Abstract
A real-time polymerase chain reaction (qPCR) is considered the gold standard for the laboratory diagnosis of canine parvovirus (CPV) infection but can only be performed in specialized laboratories. Several point-of-care tests (POCT), detecting CPV antigens in faeces within minutes, are commercially available. The aim of this study was to evaluate eight POCT in comparison with qPCR. Faecal samples of 150 dogs from three groups (H: 50 client-owned, healthy dogs, not vaccinated within the last four weeks; S: 50 shelter dogs, healthy, not vaccinated within the last four weeks; p = 50 dogs with clinical signs of CPV infection) were tested with eight POCT and qPCR. Practicability, sensitivity, specificity, positive (PPV) and negative predictive values (NPV), as well as overall accuracy were determined. To assess the differences between and agreement among POCT, McNemar’s test and Cohen’s Kappa statistic were performed. Specificity and PPV were 100.0% in all POCT. Sensitivity varied from 22.9–34.3% overall and from 32.7–49.0% in group P. VetexpertRapidTestCPVAg® had the highest sensitivity (34.3% overall, 49.0% group P) and differed significantly from the 3 POCT with the lowest sensitivities (Fassisi®Parvo (27.7% overall, 36.7% group P), Primagnost®ParvoH+K (24.3% overall, 34.7% group P), FASTest®PARVOCard (22.9% overall, 32.7% group P)). The agreement among all POCT was at least substantial (kappa >0.80). A positive POCT result confirmed the infection with CPV in unvaccinated dogs, whereas a negative POCT result did not definitely exclude CPV infection due to the low sensitivity of all POCT.
1. Introduction
Canine parvovirus (CPV) is a common enteric virus in dogs. It emerged from the feline panleukopenia-like virus (FPV-like), CPV-2, in the 1970s, causing an acute haemorrhagic enteritis [1,2,3]. In the following years, the original type CPV-2 was replaced by two antigenic variants: CPV-2a and CPV-2b [2,4,5]. In 2000, a third antigenic type, CPV-2c, was identified which also spread worldwide in the meantime [6,7]. In this paper, the term CPV is used for all canine field and vaccination parvovirus strains (CPV-2a, CPV-2b and CPV-2c). CPV is highly contagious [8] and causes an often fatal disease, especially in puppies. Clinical signs and laboratory findings include diarrhea and vomiting [9], dehydration, as well as leukopenia [10]. Complications, such as bacterial translocation with consecutive septicemia, systemic inflammatory response syndrome (SIRS), hypercoagulability and multiorgan disfunction, can occur, causing high mortality rates in untreated dogs [10,11].
In order to isolate infected patients and to provide an immediate adequate therapy for dogs with this life-threatening infection, a rapid diagnosis is essential. Thus, reliable diagnostic methods to detect CPV infection are highly important. A polymerase chain reaction (PCR) shows the highest sensitivity compared to traditional methods, such as haemagglutination or virus isolation [12]. A quantitative real-time (q)PCR is considered the gold standard to diagnose CPV infection [12,13,14]. However, qPCR can only be performed in specialized laboratories which delays the diagnosis.
As infected dogs shed high amounts of CPV in their faeces [15], several point-of-care tests (POCT) are commercially available for the detection of the CPV antigen in-house. These POCT are based on an enzyme-linked immunosorbent assay (ELISA) or immunomigration technology and detect CPV antigen in faeces within a few minutes.
For test performance, sensitivity is the most important parameter in this scenario to identify and isolate all the affected dogs before infecting other contact animals. Recent studies showed a high specificity of some of these POCT with over 95.1% [14,16], but sensitivity was low, varying between 15.8% and 80.4% compared to the gold standard qPCR [12,14,16,17]. However, only a few POCT (IDEXX SNAP® Parvo, FASTest® parvo strip, and Witness® parvo card) were evaluated in independent studies so far and studies comparing the tests have yet to be performed.
Therefore, the aim of the present study was to evaluate eight commercially available POCT for the detection of the CPV antigen in the faeces of dogs in comparison with the reference standard qPCR. Practicability, sensitivity, specificity, positive (PPV) and negative predictive values (NPV), as well as overall accuracy, were evaluated.
2. Materials and Methods
2.1. Faecal Samples
A total of 150 faecal samples from three groups of dogs were included in the study. Ages of the dogs (see Table S1 in the Supplementary Materials) were from 4 weeks up to 13 years (median age of 3 years). Group H included 50 healthy, client-owned dogs (age: 1–13 years, median: 5 years). Dogs were only included in this group if there were no abnormalities in physical examination and history and the dogs had not been vaccinated against CPV within the last 4 weeks. Group S consisted of 50 shelter dogs (age: 7 months–12 years, median: 7 years), that were also healthy in their physical examinations and not vaccinated against CPV within the last 4 weeks. These dogs were considered to have a high risk of CPV infection because of high population density and stress. Group P included 50 dogs (age: 4 weeks–2 years, median: 3 months) which were presented to veterinary hospitals with suspicion of CPV infection. The dogs of group P had to meet at least 3 of the following criteria: diarrhea, vomiting, bad general condition, fever, neutropenia and/or incomplete vaccination status according to the WSAVA vaccination guidelines [18]. Vaccination status was known for 28 dogs of group P. Three dogs were vaccinated against CPV within the last 4 weeks, 25 dogs were not vaccinated against CPV within the last 4 weeks. For the remaining 22 dogs, vaccination status was unknown. Vaccination status of the dogs of group P is presented in Table S1 in the Supplementary Materials. All faecal samples were stored at −80 °C until further processing. The study protocol for this study was approved by the ethical committee of the Centre for Veterinary Clinical Medicine, LMU Munich, Germany (reference number 53-09-09-2015).
2.2. Real-Time PCR, Faecal CPV Load
DNA from faecal samples was extracted using the QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturers’ recommendations. QPCR for detection and quantification of faecal CPV DNA was performed as described by Streck and colleagues (2013) [19] by a person blinded to the results of the POCT. All samples were run in duplicates. To compare results, the number of DNA copies/template was converted to the number of copies/g faeces, based on the individual sample weight.
2.3. Virus Culture
Virus cultures were performed for all qPCR-positive faecal samples. Two hundred milligram of faeces were suspended in 2.0 mL phosphate-buffered saline (pH 7.2), centrifuged at 3000× g for five minutes and the supernatant was filtered through a 0.22 µm syringe filter. One hundred microlitres of these filter suspensions were used to inoculate Crandell Rees feline kidney cells maintained in Dulbecco’s medium (Biochrom) supplemented with 5% fetal calf serum (Sigma Aldrich), 1% non-essential amino acids (Biochrom) and 1% penicillin-streptomycin (Biochrom). Cultures were incubated at 37 °C, 5% CO2. After seven days of incubation, each culture was subcultured.
2.4. Point-of-Care Tests
All faecal samples were analyzed with the eight POCT detecting CPV antigen (details in Table 1) by the same author (J.W.-W.) who was blinded to the results of the qPCR. Samples were only tested once with each of the POCT.

Table 1.
Eight point-of-care tests for the detection of canine parvovirus in faeces and the respective manufacturers’ instructions.
POCT were performed according to the manufacturers’ instructions. Testing delivered results after 5–10 min.
The IDEXX SNAP® Parvo is an ELISA, in which a conjugate (an enzyme-labeled antibody) forms an immune complex with the antigen in the sample and matrix-bound antibodies. Subsequently, a washing step removes unbound debris and unreacted conjugate. Finally, a substrate-based enzymatic reaction generates a blue point in case of a positive test result [20]. All other POCT are lateral flow immunoassays, using immunomigration technologies. In this technology, the antigen in the sample reacts with mobile, monoclonal anti-CPV antibodies conjugated with gold particles. After migration along a nitrocellulose membrane, the antigen antibody complexes are captured by fixed anti-CPV monoclonal antibodies creating a test line in case of a positive result.
2.5. Data Analysis
Test results of the eight POCT were compared with results of the qPCR. Practicability, difficulties in test result interpretation, sensitivity (true positive rate), specificity (true negative rate), negative predictive value (NPV) (proportion of predicted negatives that were true negatives), positive predictive value (PPV) (proportion of predicted positives that were true positives) and overall accuracy (OA) (probability that a dog will be correctly classified by the tests; sum of true positives plus true negatives divided by the total number of dogs tested) were calculated and used for comparison of test performances. Sensitivity was considered as most important parameter. The differences between the POCT performance were tested for statistical significance using Cochran’s Q omnibus test at the group level. In case of significant results, pairwise comparison was performed using McNemar’s test to determine significant differences in sensitivity of the POCT. Cohen’s Kappa statistic was performed to assess agreement of the results among the POCT. Values < 0 indicated poor agreement, 0.00–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect agreement [21]. Logistic regression was performed to assess the detection probability of each POCT as a function of the virus load. Log transform was applied to the number of virus copies prior to regression analysis. The regression results were presented along with the decision threshold of virus copies/g faeces corresponding to a 50% detection probability. Error bounds with 95% confidence were calculated based on the fit statistics. Virus loads in qPCR-positive samples were statistically compared between loads in the virus culture-positive and loads in virus culture-negative using Mann–Whitney U Test. Results were being considered significant for p < 0.05. All statistical analyses were performed with Matlab (R2020b, MathWorks, Natick, MA, USA).
3. Results
3.1. Detection of CPV by Real-Time PCR
In total, 46.6% (70/150) of all faecal samples showed positive qPCR results. In group H (healthy dogs), 24.0% (12/50), in group S (shelter dogs), 18.0% (9/50), and in group P (dogs suspected to have parvovirosis), 98.0% (49/50) of faecal samples were qPCR-positive. The mean CPV virus load was 2.4 × 106 copies/g faeces and 2.7 × 106 copies/g faeces for all qPCR-positive dogs in group H and S, respectively, and 5.3 × 1013 for all qPCR-positive dogs in group P.
3.2. Practicability of the Point-of-Care Tests
All eight POCT were easy to perform. In two POCT (Primagnost® Parvo H + K and FASTest® PARVO Card), faecal samples had to be collected using plastic spiral sticks that had to be introduced several times at various points in the faeces. The cotton swabs of the other tests had to be inserted only one time. There was no difference in the practicability of the tests. There were, however, some differences in the interpretation. The control fields of all POCT could always be clearly detected. Primagnost® Parvo H + K (POCT-C) and FASTest® PARVO Card (POCT-D) showed clear positive and negative results in all samples tested. However, the interpretation of some positive results in the other POCT was difficult due to very light test bands or points (Table 2): One WITNESS® Parvo (POCT-H), two IDEXX Snap® Parvo (POCT-A), two ImmunoRun® Parvovirus Antigen Detection Kit (POCT-G) tests results, three Fassisi® Parvo (POCT-B) test results, four Vetexpert Rapid Test CPV Ag® (POCT-E) test results, and five Anigen Rapid CPV Ag Test Kit® (POCT-F) test results were difficult to interpret.

Table 2.
Performance parameters of the eight point-of-care tests to detect canine parvovirus antigen in faeces when considering all 150 samples: tests which were difficult to interpret, sensitivity, specificity, positive and negative predictive values, as well as overall accuracy, were calculated using real-time polymerase chain reaction as gold standard.
3.3. Sensitivity, Specificity and Predictive Values of CPV by Point-of-Care Tests
The results of the sensitivity, specificity, predictive values and overall accuracy tested by the eight POCT compared to qPCR are shown in Table 2 and Table 3.

Table 3.
Results of eight canine parvovirus point-of-care tests of 150 faecal samples in comparison with real-time polymerase chain reaction as gold standard.
The specificity was excellent for all eight POCT (100.0% in all tests), as was the PPV (100.0% in all tests). There were no false positive results.
The Vetexpert Rapid Test CPV Ag® (POCT-E) and Anigen Rapid CPV Ag Test Kit® (POCT-F) had the highest sensitivity (34.3% and 32.9%, respectively) and highest NPV (63.5% and 63.0%, respectively). The Primagnost® Parvo H + K (POCT-C) and FASTest® PARVO Card (POCT-D) showed the lowest sensitivity (24.3% and 22.9%, respectively) and lowest NPV (60.2% and 59.7%, respectively).
The sensitivity and specificity of the POCT results within the three groups (healthy dogs, shelter dogs, CPV infection suspected dogs) are shown in Table 4. As none of the dogs of group H and S were tested positive in any of the POCT, all qPCR-positive faecal samples of group H, as well as all qPCR-positive faecal samples of group S were false negative in every POCT. In group P, the sensitivity of the POCT varied between 32.7% (FASTest® PARVO Card (POCT-D)) and 49.0% (Vetexpert Rapid Test CPV Ag® (POCT-E)).

Table 4.
Sensitivity and specificity of eight canine parvovirus point-of-care tests to detect canine parvovirus antigen in faeces in comparison with real-time polymerase chain reaction as gold standard for three groups (each 50 dogs).
3.4. Comparison of the Point-of-Care Tests
The differences among the POCT were found to be significant in group comparisons (p = 0.0003). The significant differences in sensitivities (shown in Table 5) were detected between the Vetexpert Rapid Test CPV Ag® (POCT-E) and Fassisi® Parvo (POCT-B) (McNemar’s p-value: 0.04), as well as between the Primagnost® Parvo H + K (POCT-C) (McNemar’s p-value: 0.02) and FASTest® PARVO Card (POCT-D) (McNemar’s p-value: 0.01). The sensitivity of the Anigen Rapid CPV Ag Test Kit® (POCT-F) was significantly different from that of the Primagnost® Parvo H + K (POCT-C) (McNemar’s p-value: 0.04) and FASTest® PARVO Card (POCT-D) (McNemar’s p-value: 0.02). The IDEXX Snap® Parvo (POCT-A) and FASTest® PARVO Card (POCT-D) were also found to differ significantly in sensitivity (McNemar’s p-value: 0.04).

Table 5.
Results of McNemar’s statistic to determine differences in sensitivity of the eight point-of-care tests detecting canine parvovirus antigen in faeces with overall consistency and kappa coefficent of the point-of-care test results in direct comparison.
The agreement of the POCT results is shown in Table 5. For the Fassisi® Parvo (POCT-B) and Anigen Rapid CPV Ag Test Kit® (POCT-F); the Primagnost® Parvo H + K (POCT-C) and Vetexpert Rapid Test CPV Ag® (POCT-E), as well as the FASTest® PARVO Card (POCT-D) and Anigen Rapid CPV Ag Test Kit® (POCT-F) the agreement was substantial. For all other POCT, the agreement was almost perfect with Kappa values higher of than 0.80. The overall consistency of the POCT results in pairwise comparison of the eight evaluated test kits varied between 94.7% and 99.3%.
3.5. Detection Probability and Virus Culture
A regression analysis showed a significant dependence on the detection probability of the eight POCT on the virus load with p values < 0.001. A decision threshold (in the number of virus copies/g faeces) for every POCT was calculated. The threshold identified the necessary amount of virus copies/g faeces to reach a detection probability of 50% (50% correctly identified positive faecal samples). Decision thresholds varied between 1.406 × 1012 virus copies/g faeces for the POCT with the highest sensitivity (Vetexpert Rapid Test CPV Ag® (POCT-E)) and 1.931 × 1013 for the POCT with the lowest sensitivity (FASTest® PARVO Card (POCT-D)). The curves of all POCT with decision thresholds are shown in Figure 1.
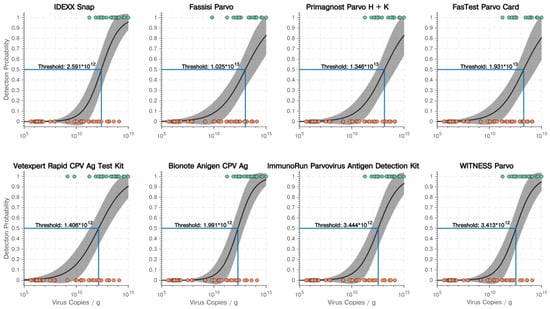
Figure 1.
Results of the regression analysis showing the dependence of the detection probability on the virus load with decision thresholds (detection probability of 50%) for all eight point-of-care tests. Green points indicate correct positive point-of-care test results, red points false negative point-of-care test results.
A viral replication in culture was not observed in any faecal sample of group H (healthy dogs) and S (shelter dogs). In group P (dogs suspected to have parvovirosis), faecal culture was positive in 36.0% (18/50) of the faecal samples, and thus in 36.7% of the qPCR-positive samples. The results of virus culture can be seen in Table S1 of the Supplementary Materials. The virus culture-positive faecal samples had significantly higher viral loads compared to those in virus culture-negative faecal samples (p < 0.05) (Figure 2).
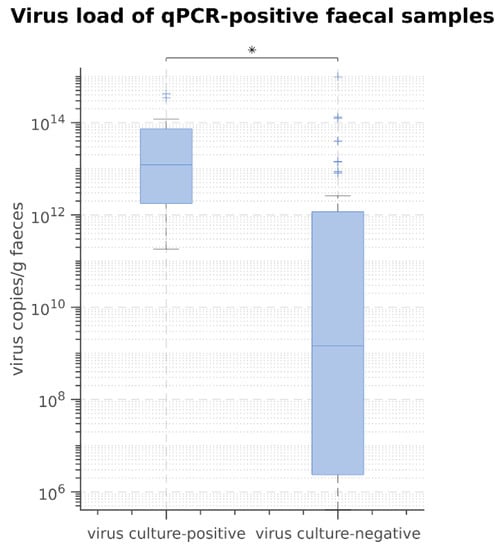
Figure 2.
Results of comparison of viral loads in qPCR-positive samples between virus culture-positive and virus culture-negative faecal samples using Mann–Whitney-U-Test. * p = 0.01.
Sensitivities of the eight POCT of qPCR-positive and culture-positive faecal samples were between 83.3 and 94.4%; those of qPCR-positive and culture-negative faecal samples were between 1.9 and 9.5% (Table 6).

Table 6.
Sensitivities of the eight point-of-care-tests in groups of real-time polymerase chain reaction-positive, virus culture-positive, real-time polymerase chain reaction-positive, and virus culture-negative faecal samples.
4. Discussion
CPV infection causes an acute and severe disease with a high mortality. Therefore, a fast and correct diagnosis is extremely important. The reference standard qPCR has to be performed in specialized laboratories and results are available generally no earlier than after a few days. Thus, POCT for in-house testing are an important measure to diagnose infected dogs immediately and directly at the veterinarian. Therefore, the aim of this study was to evaluate eight POCT regarding practicability, sensitivity, specificity, NPV, PPV, and overall accuracy when compared to qPCR.
The current study showed an excellent specificity of 100.0% of all eight POCT, with no false positive test results in all POCT. However, it has to be considered, that only dogs not vaccinated against CPV during the last four weeks, were included in groups H and S. A previous study showed the shedding of field and vaccination strains of CPV in 23.0% of dogs up to 28 days after modified-live vaccination, detected by qPCR [22]. This could lead to positive POCT results in the post-vaccination period, although in previous studies no positive POCT results after vaccination were observed despite high viral titres [14,17,23]. Three dogs of group P were vaccinated against CPV during the last four weeks. As described above, this could potentially have led to false positive test results in POCT and qPCR. All dogs of group P showed clinical signs of an infection with CPV. Consequently, it was assumed that these three dogs were not only shedding the vaccination virus but were infected with CPV and therefore tested correctly positive.
All eight POCT used in this study were easy to perform and practicability was comparable for all of them. However, the overall sensitivity of the POCT for all dogs (group H, S and P) was very low (between 22.9% and 34.3%) with the Vetexpert Rapid Test CPV Ag® (POCT-E) being the test with the highest sensitivity, and the FASTest® PARVO Card (POCT-D) being the test with the lowest sensitivity. For the test performance, sensitivity (correctly positive-identified faecal samples) was considered as the most important parameter to avoid virus spread by unrecognized infected dogs and to provide intensive treatment to every dog with a symptomatic CPV infection. It must be considered that the POCT were only performed once and not repeated on the same fecal sample which, however, is the normal situation in the field where POCT are not repeated. In former studies, the sensitivity of the POCT showed great variability with values between 15.8% and 80.4% [12,14,16,17]. The reasons for the variability of sensitivities might be differences in the virus load of the faecal samples. Some former studies [12,16] did not rule out the correlation between POCT results and virus load which limits the comparability. In the present study, the detection probability of all POCT depended on the virus load. The lower the number of virus copies/g faeces, the lower the detection probability of the POCT. Therefore, a correlation between false negative POCT results and low virus loads was clearly shown in the present study which was in line with the results from Decaro and colleagues (2010) [17] and Proksch and colleagues (2015) [15]. Proksch and colleagues (2015) [15] showed that 51.3% of the IDEXX Snap® Parvo test results compared to qPCR were false negative, with the false negative faecal samples in POCT having significantly lower viral loads. Decaro and colleagues (2010) [17] demonstrated that the IDEXX Snap® Parvo had a good sensitivity of 80.4%, 78.0% and 77.0% for the detection of CPV 2-a, -b and -c compared to qPCR. However, the relatively high sensitivity rate in that study was likely the result of the preselection of samples to only include those containing high viral loads (>108 DNA copies/g faeces). In the present study, the mean virus load in group H and S was 106 DNA copies/g faeces and consequently significantly lower than the one in the preselected samples of Decaro and colleagues (2010) [17]. All qPCR-positive faecal samples of groups H and S were tested as false negative in every POCT. Therefore, the present study confirmed that samples with low virus loads could not be detected by POCT, only by qPCR. However, it must be mentioned that none of the samples of group H and S contained intact and infectious CPV in virus culture.
The overall low sensitivity of all POCT in the present study was partially caused by the high number of false negative results in groups H and S, presumably due to low virus loads. The dogs from both groups were healthy and showed no gastrointestinal signs. Schmitz and colleagues (2009) [16] also evaluated POCT with faeces from different groups of dogs, including dogs with acute hemorrhagic diarrhea, chronic hemorrhagic diarrhea and without gastrointestinal signs. The sensitivities of the POCT in this study were comparable to the results of the present study, with 18.4% for the IDEXX Snap® Parvo, 15.8% for the FASTest® PARVO Strip and 26.3% for the WITNESS® Parvo card, and false negative POCT results also continuously occurred in the group of dogs without gastrointestinal signs, as well as in dogs with chronic haemorrhagaic diarrhea. The testing of clinically healthy animals was performed to identify asymptomatic virus shedders, for example in animal shelters [24]. The results of the present study showed that POCT were not useful at least in healthy dogs.
It was remarkable that 24.0% of the dogs in group H and 18.0% of the dogs in group S were qPCR-positive, respectively. None of these dogs were vaccinated against CPV within the last four weeks, and all dogs were healthy without any clinical signs. In a study by Freisl and colleagues (2017) [22], CPV shedding was also detected in healthy, client-owned dogs, but their prevalence was lower with 2.0%. Subclinical infections are the most likely reason for these positive PCR results. The role of subclinically infected dogs is not clear yet. Bergmann and colleagues (2019) [25] found FPV and CPV field virus DNA in clinically healthy cats. In that study, virus replication in virus culture showed that these cats shed an intact and infectious form of the virus and this is most likely possible for clinically asymptomatic dogs as well. With shedding infectious virus, these dogs might infect other susceptible animals and cause environmental contamination. However, in the present study, virus growth in virus cultures and, consequently, the excretion of the infectious field virus could not be detected for any of the asymptomatic dogs of group H and S. It must be considered that the qPCR detects viral DNA and not infectious viruses. Therefore, the role of positive qPCR results in asymptomatic dogs, as well as the importance of these dogs for environmental contamination should further be clarified in future studies.
In practice, dogs that are being tested by the veterinarian for CPV infection commonly show gastrointestinal signs. Thus, in the present study, group P represents the group, where POCT are most commonly used. In this group, 98.0% of the faecal samples were qPCR-positive and, consequently, these dogs suffered from a clinically relevant parvovirosis. The mean virus load of this group was 1013 DNA copies/g faeces and notably higher than that in groups H and S (106 DNA copies/g faeces). The results of the virus culture showed a growth of intact CPV in 36.0% of all faecal samples of group P. The sensitivities of the POCT using only samples from group P were between 32.7 and 49.0%. This is still much too low and comparable with the results of a study from Proksch and colleagues (2015) evaluating a POCT only with samples from dogs with confirmed parvovirosis [16].
A reason for the false negative POCT results in group P could be the time of testing. Experimental studies showed that CPV shedding started 3–4 days after inoculation [26,27] with the highest virus shedding 4–7 days after inoculation [5,26]. As qPCR was able to detect lower virus loads, infections in the early and late stages might only be recognized by qPCR, but not by POCT. Despite the high viral loads, some faecal samples of group P tested negative in all POCT. One possible explanation for this finding might be the consinstency of the faeces of these samples. Their texture was very bloody with significant mucous membrane particles which could cause detection problems. In addition, POCT detected virions, whereas qPCR detected DNA particles; therefore, infected cells might contain more DNA particles than virions [14]. Another reason for false negative POCT results could be high titres of interfering antibodies, which sequestrate viral antigen. Thus, the antigen might not be detected by POCT [15,17]. This could explain, why some faecal samples of group P in this study were not detected by any POCT in spite of high viral loads and positive faecal virus cultures.
One limitation is that all POC tests were only performed once. Thus, operating errors, which could have been minimized with multiple test runs, must be taken into consideration. Accordingly, the sensitivity and specificity of the POCT, as reported in the present study could be subject to inter-operator variability and could deviate in clinical practice depending on the exact operating conditions. Therefore, the sensitivities of the POCT determined in the present study represented only a general statement and no absolute rating. However, POCT were also generally not repeated in veterinary practice, and thus the present study mimics the practice situation in which the POCT are used. Larger clinical studies including the analysis of inter-operator and inter-reader variability would be useful to ascertain the clinical accuracy of the POCT.
5. Conclusions
In conclusion, all POCT were easy to perform and were suitable as screening tests in veterinary practices and clinics. A positive POCT result confirmed an infection with CPV in dogs not vaccinated against CPV within the last weeks. However, due to the low sensitivities and following false negative test results in all POCT, a negative POCT result could not definitely rule out a parvovirus infection. In this study, the sensitivity of all POCT in group P ranged from 32.7% to 49.0%. Based on these results, in the case of a negative POCT, a confirmatory PCR test should be performed in dogs showing clinical signs of CPV infection. In healthy dogs, POCT are not useful. Although the Vetexpert Rapid Test CPV Ag® (POCT-E) showed the highest sensitivity compared to the other POCT, the sensitivity of all POCT was too low and must be improved to avoid false negative test results. The qPCR was able to detect very small amounts of viral DNA and, consequently, detect subclinical infections. The importance of a positive qPCR result in clinically healthy dogs still has to be clarified.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13102080/s1, Table S1: Age and vaccination status of the 150 dogs, results of eight point-of-care tests, real-time polymerase chain reaction (incl. virus load/g faeces) and virus culture for detection of canine parvovirus of 150 faecal samples (three groups; group H: healthy dogs, group S: shelter dogs, group p: dogs with suspicion of parvovirosis).
Author Contributions
Conceptualization, K.H. and M.B.; methodology, K.H., M.B. and K.W.; formal analysis, J.W.-W.; investigation, J.W.-W., K.W. and U.T.; resources, K.H. and U.T.; data curation, J.W.-W. and C.M.; writing—original draft preparation, J.W.-W., M.B. and K.H.; writing—review and editing, K.H., M.B., K.W., C.M. and U.T.; visualization, J.W.-W.; supervision, K.H. and M.B.; project administration, K.H. and M.B.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
IDEXX Snap® Parvo, Fassisi® Parvo, Primagnost® Parvo H + K, FASTest® PARVO Card, Vetexpert Rapid Test CPV Ag®, Anigen Rapid CPV Ag Test Kit®, and WITNESS® Parvo were kindly provided for free by the companies. The companies played no role in the interpretation of data or in the decision to submit the manuscript for publication. Other than receiving the POC tests, this research received no external founding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the LMU Munich (protocol code 53-09-09-2015).
Informed Consent Statement
Informed consent statement was not necessary because only faecal samples were collected.
Data Availability Statement
The authors confirm that the datasets analyzed during the study are available from the first and corresponding author upon reasonable request.
Acknowledgments
We thank Dana Rüster from the Institute of Animal Hygiene and Veterinary Public Health, University of Leipzig, for processing of the samples by qPCR and virus culture. We thank IDEXX, Fassisi, Dechra, Megacor, Bionote, Vetexpert and Zoetis for providing the POCT for free.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article. Although the POCT were provided for free by IDEXX, Fassisi, Dechra, Megacor, Bionote, Vetexpert and Zoetis, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. There is no commercial conflict of interest as the information generated here is solely for scientific dissemination. The authors declare that they have no competing interests.
References
- Appel, M.; Scott, F.; Carmichael, L. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Vet. Rec. 1979, 105, 156. [Google Scholar] [CrossRef]
- Truyen, U. Evolution of canine parvovirus—a need for new vaccines? Vet. Microbiol. 2006, 117, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Truyen, U. Emergence and recent evolution of canine parvovirus. Vet. Microbiol. 1999, 69, 47–50. [Google Scholar] [CrossRef]
- Parrish, C.R.; Aquadro, C.F.; Strassheim, M.; Evermann, J.; Sgro, J.; Mohammed, H. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 1991, 65, 6544–6552. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.; Leisewitz, A.L. Canine parvovirus. Vet. Clin. Small Anim. Pract. 2010, 40, 1041–1053. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef]
- McCandlish, I.A.; Thompson, H.; Fisher, E.; Cornwell, H.; Macartney, J.; Walton, I. Canine parvovirus infection. Practice 1981, 3, 5–14. [Google Scholar] [CrossRef]
- Macintire, D.K.; Smith-Carr, S. Canine parvovirus. II. Clinical signs, diagnosis, and treatment. Compend. Contin. Educ. Pract. Vet. 1997, 19, 291–302. [Google Scholar]
- Sykes, J.E. Canine parvovirus infections and other viral enteritides. In Canine and Feline Infectious Diseases, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 141–151. [Google Scholar]
- Kalli, I.; Leontides, L.S.; Mylonakis, M.E.; Adamama-Moraitou, K.; Rallis, T.; Koutinas, A.F. Factors affecting the occurrence, duration of hospitalization and final outcome in canine parvovirus infection. Res. Vet. Sci. 2010, 89, 174–178. [Google Scholar] [CrossRef]
- Desario, C.; Decaro, N.; Campolo, M.; Cavalli, A.; Cirone, F.; Elia, G.; Martella, V.; Lorusso, E.; Camero, M.; Buonavoglia, C. Canine parvovirus infection: Which diagnostic test for virus? J. Virol. Methods 2005, 126, 179–185. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Desario, C.; Billi, M.; Lorusso, E.; Colaianni, M.; Colao, V.; Elia, G.; Ventrella, G.; Kusi, I.; Bo, S. Evaluation of an in-clinic assay for the diagnosis of canine parvovirus. Vet. J. 2013, 198, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Proksch, A.; Unterer, S.; Speck, S.; Truyen, U.; Hartmann, K. Influence of clinical and laboratory variables on faecal antigen ELISA results in dogs with canine parvovirus infection. Vet. J. 2015, 204, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Coenen, C.; Matthias, K.; Heinz-Jürgen, T.; Neiger, R. Comparison of three rapid commercial canine parvovirus antigen detection tests with electron microscopy and polymerase chain reaction. J. Vet. Diagn. Investig. 2009, 21, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Desario, C.; Beall, M.J.; Cavalli, A.; Campolo, M.; DiMarco, A.A.; Amorisco, F.; Colaianni, M.L.; Buonavoglia, C. Detection of canine parvovirus type 2c by a commercially available in-house rapid test. Vet. J. 2010, 184, 373–375. [Google Scholar] [CrossRef]
- Day, M.J.; Horzinek, M.; Schultz, R.; Squires, R. WSAVA guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1. [Google Scholar] [CrossRef]
- Streck, A.F.; Rüster, D.; Truyen, U.; Homeier, T. An updated TaqMan real-time PCR for canine and feline parvoviruses. J. Virol. Methods 2013, 193, 6–8. [Google Scholar] [CrossRef]
- O’Connor, T.P. SNAP assay technology. Top. Companion Anim. Med. 2015, 30, 132–138. [Google Scholar] [CrossRef][Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Freisl, M.; Speck, S.; Truyen, U.; Reese, S.; Proksch, A.-L.; Hartmann, K. Faecal shedding of canine parvovirus after modified-live vaccination in healthy adult dogs. Vet. J. 2017, 219, 15–21. [Google Scholar] [CrossRef]
- Decaro, N.; Crescenzo, G.; Desario, C.; Cavalli, A.; Losurdo, M.; Colaianni, M.L.; Ventrella, G.; Rizzi, S.; Aulicino, S.; Lucente, M.S. Long-term viremia and fecal shedding in pups after modified-live canine parvovirus vaccination. Vaccine 2014, 32, 3850–3853. [Google Scholar] [CrossRef]
- Appel, L.D.; Barr, S.C. Canine parvovirus and coronavirus. In Infectious Disease Management in Animal Shelters; Wiley-Blackwell Ames, IA: Hoboken, NJ, USA, 2009; pp. 197–208. [Google Scholar]
- Bergmann, M.; Schwertler, S.; Speck, S.; Truyen, U.; Reese, S.; Hartmann, K. Faecal shedding of parvovirus deoxyribonucleic acid following modified live feline panleucopenia virus vaccination in healthy cats. Vet. Rec. 2019, 185, 83. [Google Scholar] [CrossRef] [PubMed]
- Macartney, L.; McCandlish, I.; Thompson, H.; Cornwell, H. Canine parvovirus enteritis 2: Pathogenesis. Vet. Rec. 1984, 115, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.; Cooper, B.; Appel, M.; Slauson, D. Pathogenesis of canine parvovirus enteritis: The importance of viremia. Vet. Pathol. 1985, 22, 60–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).