology 2014, 456–457, 220–226. [Google Scholar] [CrossRef]
- Eusebio-Cope, A.; Suzuki, N. Mycoreovirus genome rearrangements associated with RNA silencing deficiency. Nucleic Acids Res. 2015, 43, 3802–3813. [Google Scholar] [CrossRef]
- Nibert, M.L.; Ghabrial, S.A.; Maiss, E.; Lesker, T.; Vainio, E.J.; Jiang, D.; Suzuki, N. Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research. Virus Res. 2014, 188, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Telengech, P.; Hisano, S.; Mugambi, C.; Hyodo, K.; Arjona-López, J.M.; López-Herrera, C.J.; Kanematsu, S.; Kondo, H.; Suzuki, N. Diverse partitiviruses from the phytopathogenic fungus, Rosellinia necatrix. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Lyu, R.; Zhang, Y.; Tang, Q.; Li, Y.; Cheng, J.; Fu, Y.; Chen, T.; Jiang, D.; Xie, J. Two alphapartitiviruses co-infecting a single isolate of the plant pathogenic fungus Rhizoctonia solani. Arch. Virol. 2018, 163, 515–520. [Google Scholar] [CrossRef]
- Picarelli, M.A.S.C.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from brazil. Front. Cell. Infect. Microbiol. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, M.; Zhang, M.; Shu, C.; Zhou, E. Complete nucleotide sequence of a partitivirus from Rhizoctonia solani AG-1 IA strain C24. Viruses 2018, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Abdoulaye, A.H.; Hai, D.; Tang, Q.; Jiang, D.; Fu, Y.; Cheng, J.; Lin, Y.; Li, B.; Kotta-Loizou, I.; Xie, J. Two distant helicases in one mycovirus: Evidence of horizontal gene transfer between mycoviruses, coronaviruses and other nidoviruses. Virus Evol. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, L.; Liu, C.; Shu, C.; Zhou, E. Characterization of a novel dsRNA mycovirus isolated from strain A105 of Rhizoctonia solani AG-1 IA. Arch. Virol. 2018, 163, 427–430. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, H.; Zhang, M.; Cao, X.; Zhou, E. The complete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 IA strain B275. Arch. Virol. 2013, 158, 1609–1612. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, C.-Y.; Gao, B.-D. Genome sequence of a novel mycovirus of Rhizoctonia solani, a plant pathogenic fungus. Virus Genes 2015, 51, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong Gai, X.; Xing Chen, R.; Li, C.X.; Zhao, G.K.; Yuan Xia, Z.; Zou, C.M.; Zhong, J. Characterization of three novel betapartitiviruses co-infecting the phytopathogenic fungus Rhizoctonia solani. Virus Res. 2019, 270, 197649. [Google Scholar] [CrossRef]
- Ihrmark, K.; Stenström, E.; Stenlid, J. Double-stranded RNA transmission through basidiospores of Heterobasidion annosum. Mycol. Res. 2004, 108, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, S.; Sasaki, A.; Onoue, M.; Oikawa, Y.; Ito, T. Extending the fungal host range of a partitivirus and a mycoreovirus from Rosellinia necatrix by inoculation of protoplasts with virus particles. Phytopathology 2010, 100, 922–930. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Vilgalys, R. Population biology of the Rhizoctonia solani complex. Phytopathology 1997, 87, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, A. Ecology and pathogenicity of anastomosis and interspecific groups of Rhizoctonia solani Kühn. Annu. Rev. Phytopathol. 1987, 25, 124–143. [Google Scholar] [CrossRef]
- Taheri, P.; Gnanamanickam, S.; Höfte, M. Characterization, genetic structure, and pathogenicity of Rhizoctonia spp. associated with rice sheath diseases in India. Phytopathology 2007, 97, 373–383. [Google Scholar] [CrossRef]
- Chiba, S.; Lin, Y.-H.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel victorivirus from a phytopathogenic fungus, Rosellinia necatrix, is infectious as particles and targeted by RNA silencing. J. Virol. 2013, 87, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Lin, Y.-H.; Kondo, H.; Kanematsu, S.; Suzuki, N. Effects of defective interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus, Rosellinia necatrix. J. Virol. 2013, 87, 2330–2341. [Google Scholar] [CrossRef]
- Chiba, S.; Lin, Y.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel betapartitivirus RnPV6 from Rosellinia necatrix tolerates host RNA silencing but is interfered by its defective RNAs. Virus Res. 2016, 219, 62–72. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hakanpää, J.; Dai, Y.-C.; Hansen, E.; Korhonen, K.; Hantula, J. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biol. 2011, 115, 1234–1243. [Google Scholar] [CrossRef]
- Cubeta, M.A. Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 1991, 81, 1395. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Zhu, H.J.; Gao, B.D.; Zhou, Q. Hypovirulence of Sclerotium rolfsii caused by associated RNA mycovirus. Front. Microbiol. 2016, 7, 1–18. [Google Scholar] [CrossRef]
- Kondo, H.; Kanematsu, S.; Suzuki, N. Viruses of the White Root Rot Fungus, Rosellinia necatrix. Adv. Virus Res. 2013, 86, 177–214. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.I.; Annisa, A.; Suzuki, N. Viruses of Plant-Interacting Fungi. Adv. Virus Res. 2018, 100, 99–116. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Luo, C.; Jiang, D.; Li, G.; Li, Q.; Hsiang, T.; Huang, J. Prevalence and diversity of mycoviruses infecting the plant pathogen Ustilaginoidea virens. Virus Res. 2015, 195, 47–56. [Google Scholar] [CrossRef]
- Parker, W.B. Metabolism and antiviral activity of ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef]
- Schneider-Poetsch, T.; Ju, J.; Eyler, D.E.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Yang, H.-E.; Kim, D.-H. Identification of a novel partitivirus of Trichoderma harzianum NFCF319 and evidence for the related antifungal activity. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Sasaki, A.; Nakamura, H.; Suzuki, N.; Kanematsu, S. Characterization of a new megabirnavirus that confers hypovirulence with the aid of a co-infecting partitivirus to the host fungus, Rosellinia necatrix. Virus Res. 2016, 219, 73–82. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).