Efficient Control of Zika Virus Infection Induced by a Non-Replicating Adenovector Encoding Zika Virus NS1/NS2 Antigens Fused to the MHC Class II-Associated Invariant Chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Virus Preparation and Quantitation
2.3. Recombinant Adenoviral Vectors
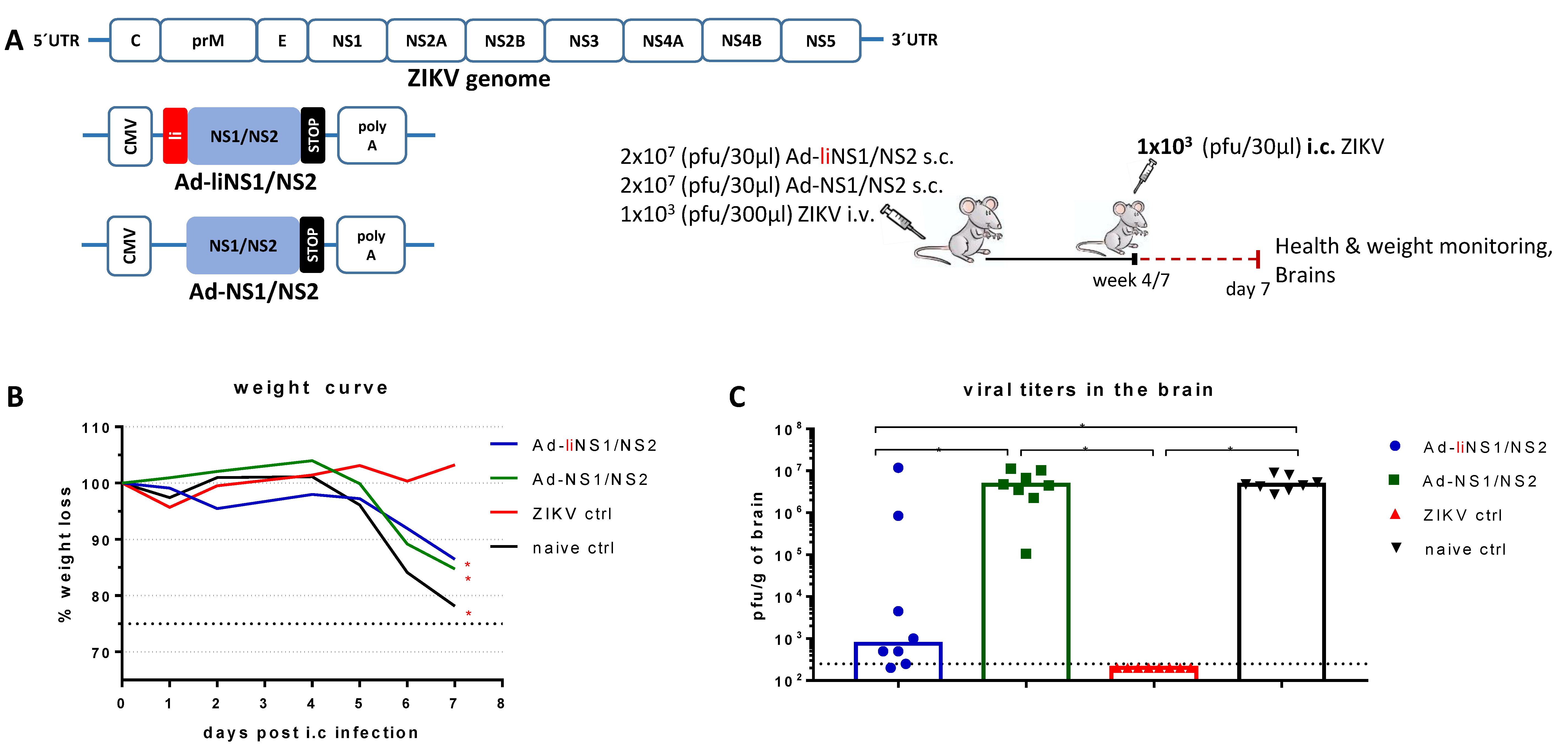
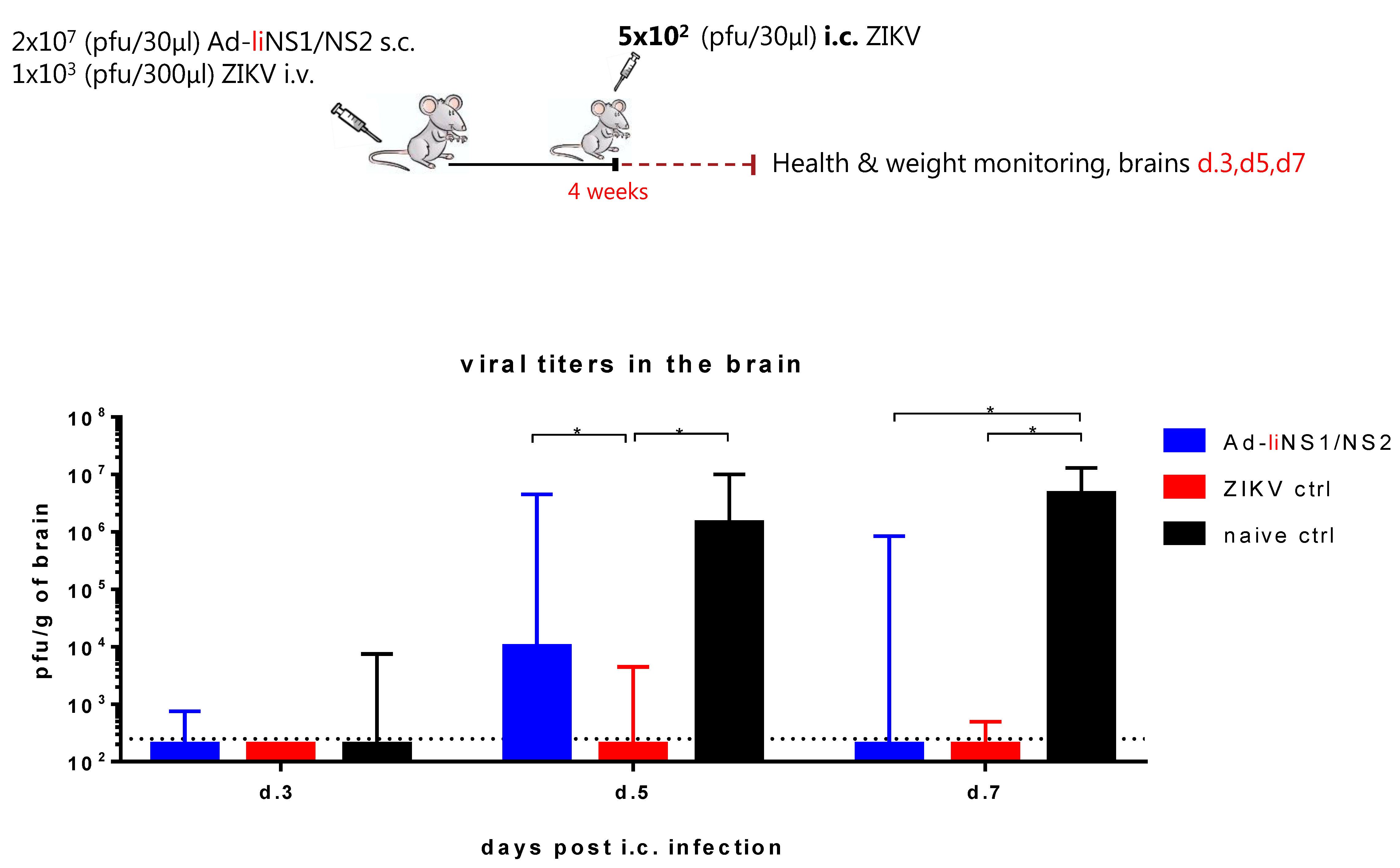
2.4. Immunization and Viral Intracranial Challenge
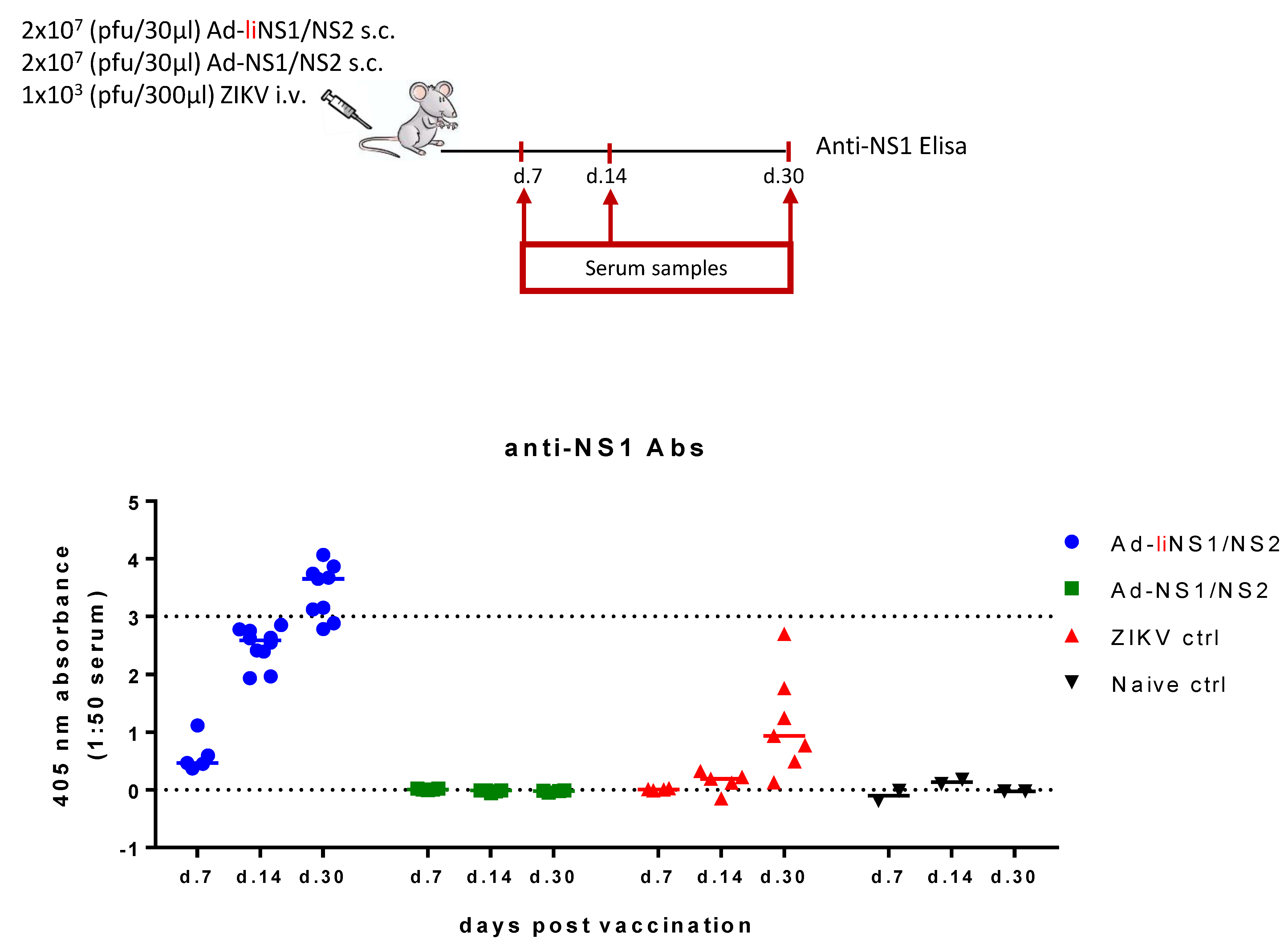
2.5. Anti-ZIKV NS1 Antibody Response
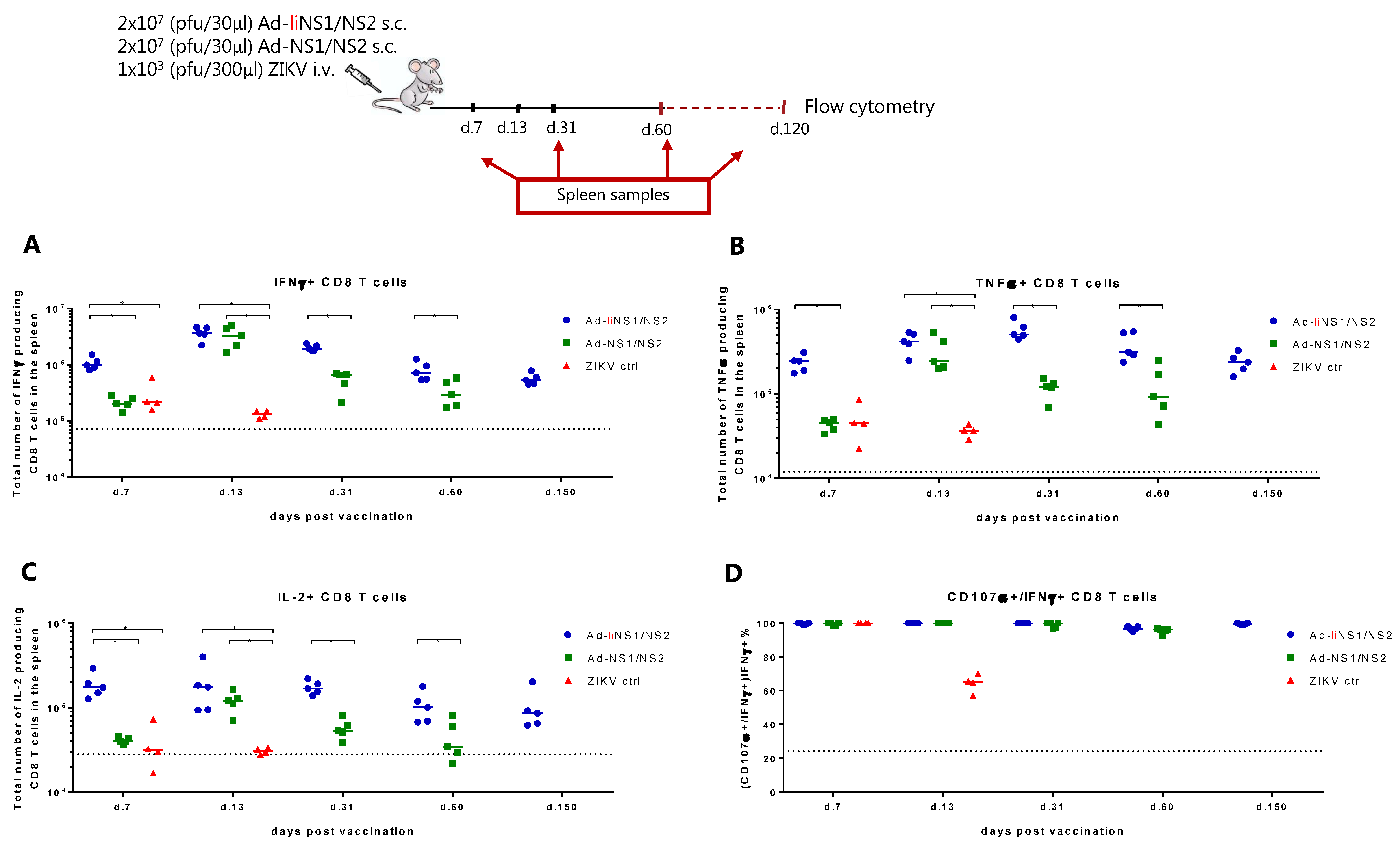
2.6. Flow Cytometry Analyses
2.7. Peptide and Antibodies
2.8. In Vivo CD8 T Cell Depletion
2.9. Serum Transfer
2.10. Statistical Evaluation
3. Results
3.1. Adenovirus Vectors Expressing ZIKV NS1/NS2 with and without Li-Linkage
3.2. Quality of Protection Conferred by the Ad-liNS1/NS2 and Ad-NS1/NS2 Vaccines
3.3. Immunogenicity of Ad-liNS1/NS2 and Ad-NS1/NS2 Vaccines
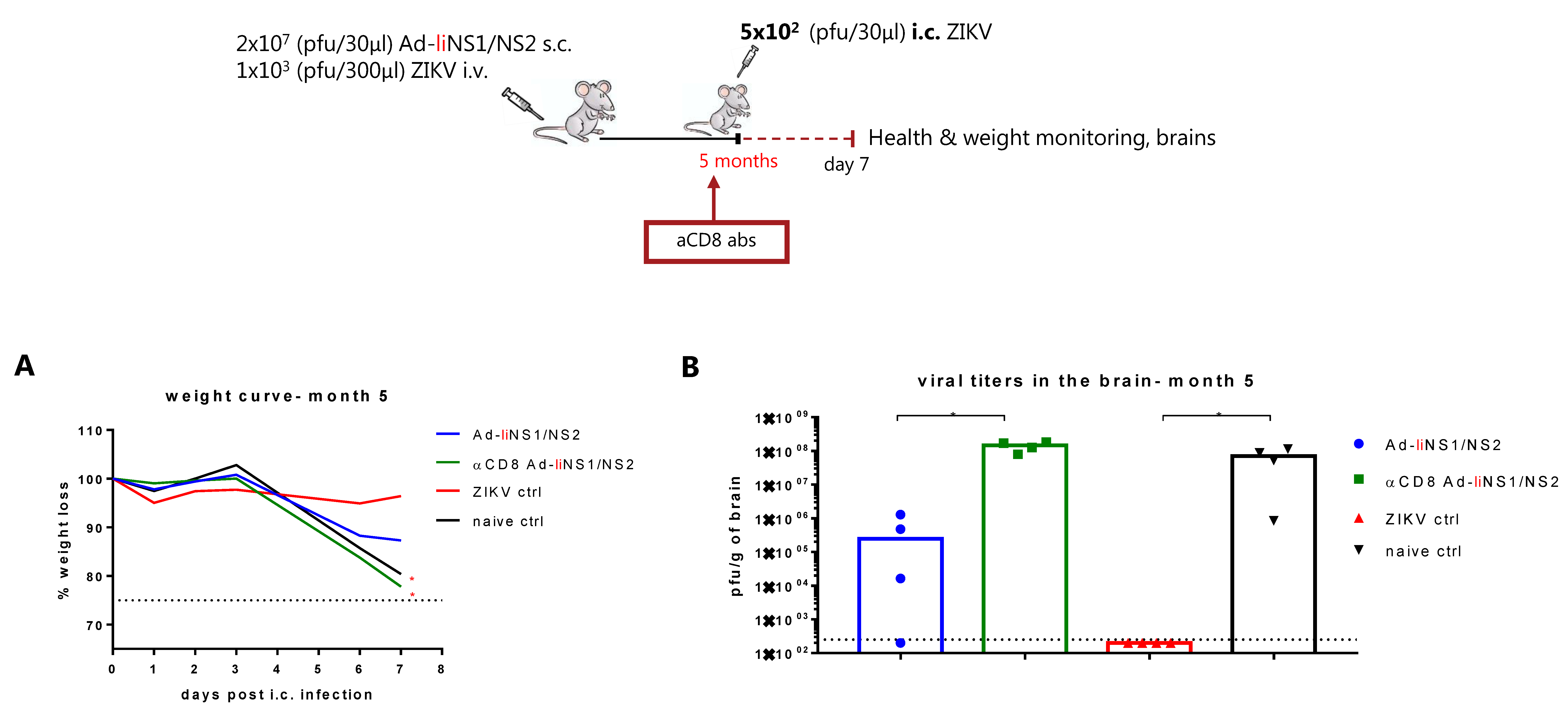
3.4. Kinetic of Viral Control in Ad-liNS1/NS2 Vaccinated Mice
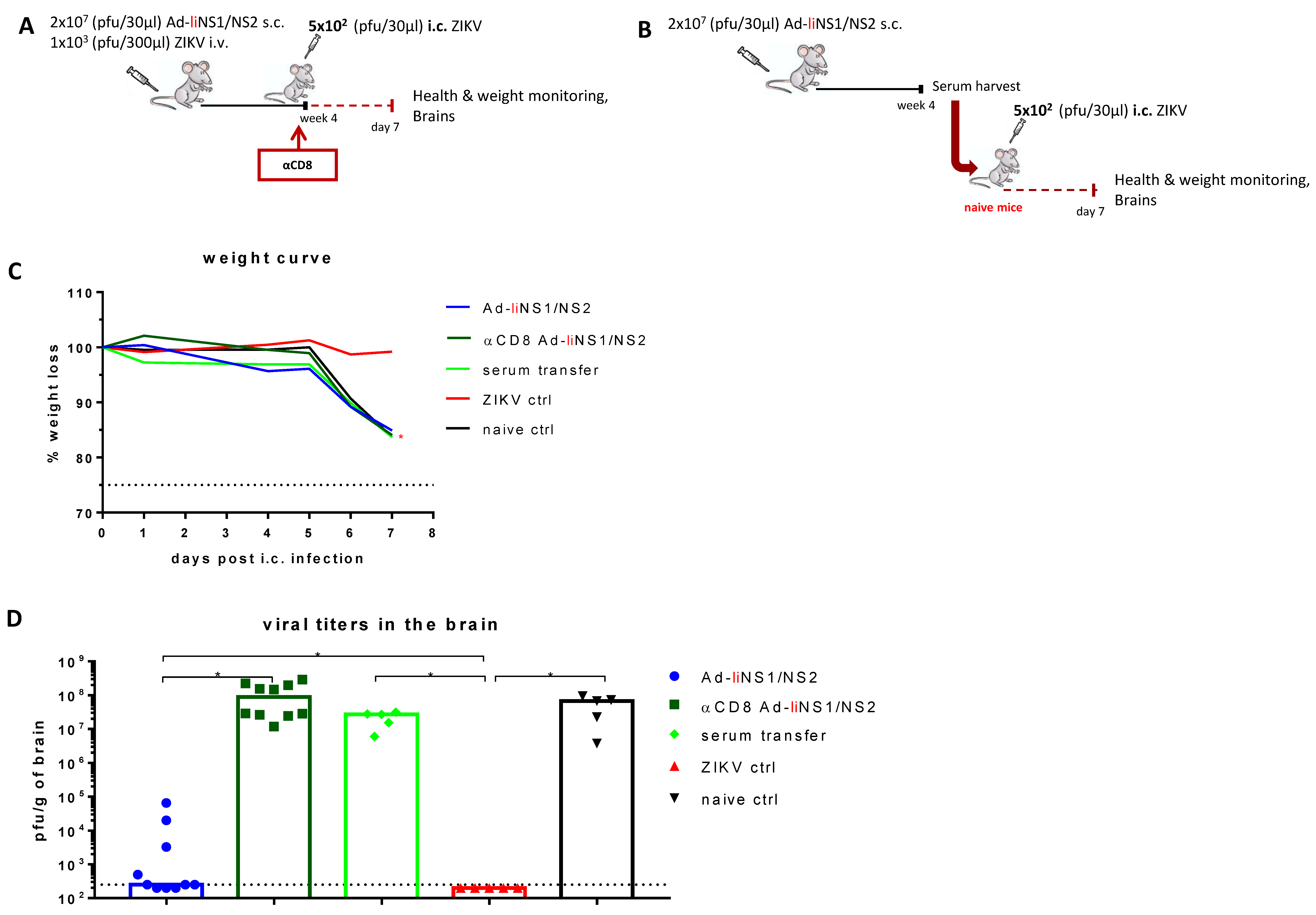
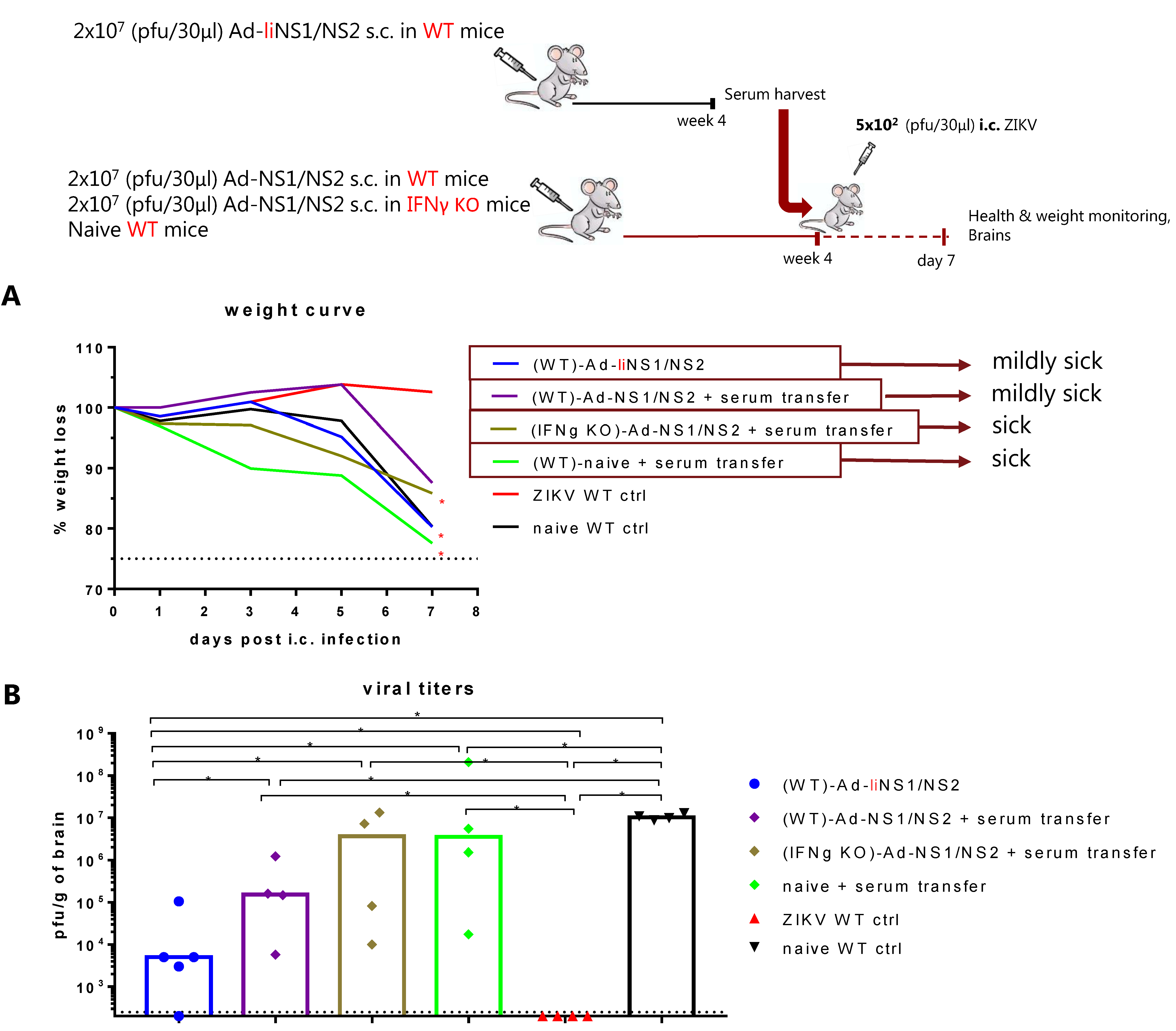
3.5. Contribution of CD8 T Cells and Anti-NS1 Antibodies in Ad-liNS1/NS2 Mediated Protection
3.6. Potential Mechanism of Protection Mediated by the Ad-liNS1/NS2 and Ad-NS1/NS2 Vaccines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016, 130, 69–80. [Google Scholar]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Dowd, K.A.; DeMaso, C.R.; Pelc, R.; Speer, S.D.; Smith, A.R.; Goo, L.; Platt, D.; Mascola, J.R.; Graham, B.S.; Mulligan, M.J.; et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016, 16, 1485–1491. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.D.T. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. npj Vaccines 2018, 3, 24. [Google Scholar] [CrossRef]
- Nazerai, L.; Christensen, J.P.; Thomsen, A.R. A ‘Furry-Tale’ of Zika Virus Infection: What have we learned from animal models? Viruses 2019, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Nazerai, L.; Schøller, A.S.; Rasmussen, P.O.S.; Buus, S.; Stryhn, A.; Christensen, J.; Thomsen, A.R. A New In vivo model to study protective immunity to zika virus infection in mice with intact type i interferon signaling. Front. Immunol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Martin-Acebes, M.A.; Saiz, J.C.; Jimenez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Men-ace? Front. Cell Infect. Microbiol. 2018, 8, 44. [Google Scholar]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Spaeth, G.B.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Watanabe, S.; Tan, N.W.W.; Chan, K.W.K.; Vasudevan, S. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J. Infect. Dis. 2018, 219, 223–233. [Google Scholar] [CrossRef]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [Green Version]
- McCracken, M.; Gromowski, G.D.; Friberg, H.L.; Lin, X.; Abbink, P.; De La Barrera, R.; Eckles, K.H.; Garver, L.S.; Boyd, M.; Jetton, D.; et al. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 2017, 13, e1006487. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar]
- Bonheur, A.N.; Thomas, S.; Soshnick, S.H.; McGibbon, E.; Dupuis, A.P.; Hull, R.; Slavinski, S.; Del Rosso, P.E.; Weiss, D.; Hunt, D.T.; et al. A fatal case report of antibody-dependent enhancement of dengue virus type 1 following remote Zika virus infection. BMC Infect Dis. 2021, 21, 749. [Google Scholar]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Mercado, B.L.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Carillo, F.B.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Post, P.R.; Carvalho, R.; Galler, R. Glycosylation and secretion of yellow fever virus nonstructural protein NS1. Virus Res. 1991, 18, 291–302. [Google Scholar]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef] [Green Version]
- Avirutnan, P.; Zhang, L.; Punyadee, N.; Manuyakorn, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Atkinson, J.P.; Diamond, M.S. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin Sulfate, E. PLoS Pathog. 2007, 3, e183. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Shin, O.S. Advances in Zika virus–host cell interaction: Current knowledge and future perspectives. Int. J. Mol. Sci. 2019, 20, 1101. [Google Scholar] [CrossRef] [Green Version]
- Brault, A.C.; Domi, A.; McDonald, E.M.; Talmi-Frank, D.; McCurley, N.; Basu, R.; Robinson, H.L.; Hellerstein, M.; Duggal, N.K.; Bowen, R.A.; et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Qu, L.; Ye, X.; Yi, C.; Zheng, X.; Hao, M.; Su, W.; Yao, Z.; Chen, P.; Zhang, S.; et al. Incorporation of NS1 and prM/M are important to confer effective protection of adenovirus-vectored Zika virus vaccine carrying E protein. Npj Vaccines 2018, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Yu, J.; Lu, M.; Ma, Y.; Attia, Z.; Shan, C.; Xue, M.; Liang, X.; Craig, K.; Makadiya, N.; et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA vaccination protects against Zika infection through T cell–mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.R.; Alves, R.P.D.S.; Sales, N.S.; Andreata-Santos, R.; Venceslau-Carvalho, A.A.; Pereira, S.S.; Castro-Amarante, M.F.; Rodrigues-Jesus, M.J.; Favaro, M.T.D.P.; Chura-Chambi, R.M.; et al. Enhanced immune responses and protective immunity to zika virus induced by a DNA vaccine encoding a chimeric NS1 fused with type 1 Herpes Virus gD Protein. Front. Med. Technol. 2020, 2, 4160. [Google Scholar] [CrossRef]
- Fougeroux, C.; Turner, L.; Bojesen, A.M.; Lavstsen, T.; Holst, P.J. Modified MHC Class II-Associated Invariant Chain Induces In-creased Antibody Responses against Plasmodium falciparum Antigens after Adenoviral Vaccination. J Immunol. 2019, 202, 2320–2331. [Google Scholar]
- Germain, R.N. Uncovering the Role of Invariant Chain in Controlling MHC Class II Antigen Capture. J. Immunol. 2011, 187, 1073–1075. [Google Scholar] [CrossRef] [Green Version]
- Holst, P.J.; Sørensen, M.R.; Jensen, C.M.M.; Orskov, C.; Thomsen, A.R.; Christensen, J. MHC Class II-Associated Invariant Chain Linkage of Antigen Dramatically Improves Cell-Mediated Immunity Induced by Adenovirus Vaccines. J. Immunol. 2008, 180, 3339–3346. [Google Scholar] [CrossRef]
- Jensen, S.; Steffensen, M.A.; Jensen, B.A.H.; Schlüter, D.; Christensen, J.P.; Thomsen, A.R. Adenovirus-Based Vaccine against Listeria monocytogenes: Extending the Concept of Invariant Chain Linkage. J. Immunol. 2013, 191, 4152–4164. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, M.R.; Holst, P.J.; Pircher, H.; Christensen, J.P.; Thomsen, A.R. Vaccination with an adenoviral vector encoding the tumor antigen directly linked to invariant chain induces potent CD4+T-cell-independent CD8+T-cell-mediated tumor control. Eur. J. Immunol. 2009, 39, 2725–2736. [Google Scholar] [CrossRef]
- Spencer, A.J.; Cottingham, M.; Jenks, J.; Longley, R.; Capone, S.; Colloca, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Bregu, M.; et al. Enhanced Vaccine-Induced CD8+ T Cell Responses to Malaria Antigen ME-TRAP by Fusion to MHC Class II Invariant Chain. PLoS ONE 2014, 9, e100538. [Google Scholar] [CrossRef] [Green Version]
- Esposito, I.; Cicconi, P.; D’Alise, A.M.; Brown, A.; Esposito, M.; Swadling, L.; Holst, P.J.; Bassi, M.R.; Stornaiuolo, M.; Mori, F.; et al. MHC class II invariant chain–adjuvanted viral vectored vaccines enhances T cell responses in humans. Sci. Transl. Med. 2020, 12, 7715. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Decman, V.; Ali, M.-A.A.; Abt, M.C.; Wolf, A.I.; Monticelli, L.A.; Mozdzanowska, K.; Angelosanto, J.M.; Artis, D.; Erikson, J.; et al. Cooperativity Between CD8+ T Cells, Non-Neutralizing Antibodies, and Alveolar Macrophages Is Important for Heterosubtypic Influenza Virus Immunity. PLoS Pathog. 2013, 9, e1003207. [Google Scholar] [CrossRef]
- Lucas, C.G.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4(+) T cells and IFNgamma signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun. 2018, 9, 3136. [Google Scholar]
- Cavazzoni, C.B.; Bozza, V.B.; Lucas, T.C.; Conde, L.; Maia, B.; Mesin, L.; Schiepers, A.; Ersching, J.; Neris, R.L.; Conde, J.N.; et al. The immunodominant antibody response to Zika virus NS1 protein is characterized by cross-reactivity to self. J. Exp. Med. 2021, 218, 580. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazerai, L.; Buus, S.; Stryhn, A.; Thomsen, A.R.; Christensen, J.P. Efficient Control of Zika Virus Infection Induced by a Non-Replicating Adenovector Encoding Zika Virus NS1/NS2 Antigens Fused to the MHC Class II-Associated Invariant Chain. Viruses 2021, 13, 2215. https://doi.org/10.3390/v13112215
Nazerai L, Buus S, Stryhn A, Thomsen AR, Christensen JP. Efficient Control of Zika Virus Infection Induced by a Non-Replicating Adenovector Encoding Zika Virus NS1/NS2 Antigens Fused to the MHC Class II-Associated Invariant Chain. Viruses. 2021; 13(11):2215. https://doi.org/10.3390/v13112215
Chicago/Turabian StyleNazerai, Loulieta, Søren Buus, Anette Stryhn, Allan Randrup Thomsen, and Jan Pravsgaard Christensen. 2021. "Efficient Control of Zika Virus Infection Induced by a Non-Replicating Adenovector Encoding Zika Virus NS1/NS2 Antigens Fused to the MHC Class II-Associated Invariant Chain" Viruses 13, no. 11: 2215. https://doi.org/10.3390/v13112215
APA StyleNazerai, L., Buus, S., Stryhn, A., Thomsen, A. R., & Christensen, J. P. (2021). Efficient Control of Zika Virus Infection Induced by a Non-Replicating Adenovector Encoding Zika Virus NS1/NS2 Antigens Fused to the MHC Class II-Associated Invariant Chain. Viruses, 13(11), 2215. https://doi.org/10.3390/v13112215





