The Use of Electronic Medical Records-Based Big-Data Informatics to Describe ALT Elevations Higher than 1000 IU/L in Patients with or without Hepatitis B Virus Infection
Abstract
1. Introduction
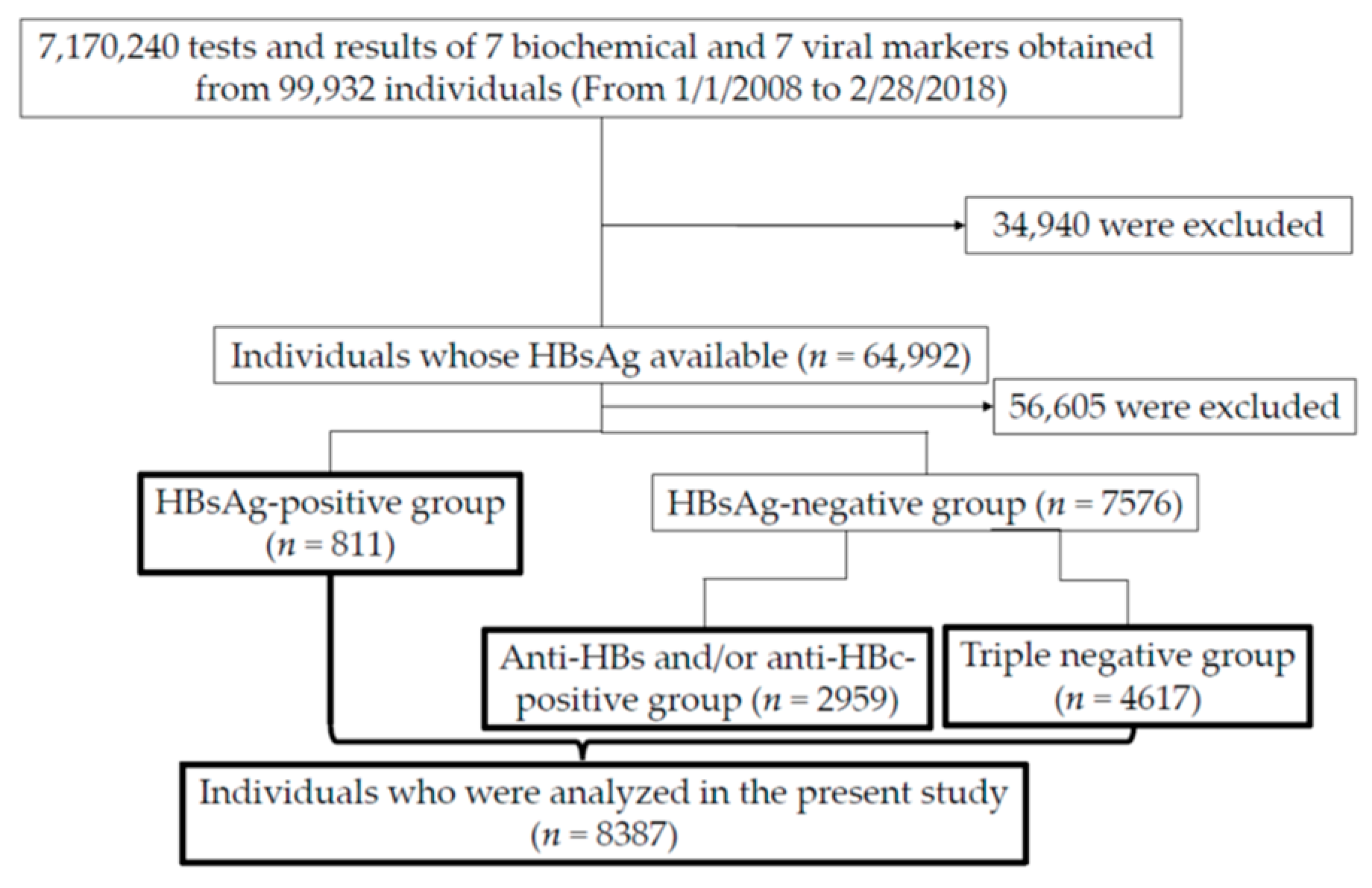
2. Patients and Methods
2.1. Patients and Data Collection
2.2. Clinical and Laboratory Assessments
2.3. Measurement of HBV DNA Levels
2.4. Definition of Highly Suspected HBV Individuals
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics of Three Groups (HBsAg-Positive, HBsAg-Negative but Positive for Anti-HBs/Anti-HBc, and Triple-Negative Groups) Classified by Three Principal HBV Markers
3.2. Highly Suspected HBV-Causing ALT Elevations Higher than 1000 IU/L among HBsAg-Positive Patients
3.3. Highly Suspected HBV-Causing ALT Elevations Higher than 1000 IU/L among HBsAg-Negative, but Positive for Anti-HBs/Anti-HBc Patients
3.4. Highly Suspected Non-HBV-Causing ALT Elevations Higher than 1000 IU/L among Triple-Negative Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Bisceglie, A.M. Screening for hepatocellular carcinoma: Being old is not all bad. Am. J. Gastroenterol. 2004, 99, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Ehata, T.; Yokosuka, O.; Hosoda, K.; Ohto, M. Mutations in the precore region of hepatitis B virus DNA in patients with fulminant and severe hepatitis. N. Engl. J. Med. 1991, 324, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Afroudakis, A.; Liew, C.T.; Ashcavai, M.; Peters, R.L. Comparison of serum hepatitis B surface antigen (HBsAg) and serum anticore with tissue HBsAg and hepatitis B core antigen (HBcAg). Gastroenterology 1978, 75, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.W.; Chen, Y.P.; Huang, X.D.; Zhou, G.Q.; Chen, L.; Li, W.F.; Tang, L.L.; Mao, Y.P.; Guo, Y.; Xu, R.H.; et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer 2017, 123, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Sugiyama, M.; Sato, Y.; Nagashima, K.; Takahashi, S.; Mizokami, M.; Hata, A. Hepatitis B virus reactivation in patients with rheumatoid arthritis: Analysis of the National Database of Japan. J. Viral Hepat. 2018, 25, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Downs, L.O.; Smith, D.A.; Lumley, S.F.; Patel, M.; McNaughton, A.L.; Mokaya, J.; Ansari, M.A.; Salih, H.; Várnai, K.A.; Freeman, O.; et al. Electronic Health Informatics Data to Describe Clearance Dynamics of Hepatitis B Surface Antigen (HBsAg) and e Antigen (HBeAg) in Chronic Hepatitis B Virus Infection. mBio 2019, 10, e00699-19. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, K.; Ho, T.B.; Yokoi, H.; Nguyen, T.D.; Kawasaki, S.; Le, S.Q.; Suzuki, T.; Yokosuka, O. Temporal abstraction and data mining with visualization of laboratory data. Stud. Health Technol. Inform. 2007, 129 Pt 2, 1304–1308. [Google Scholar] [PubMed]
- Thang, B.N.; Ho, T.B.; Kanda, T. A semi-supervised tensor regression model for siRNA efficacy prediction. BMC Bioinform. 2015, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, X.; Zhou, H.; Gao, Y.; Zheng, J.; Tong, M.; Wu, C.; Liu, C.; Huang, L.; Jiang, T.; et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: A prospective multicentre study. Gut 2019, 68, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Mastrodomenico, M.; Muselli, M.; Provvidenti, L.; Scatigna, M.; Bianchi, S.; Fabiani, L. Long-term immune protection against HBV: Associated factors and determinants. Hum. Vaccin. Immunother. 2021, 17, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Sasaki, K.; Yoshikawa, N.; Enami, T.; Shobayashi, T. Introduction of a hepatitis B vaccine into the national routine immunisation programme of Japan. Lancet Infect. Dis. 2016, 16, 1325. [Google Scholar] [CrossRef] [PubMed]
- Free Software Foundation. Bash (3.2.48). Available online: http://ftp.gnu.org/gnu/bash/bash-3.2.48.tar.gz (accessed on 7 April 2020).
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comp. Graph. Stat. 1996, 5, 299–314. Available online: https://www.R-project.org/ (accessed on 26 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 26 April 2020).
- Miyauchi, T.; Kanda, T.; Shinozaki, M.; Kamezaki, H.; Wu, S.; Nakamoto, S.; Kato, K.; Arai, M.; Mikami, S.; Sugiura, N.; et al. Efficacy of lamivudine or entecavir against virological rebound after achieving HBV DNA negativity in chronic hepatitis B patients. Int. J. Med. Sci. 2013, 10, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Udo, K.; Takahashi, Y.; Yoshizawa, H.; Tsuda, F.; Itoh, Y.; Miyakawa, Y.; Mayumi, M. Correlation between titer of antibody to hepatitis B core antigen and presence of viral antigens in the liver. Gastroenterology 1977, 73 Pt 1, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Shimojima, S.; Gotoh, K.; Shikata, T.; Mima, S. Pathology of livers infected with “silent” hepatitis B virus mutant. Liver 1994, 14, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Fukai, K.; Yokosuka, O.; Fujiwara, K.; Tagawa, M.; Imazeki, F.; Saisho, H.; Omata, M. Etiologic considerations of fulminant non-A, non-B viral hepatitis in Japan: Analyses by nucleic acid amplification method. J. Infect. Dis. 1998, 178, 325–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, M.; Kanda, T.; Nakamoto, S.; Haga, Y.; Sasaki, R.; Jiang, X.; Yasui, S.; Arai, M.; Yokosuka, O. Reappearance of serum hepatitis B viral DNA in patients with hepatitis B surface antigen seroclearance. Hepatology 2015, 62, 1329. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Hsu, Y.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. Effect of Nucleos(t)ide Analogue Therapy on Risk of Intrahepatic Cholangiocarcinoma in Patients with Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2018, 16, 947–954.e4. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Hsu, Y.C.; Tseng, H.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. Association of Daily Aspirin Therapy with Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. JAMA Intern. Med. 2019, 179, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Z.H.; Lockart, I.; Abdel Shaheed, C.; Danta, M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 54, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Su, F.H.; Wang, W.C.; Lin, C.P.; Kamiza, A.B.; Chang, S.N.; Yeh, C.C. Association of chronic hepatitis B virus infection with age-related macular degeneration. Acta Ophthalmol. 2019, 97, e713–e718. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jing, M.; Wang, C.; Wang, M.; You, S.; Lin, S.; Zhu, Y. The impact of hepatitis B virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study. J. Med. Virol. 2020, 92, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.; Yu, M.L.; Wong, G.; Thompson, A.; Ghazinian, H.; Hou, J.L.; Piratvisuth, T.; Jia, J.D.; Mizokami, M.; Cheng, G.; et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol. Int. 2021, 15, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kanda, T.; Nakaseko, C.; Sakaida, E.; Ohwada, C.; Takeuchi, M.; Takeda, Y.; Mimura, N.; Iseki, T.; Wu, S.; et al. Reactivation of hepatitis B virus in hematopoietic stem cell transplant recipients in Japan: Efficacy of nucleos(t)ide analogues for prevention and treatment. Int. J. Mol. Sci. 2014, 5, 21455–21467. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wu, C.S.; Chen, M.H.; Tsai, C.Y.; Lee, F.Y.; Huang, Y.H. High Risk of Viral Reactivation in Hepatitis B Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2021, 22, 9116. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, Y. Novel biomarkers for the management of chronic hepatitis B. Clin. Mol. Hepatol. 2020, 26, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Taida, T.; Arai, M.; Kanda, T.; Hige, S.; Ueno, Y.; Imazeki, F.; Izumi, N.; Tanaka, E.; Shinkai, N.; Yoshioka, K.; et al. The prognosis of hepatitis B inactive carriers in Japan: A multicenter prospective study. J. Gastroenterol. 2017, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
| Patient Groups | HBsAg-Positive | HBsAg-Negative but Anti-HBs and/or Anti-HBc-Positive | Triple-Negative | p-Values |
|---|---|---|---|---|
| Number | 811 | 2959 | 4617 | |
| Sex (male/female) | 458/353 | 1505/1454 | 2351/2266 | NS |
| Age (years) | 59.6 ± 16.1 | 55.6 ± 21.6 | 58.1 ± 17.1 | 0.0201 |
| AST (IU/L) | 75.6 ± 255.3 | 41.0 ± 80.4 | 58.9 ± 264.7 | NS |
| ALT (IU/L) | 74.4 ± 342.4 | 38.8 ± 93.4 | 57.5 ± 220.8 | NS |
| γ-GTP (IU/L) | 47.7 ± 76.1 | 73.0 ± 189.5 | 86.7 ± 180.7 | 0.00159 |
| Albumin (g/dL) | 4.1 ± 0.6 | 3.9 ± 0.7 | 4.0 ± 0.7 | <0.001 |
| Total bilirubin (mg/dL) | 1.1 ± 1.7 | 1.1 ± 1.8 | 1.1 ± 1.7 | NS |
| Platelets (×104/μL) | 19.9 ± 9.0 | 21.5 ± 9.9 | 23.0 ± 11.5 | <0.001 |
| Hemoglobin (g/dL) | 13.3 ± 2.0 | 13.0 ± 2.1 | 12.8 ± 2.4 | <0.001 |
| Case | Age (Years)/Sex | Type of Diseases | Outcomes/NUC | HBV DNA (LIU/mL) | Anti-HBs/ Anti-HBc | HBeAg/ Anti-HBe |
|---|---|---|---|---|---|---|
| 1 | 21/male | Acute hepatitis | Recovery/ETV (1.0) | 5.5 | −/+ | +/− |
| 2 | 23/female | Acute hepatitis | Recovery/None | 5.9 | −/+ | +/− |
| 3 | 25/male | Acute hepatitis | Recovery/ETV (1.0) | 7.5 | −/+ | +/+ |
| 4 | 28/male | Acute hepatitis | Recovery/ETV (0.5) | 8.2 | −/+ | +/− |
| 5 | 28/female | Acute hepatitis | Recovery/ETV (0.5) | 8.2 | −/+ | −/− |
| 6 | 28/male | Acute hepatitis | Recovery/None | 6.0 | −/+ | +/− |
| 7 | 31/male | Acute hepatitis | Recovery/None | 7 | −/+ | +/+ |
| 8 | 33/male | Acute hepatitis | Recovery/None | 6.2 | −/+ | −/+ |
| 9 | 35/male | Acute hepatitis | Recovery/ETV (0.5) | 5.4 | −/+ | +/− |
| 10 | 36/male | Acute hepatitis | Recovery/ETV (0.5) | 9.1 | −/+ | +/− |
| 11 | 45/male | Liver failure | Deceased/ETV (1.0) | 7.5 | −/+ | −/+ |
| 12 | 49/male | Acute hepatitis | Recovery/ETV (0.5) | 6 | −/+ | +/− |
| 13 | 50/male | Acute hepatitis | Recovery/ETV (1.0) | 2.5 | −/+ | −/+ |
| 14 | 51/male | Acute hepatitis | Recovery/None | 6.5 | +/+ | +/− |
| 15 | 51/male | Acute hepatitis | Recovery/ETV (0.5) | 4.4 | −/+ | −/+ |
| 16 | 52/male | Acute hepatitis | Recovery/Unknown | 2.5 | −/+ | +/− |
| 17 | 54/male | Acute hepatitis | Recovery/None | 5.6 | −/+ | −/+ |
| 18 | 54/male | Acute hepatitis | Recovery/None | 6.0 | −/+ | +/− |
| 19 | 55/male | Liver failure | Deceased/ETV (0.5) | 6.7 | −/+ | −/+ |
| 20 | 56/male | Acute hepatitis | Recovery/ETV (0.5) | 4.2 | −/+ | +/− |
| 21 | 64/male | Acute hepatitis | Recovery/ETV (0.5) | 5.4 | −/+ | NA/NA |
| 22 | 65/male | Acute hepatitis | Recovery/Unknown | 2.1 | −/+ | −/+ |
| Case | AGE (Years)/Sex | Type of Diseases | Outcomes/ NUC | HBsAg/HBV DNA (LIU/mL) | Anti-HBs/ Anti-HBc |
|---|---|---|---|---|---|
| 23 | 64/male | Ischemic liver injury | Recovery/None | +/NA | NA/NA |
| 24 | 79/male | Ischemic liver injury | Recovery/None | +/NA | NA/− |
| Case | Age (Years)/Sex | Type of Diseases | Outcomes/NUC | Anti-HBs/ Anti-HBc | HBeAg/ Anti-HBe |
|---|---|---|---|---|---|
| 25 | 52/male | Acute hepatitis | Recovery/None | +/+ | NA/NA |
| 26 | 55/male | Liver failure | Deceased/None | +/+ | NA/NA |
| 27 | 65/male | Acute hepatitis | Recovery/None | NA/+ | NA |
| 28 | 70/male | Acute hepatitis | Deceased/ETV (0.5) | +/+ | NA |
| 29 | 76/male | Acute hepatitis | Unknown/None | +/+ | +/+ |
| 30 | 81/female | Acute hepatitis | Recovery/None | NA/+ | NA/NA |
| 31 | 82/male | Liver failure | Deceased/None | −/+ | NA/NA |
| Number of Patients among the HBsAg-Negative but Anti-HBs/Anti-HBc-Positive Group (n = 23) | Number of Patients among Triple Negative Group (n = 75) | |
|---|---|---|
| Ischemic liver injury | 13 | 24 |
| Drug-induced liver injury | 3 | 22 |
| Autoimmune hepatitis | 0 | 6 |
| Obstructive jaundice | 2 | 4 |
| Post hepatectomy | 0 | 4 |
| Alcoholic cirrhosis | 0 | 5 |
| Acute viral hepatitis caused by non-HBV | 3 | 7 |
| Idiopathic thrombocytopenic purpura | 0 | 1 |
| Traumatic liver injury | 0 | 1 |
| Hemophagocytic syndrome | 0 | 1 |
| Unknown etiology | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, H.; Kanda, T.; Mochizuki, H.; Kojima, Y.; Suzuki, Y.; Hosoda, K.; Ashizawa, H.; Miura, Y.; Tsunoda, S.; Hirotsu, Y.; et al. The Use of Electronic Medical Records-Based Big-Data Informatics to Describe ALT Elevations Higher than 1000 IU/L in Patients with or without Hepatitis B Virus Infection. Viruses 2021, 13, 2216. https://doi.org/10.3390/v13112216
Amano H, Kanda T, Mochizuki H, Kojima Y, Suzuki Y, Hosoda K, Ashizawa H, Miura Y, Tsunoda S, Hirotsu Y, et al. The Use of Electronic Medical Records-Based Big-Data Informatics to Describe ALT Elevations Higher than 1000 IU/L in Patients with or without Hepatitis B Virus Infection. Viruses. 2021; 13(11):2216. https://doi.org/10.3390/v13112216
Chicago/Turabian StyleAmano, Hiroyuki, Tatsuo Kanda, Hitoshi Mochizuki, Yuichiro Kojima, Yoji Suzuki, Kenji Hosoda, Hiroshi Ashizawa, Yuko Miura, Shotaro Tsunoda, Yosuke Hirotsu, and et al. 2021. "The Use of Electronic Medical Records-Based Big-Data Informatics to Describe ALT Elevations Higher than 1000 IU/L in Patients with or without Hepatitis B Virus Infection" Viruses 13, no. 11: 2216. https://doi.org/10.3390/v13112216
APA StyleAmano, H., Kanda, T., Mochizuki, H., Kojima, Y., Suzuki, Y., Hosoda, K., Ashizawa, H., Miura, Y., Tsunoda, S., Hirotsu, Y., Ohyama, H., Kato, N., Moriyama, M., Obi, S., & Omata, M. (2021). The Use of Electronic Medical Records-Based Big-Data Informatics to Describe ALT Elevations Higher than 1000 IU/L in Patients with or without Hepatitis B Virus Infection. Viruses, 13(11), 2216. https://doi.org/10.3390/v13112216







