A Longitudinal Analysis of Cerebral Blood Flow in Perinatally HIV Infected Adolescents as Compared to Matched Healthy Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
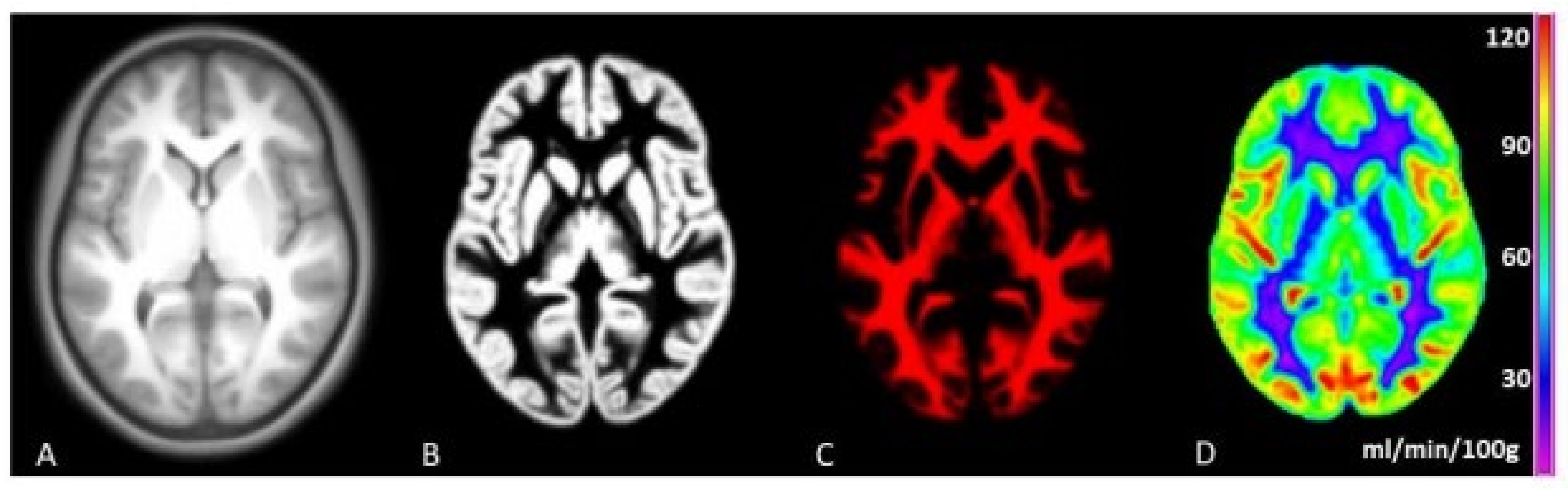
2.2. MRI Data Acquisition
2.3. Image Processing
2.4. Demographic and HIV Related Variables
2.5. Cognitive Functioning
2.6. Statistical Analyses
3. Results
3.1. Differences in CBF between Baseline and Follow-Up
3.2. Determinants of Changes in CBF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Hof, M.V.D.; Ter Haar, A.M.; Caan, M.W.; Spijker, R.; Van Der Lee, J.H.; Pajkrt, D. Brain structure of perinatally HIV-infected patients on long-term treatment. Neurol. Clin. Pract. 2019, 9, 433–442. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Valdés Hernández, M.C.; Muñoz-Maniega, S. What Are White Matter Hyperintensities Made of? Relevance to Vascular Cognitive Impairment. J. Am. Heart Assocation 2015, 4, e001140. [Google Scholar]
- Blokhuis, C.; Mutsaerts, H.J.; Cohen, S.; Scherpbier, H.J.; Caan, M.W.; Majoie, C.B.; Kuijpers, T.W.; Reiss, P.; Wit, F.W.; Pajkrt, D. Higher subcortical and white matter cerebral blood flow in perinatally HIV-infected children. Medicine 2017, 96, e5891. [Google Scholar] [CrossRef]
- Van Dalen, Y.W.; Blokhuis, C.; Cohen, S.; Ter Stege, J.A.; Teunissen, C.E.; Kuhle, J.; Kootstra, N.A.; Scherpbier, H.J.; Kuijpers, T.W.; Reiss, P.; et al. Neurometabolite Alterations Associated With Cognitive Performance in Perinatally HIV-Infected Children. Medicine 2016, 95, e3093. [Google Scholar] [CrossRef]
- Graham, A.S.; Holmes, M.J.; Little, F.; Dobbels, E.; Cotton, M.F.; Laughton, B.; van der Kouwe, A.; Meintjes, E.M.; Robertson, F.C. MRS suggests multi-regional inflammation and white matter axonal damage at 11 years following perinatal HIV infection. NeuroImage Clin. 2020, 28, 102505. [Google Scholar] [CrossRef]
- Blokhuis, C.; Demirkaya, N.; Cohen, S.; Wit, F.W.N.M.; Scherpbier, J.; Reiss, P.; Abramoff, M.D.; Caan, M.W.A.; Majoie, C.B.L.M.; Verbraak, F.D.; et al. The Eye as a Window to the Brain: Neuroretinal Thickness Is Associated With The Eye as a Window to the Brain: Neuroretinal Thickness Is Associated With Microstructural White Matter Injury in HIV-infected children. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3864–3871. [Google Scholar] [CrossRef] [Green Version]
- Dean, O.; Buda, A.; Adams, H.R.; Mwanza-kabaghe, S.; Potchen, M.J.; Mbewe, E.G.; Kabundula, P.P.; Moghaddam, S.M.; Birbeck, G.L.; Bearden, D.R. Pediatric Neurology Brain Magnetic Resonance Imaging Findings Associated With Cognitive Impairment in Children and Adolescents With Human Immunode fi ciency Virus in Zambia. Pediatr. Neurol. 2020, 102, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, C.; Andronikou, S.; Laughton, B.; Kidd, M.; Dobbels, E.; Innes, S.; van Toorn, R.; Cotton, M. White Matter Signal Abnormalities in Children With Suspected HIV-related Neurologic Disease on Early Combination Antiretroviral Therapy. Pediatr. Infect. Dis. J. 2014, 33, e207–e212. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201408000-00016 (accessed on 1 August 2021). [CrossRef] [Green Version]
- Hoare, J.; Fouche, J.P.; Phillips, N.; Joska, J.A.; Myer, L.; Zar, H.J.; Stein, D.J. Structural brain changes in perinatally HIV-infected young adolescents in South Africa. AIDS 2018, 32, 2707–2718. [Google Scholar] [CrossRef]
- Jankiewicz, M.; Holmes, M.J.; Taylor, P.A.; Cotton, M.F.; Laughton, B.; van der Kouwe, A.J.W.; Meintjes, E.M. White matter abnormalities in children with HIV infection and exposure. Front. Neuroanat. 2017, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Van den Hof, M.; Jellema, P.E.J.; ter Haar, A.M.; Scherpbier, H.J.; Schrantee, A.; Kaiser, A.; Caan, M.W.; Majoie, C.B.; Reiss, P.; Wit, F.W.; et al. Normal structural brain development in adolescents treated for perinatally acquired HIV: A longitudinal imaging study. AIDS 2021, 35, 1221. [Google Scholar] [CrossRef]
- Cilliers, K.; Muller, C.J.F. Effect of human immunodeficiency virus on the brain: A review. Anat. Rec. 2021, 304, 1389–1399. [Google Scholar] [CrossRef]
- Blokhuis, C.; Kootstra, N.A.; Caan, M.W.; Pajkrt, D. Neurodevelopmental delay in pediatric HIV/AIDS: Current perspectives. Neurobehav. HIV Med. 2016, 7, 1–13. [Google Scholar]
- Hof, M.V.D.; Ter Haar, A.M.; Scherpbier, H.J.; Reiss, P.; Wit, F.W.N.M.; Oostrom, K.J.; Pajkrt, D. Lower IQ and poorer cognitive profiles in treated perinatally HIV-infected children is irrespective of having a background of international adoption. PLoS ONE 2019, 14, e0224930. [Google Scholar]
- Malee, K.M.; Chernoff, M.C.; Sirois, P.A.; Williams, P.L.; Garvie, P.A.; Kammerer, B.L.; Harris, L.L.; Nozyce, M.L.; Yildirim, C.; Nichols, S.L.; et al. Impact of Perinatally Acquired HIV Disease Upon Longitudinal Changes in Memory and Executive Functioning. J. Acquir. Immune Defic. Syndr. 2017, 75, 455–464. [Google Scholar] [CrossRef]
- Rowe, K.; Buivydaite, R.; Heinsohn, T.; Rahimzadeh, M.; Wagner, R.G.; Scerif, G.; Stein, A. Executive function in HIV-affected children and adolescents: A systematic review and meta-analyses. AIDS Care 2021, 33, 833–857. [Google Scholar] [CrossRef]
- Willie, C.K.; Tzeng, Y.C.; Fisher, J.A.; Ainslie, P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef]
- Benjamin, L.A.; Bryer, A.; Emsley, H.C.A.; Khoo, S.; Solomon, T.; Connor, M.D. HIV infection and stroke: Current perspectives and future directions. Lancet Neurol. 2012, 11, 878–890. [Google Scholar] [CrossRef]
- Yoshino, Y.; Koga, I.; Kitazawa, T.; Sakurai, K.; Oba, H.; Matsuda, H.; Furui, S.; Ota, Y. Cerebral blood flow in young and middle-aged people living with HIV. Infect. Dis. 2020, 52, 75–79. [Google Scholar] [CrossRef]
- Su, T.; Mutsaerts, H.J.; Caan, M.W.; Wit, F.W.; Schouten, J.; Geurtsen, G.; Sharp, D.J.; Prins, M.; Richard, E.; Portegies, P.; et al. Cerebral blood flow and cognitive function in HIV-infected men with sustained suppressed viremia on combination antiretroviral therapy. AIDS 2017, 31, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Schmithorst, V.; Panigrahy, A. Arterial Spin Labeling in Pediatric Neuroimaging. Semin. Pediatr. Neurol. 2020, 33, 100799. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, E.; Liu, C.Y.; Yan, L.; Kim, Y.C.; Dapretto, M.; Mendez, M.F.; Wang, D.J. Relationships between Cerebral Blood Flow and IQ in Typically Developing Children and Adolescents. J. Cogn. Sci. 2011, 12, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Kazumata, K.; Tokairin, K.; Sugiyama, T.; Ito, M.; Uchino, H.; Osanai, T.; Kawabori, M.; Nakayama, N.; Houkin, K. Association of cognitive function with cerebral blood flow in children with moyamoya disease. J. Neurosurg. Pediatr. 2020, 25, 62–68. [Google Scholar] [CrossRef]
- van den, H.M.; Scherpbier, H.J.; van der, L.J.H.; Reiss, P.; Wit, F.W.N.M.; Oostrom, K.J.; Pajkrt, D. Neurocognitive Development in Perinatally Human Immunodeficiency Virus—infected Adolescents on Long-term Treatment, Compared to Healthy Matched Controls: A Longitudinal Study. Clin. Infect. Dis. 2020, 70, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Mutsaerts, H.J.M.M.; Petr, J.; Groot, P.; Vandemaele, P.; Ingala, S.; Robertson, A.D.; Václavů, L.; Groote, I.; Kuijf, H.; Zelaya, F.; et al. ExploreASL: An image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage 2020, 219, 117031. [Google Scholar] [CrossRef]
- Filley, C.M. History of Subcortical Cognitive Impairment. Front. Neurol. Neurosci. 2019, 44, 108–117. [Google Scholar]
- Stichting HIV Monitoring (SHM). Available online: https://www.hiv-monitoring.nl/english/ (accessed on 1 August 2021).
- R Core Team. R: A Language and Environment for Statistical; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 1 May 2021).
- Biagi, L.; Abbruzzese, A.; Bianchi, M.C.; Alsop, D.C.; Del Guerra, A.; Tosetti, M. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J. Magn. Reson. Imaging 2007, 25, 696–702. [Google Scholar] [CrossRef]
- Wenserski, F.; Von Giesen, H.J.; Wittsack, H.J.; Aulich, A.; Arendt, G. Human immmunodeficiency virus 1-associated minor motor disorders: Perfusion-weighted MR imaging and 1H MR spectroscopy. Radiology 2003, 228, 185–192. [Google Scholar] [CrossRef]
- Babyak, M.A. What You See May Not Be What You Get: A Brief, Nontechnical Introduction to Overfitting in Regression-Type Models. Psychosom. Med. 2004, 66, 411–421. [Google Scholar]


| PHIV (n = 21) | CONTROLS (n = 23) | p | |
|---|---|---|---|
| FU rate | 62% | 62% | |
| FU time (years) | 4.60 (0.34) | 4.60 (0.34) | 0.694 X |
| Age at baseline (years) | 13.1 (10.8–15.7) | 11.6 (11.0–14.4) | 0.181 Z |
| Age at follow-up (years) | 17.4 (15.3–20.7) | 16.2 (15.6–19.1) | 0.441 Z |
| Male sex | 12 (57%) | 9 (40%) | 0.197 Y |
| Ethnic background | |||
| Black | 15 (75%) | 13 (65%) | 0.731 Y |
| Hematocrit (l/l) | 0.43 (0.40–0.45) | 0.40 (0.39–0.44) | 0.496 Z |
| Blood pressure (mmHg) | |||
| Systolic | 123 (115–132) | 120 (113–124) | 0.600 Z |
| Diastolic | 66 (59–74) | 64 (60–73) | 0.928 Z |
| MRI scan of good quality | 20 (95%) | 20 (87%) | |
| Mean motion (mm) | 0.13 (0.10–0.18) | 0.13 (0.11–0.18) | 0.301 Z |
| GM/ICV ratio | 0.50 (0.03) | 0.49 (0.02) | 0.207 X |
| WM/ICV ratio | 0.34 (0.02) | 0.35 (0.02) | 0.686 X |
| WMH volume (mm3) | 90 (17–163) | 42 (20–72) | 0.355 Z |
| Age at HIV diagnosis (years) | 1.5 (0.8–4.1) | ||
| CDC category | |||
| NA | 8 (40%) | ||
| B | 7 (35%) | ||
| C | 5 (25%) | ||
| Undetectable viral load | 18 (90%) | ||
| Undetectable entire follow-up | 14 (70%) | ||
| HIV viral load zenith (ln) | 12.8 (11.5–13.4) | ||
| CD4+ T-cell nadir Z score | −0.83 (0.63) | ||
| Age cART initiation | 2.5 (1.2–4.3) | ||
| Current cART use | 19 (95%) |
| FA | MD | AD | RD | WMH Volume | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | |
| GM | −0.177 (−0.99 to 0.63) | 0.672 | 0.01 (−0.01 to 0.03) | 0.240 | 0.02 (−0.002 to 0.05) | 0.095 | 0.005 (−0.01 to 0.03) | 0.647 | 0.17 (−0.53 to 0.88) | 0.639 |
| WM | −0.79 (−3.3 to 1.69) | 0.542 | 0.06 (0.0003 to 0.11) | 0.062 | 0.09 (0.02 to 0.17) | 0.027 | 0.04 (−0.02 to 0.09) | 0.199 | −0.06 (−1.92 to 1.83) | 0.947 |
| IQ | Processing Speed | Learning Ability | Visual Motor Function | Executive Function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | coefficient (95%CI) | p | |
| GM | −1.63 (−11 to 8.8) | 0.751 | 13.0 (−0.78 to 27) | 0.078 | −9.3 (−30 to 11) | 0.390 | 9.18 (0.84 to 18) | 0.042 | 1.36 (−0.50 to 2.59) | 0.045 |
| WM | −2.0 (−4.9 to 0.98) | 0.195 | 1.86 (−2.3 to 6.0) | 0.397 | −3.3 (−9.1 to 2.6) | 0.295 | 1.41 (−1.1 to 4.1) | 0.296 | 0.34 (−0.16 to 0.70) | 0.121 |
| Caudate Nucleus | −5.65 (−15 to 4.01) | 0.238 | 14.9 (1.90 to 28) | 0.033 | −11 (−30 to 8.8) | 0.303 | 9.76 (1.85 to 18.5) | 0.023 | 0.47 (−1.14 to 1.51) | 0.486 |
| Putamen | −2.88 (−13 to 7.61) | 0.582 | 16.1 (1.88 to 31) | 0.036 | −6.6 (−28 to 15) | 0.562 | 9.55 (0.61 to 20) | 0.045 | 0.18 (−1.49 to 1.43) | 0.806 |
| Thalamus | −1.95 (−12 to 8.2) | 0.701 | 20 (7.8 to 33) | 0.003 | −2.15 (−21 to 16) | 0.826 | 12.5 (4.9 to 21) | 0.003 | 0.74 (−0.84 to 1.77) | 0.265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Genderen, J.G.; Van den Hof, M.; ter Haar, A.M.; Blokhuis, C.; Keil, V.C.; Pajkrt, D.; Mutsaerts, H.J.M.M. A Longitudinal Analysis of Cerebral Blood Flow in Perinatally HIV Infected Adolescents as Compared to Matched Healthy Controls. Viruses 2021, 13, 2179. https://doi.org/10.3390/v13112179
van Genderen JG, Van den Hof M, ter Haar AM, Blokhuis C, Keil VC, Pajkrt D, Mutsaerts HJMM. A Longitudinal Analysis of Cerebral Blood Flow in Perinatally HIV Infected Adolescents as Compared to Matched Healthy Controls. Viruses. 2021; 13(11):2179. https://doi.org/10.3390/v13112179
Chicago/Turabian Stylevan Genderen, Jason G., Malon Van den Hof, Anne Marleen ter Haar, Charlotte Blokhuis, Vera C. Keil, Dasja Pajkrt, and Henk J. M. M. Mutsaerts. 2021. "A Longitudinal Analysis of Cerebral Blood Flow in Perinatally HIV Infected Adolescents as Compared to Matched Healthy Controls" Viruses 13, no. 11: 2179. https://doi.org/10.3390/v13112179
APA Stylevan Genderen, J. G., Van den Hof, M., ter Haar, A. M., Blokhuis, C., Keil, V. C., Pajkrt, D., & Mutsaerts, H. J. M. M. (2021). A Longitudinal Analysis of Cerebral Blood Flow in Perinatally HIV Infected Adolescents as Compared to Matched Healthy Controls. Viruses, 13(11), 2179. https://doi.org/10.3390/v13112179







