Lower Respiratory Tract Infection and Genus Enterovirus in Children Requiring Intensive Care: Clinical Manifestations and Impact of Viral Co-Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Specimen Collection and Microbiological Diagnosis
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Lauinger, I.L.; Bible, J.M.; Halligan, E.P.; Bangalore, H.; Tosas, O.; Aarons, E.J.; MacMahon, E.; Tong, C.Y. Patient characteristics and severity of human rhinovirus infections in children. Clin. Virol. 2013, 58, 216–220. [Google Scholar] [CrossRef]
- Galanti, M.; Birger, R.; Ud-Dean, M.; Filip, I.; Morita, H.; Comito, D.; Anthony, S.; Freyer, G.A.; Ibrahim, S.; Lane, B.; et al. Longitudinal active sampling for respiratory viral infections across age groups. Influenza Other Respir. Viruses 2019, 13, 226–232. [Google Scholar] [CrossRef]
- Meskill, S.D.; O’Bryant, S.C. Respiratory virus co-infection in acute respiratory infections in children. Curr. Infect. Dis. Rep. 2020, 22, 3. [Google Scholar] [CrossRef]
- Douros, K.; Everard, M.L. Time to Say Goodbye to Bronchiolitis, Viral Wheeze, Reactive Airways Disease, Wheeze Bronchitis and All That. Front. Pediatr. 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Babady, N.E. The FilmArray® respiratory panel: An automated, broadly multiplexed molecular test for the rapid and accurate detection of respiratory pathogens. Expert. Rev. Mol. Diagn. 2013, 13, 779–788. [Google Scholar] [CrossRef]
- Casas, I.; Pozo, F.; Trallero, G.; Echevarría, J.M.; Tenoria, A. Viral diagnosis of neurological infection by RT multiplex PCR: A search for entero- and herpesviruses in a prospective study. J. Med. Virol. 1999, 57, 145–151. [Google Scholar] [CrossRef]
- Chauhan, J.C.; Slamon, N.B. The impact of multiple viral respiratory infections on outcomes for critically ill children. Pediatr. Crit. Care Med. 2017, 18, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; van der Ploeg, T.; de Groot, R.; Versteegh, F.G.; Hartwig, N.G. Single and multiple viral respiratory infections in children: Disease and management cannot be related to a specific pathogen. BMC Infect. Dis. 2017, 17, 62. [Google Scholar] [CrossRef]
- Scotta, M.C.; Chakr, V.C.; de Moura, A.; Becker, R.G.; de Souza, A.P.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Ma, J.; Zhang, J.; Hu, Y.; Dong, W.; Wan, Z.; Li, Q.; Kuang, Y.Q.; Lan, K.; et al. Altered respiratory virome and serum cytokine profile associated with recurrent respiratory tract infections in children. Nat. Commun. 2019, 10, 2288. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.; van der Meer, J.; Mhlanga, M.M.; Mulder, W.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, J.; Englund, J.A.; Magaret, A.S.; Bu, Y.; Tielsch, J.M.; Khatry, S.K.; Katz, J.; Kuypers, J.; Shrestha, L.; LeClerq, S.C.; et al. Primary and Repeated Respiratory Viral Infections Among Infants in Rural Nepal. J. Pediatric Infect. Dis. Soc. 2020, 9, 21–29. [Google Scholar] [CrossRef]
- Asner, S.A.; Rose, W.; Petrich, A.; Richardson, S.; Tran, D.J. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin. Microbiol. Infect. 2015, 21, 264.e1–264.e6. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Piedra, P.A.; Teach, S.J.; Sullivan, A.F.; Forgey, T.; Clark, S.; Espinola, J.A.; Camargo, C.A., Jr. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch. Pediatr. Adolesc. Med. 2012, 166, 700–706. [Google Scholar] [CrossRef]
- Lee, K.H.; Gordon, A.; Foxman, B. The role of respiratory viruses in the etiology of bacterial pneumonia: An ecological perspective. Evol. Med. Public Health 2016, 2016, 95–109. [Google Scholar] [CrossRef]
- van den Bergh, M.R.; Biesbroek, G.; Rossen, J.W.; de Steenhuijsen Piters, W.A.; Bosch, A.A.; van Gils, E.J.; Wang, X.; Boonacker, C.W.; Veenhoven, R.H.; Bruin, J.P.; et al. Associations between pathogens in the upper respiratory tract of young children: Interplay between viruses and bacteria. PLoS ONE 2012, 7, e47711. [Google Scholar] [CrossRef]
- Korten, I.; Mika, M.; Klenja, S.; Kieninger, E.; Mack, I.; Barbani, M.T.; Gorgievski, M.; Frey, U.; Hilty, M.; Latzin, P. Interactions of respiratory viruses and the nasal microbiota during the first year of life in healthy infants. mSphere 2016, 1, e00312–e00316. [Google Scholar] [CrossRef] [PubMed]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- de Steenhuijsen Piters, W.A.; Heinonen, S.; Hasrat, R.; Bunsow, E.; Smith, B.; Suarez-Arrabal, M.C.; Chaussabel, D.; Cohen, D.M.; Sanders, E.A.; Ramilo, O.; et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am. J. Respir. Crit. Care Med. 2016, 194, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Bont, L.; Heijnen, C.J.; Kavelaars, A.; van Aalderen, W.M.; Brus, F.; Draaisma, J.M.; Pekelharing-Berghuis, M.; van Diemen-Steenvoorde, R.A.; Kimpen, J.L. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J. Infect. Dis. 2001, 184, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Johnston, S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J. Biol. Chem. 1999, 274, 9707–9720. [Google Scholar] [CrossRef]
- Jartti, T.; Jartti, L.; Peltola, V.; Waris, M.; Ruuskanen, O. Identification of respiratory viruses in asymptomatic subjects: Asymptomatic respiratory viral infections. Pediatr. Infect. Dis. J. 2008, 27, 1103–1107. [Google Scholar] [CrossRef]
- van der Zalm, M.M.; van Ewijk, B.E.; Wilbrink, B.; Uiterwaal, C.S.; Wolfs, T.F.W.; van der Ent, C.K. Respiratory pathogens in children with and without respiratory symptoms. J. Pediatr. 2009, 154, 396–400.e1. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.R.; Pitrez, M.C.; Arruda, E.; Mattiello, R.; Sarria, E.E.; de Paula, F.E.; Proença-Modena, J.L.; Delcaro, L.S.; Cintra, O.; Jones, M.H.; et al. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: Viral etiology and co-detection as risk factors. BMC Infect. Dis. 2013, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Gern, J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Lukkarinen, M.; Koistinen, A.; Turunen, R.; Lehtinen, P.; Vuorinen, T.; Jartti, T. Rhinovirus-induced first wheezing episode predicts atopic but not non atopic asthma at school age. J. Allergy Clin. Immunol. 2017, 140, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Codez, M.I.; Moyer, K.; Benavente-Fernández, I.; Leber, A.L.; Ramilo, O.; Mejias, A. Viral Loads and Disease Severity in Children with Rhinovirus-Associated Illnesses. Viruses 2021, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Launes, C.; Armero, G.; Anton, A.; Hernandez, L.; Gimferrer, L.; Cisneros, C.; Jordan, I.; Muñoz-Almagro, C. Molecular epidemiology of severe respiratory disease by human rhinoviruses and enteroviruses at a tertiary paediatric hospital in Barcelona, Spain. Clin. Microbiol. Infect. 2015, 21, 799.e5–799.e7. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Casas, I.; García-García, M.L.; Pozo, F.; Reyes, N.; Cruz, N.; García-Cuenllas, L.; Pérez-Breña, P. Role of rhinovirus C respiratory infections in sick an healthy children in Spain. Pediatr. Infect. Dis. J. 2010, 29, 717–720. [Google Scholar] [CrossRef]
- Winther, B.; Hayden, F.G.; Hendley, J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J. Med. Virol. 2006, 78, 644–650. [Google Scholar] [CrossRef]
- Linder, J.E.; Kraft, D.C.; Mohamed, Y.; Lu, Z.; Heil, L.; Tollefson, S.; Saville, B.R.; Wright, P.F.; Williams, J.V.; Miller, E.K. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J. Allergy Clin. Immunol. 2013, 131, 69–77. [Google Scholar] [CrossRef]
- Hung, H.M.; Yang, S.L.; Chen, C.J.; Chiu, C.H.; Kuo, C.Y.; Huang, K.A.; Lin, T.Y.; Hsieh, Y.C.; Gong, Y.N.; Tsao, K.C.; et al. Molecular epidemiology and clinical features of rhinovirus infections among hospitalized patients in a medical center in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 233–241. [Google Scholar] [CrossRef]
- Caylan, E.; Weinblatt, E.; Welter, J.; Dozor, A.; Wang, G.; Nolan, S.M. Comparison of the Severity of Respiratory Disease in Children Testing Positive for Enterovirus D68 and Human Rhinovirus. J. Pediatr. 2018, 197, 147–153.e1. [Google Scholar] [CrossRef]
- González-Sanz, R.; Taravillo, I.; Reina, J.; Navascués, A.; Moreno-Docón, A.; Aranzamendi, M.; Romero, M.P.; Del Cuerpo, M.; Pérez-González, C.; Pérez-Castro, S.; et al. Enterovirus D68-associated respiratory and neurological illness in Spain, 2014–2018. Emerg. Microbes Infect. 2019, 8, 1438–1444. [Google Scholar] [CrossRef]
- Cox, D.W.; Khoo, S.K.; Zhang, G.; Lindsay, K.; Keil, A.D.; Knight, G.; Gern, J.E.; Laing, I.A.; Bizzintino, J.; Le Souëf, P.N. Rhinovirus is the most common virus and rhinovirus-C is the most common species in paediatric intensive care respiratory admissions. Eur. Respir. J. 2018, 52, 1800207. [Google Scholar] [CrossRef]
- Erkkola, R.; Turunen, R.; Räisänen, K.; Waris, M.; Vuorinen, T.; Laine, M.; Tähtinen, P.; Gern, J.E.; Bochkov, Y.A.; Ruohola, A.; et al. Rhinovirus C is associated with severe wheezing and febrile respiratory illness in young children. Pediatr. Infect. Dis. J. 2020, 39, 283–286. [Google Scholar] [CrossRef]
- Iwane, M.K.; Prill, M.M.; Lu, X.; Miller, E.K.; Edwards, K.M.; Hall, C.B.; Griffin, M.R.; Staat, M.A.; Anderson, L.J.; Williams, J.V.; et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J. Infect. Dis. 2011, 204, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Koenig-Zores, C.; Stoll-Keller, F.; Ammouche, C.; Donato, L. Does the nasopharyngeal samples virological analysis reflect the lower respiratory tract infection in children population? A PCR multiplex study. Rev. Fr. Allergol. 2013, 53, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Fu, W.; Park, D.E.; Shi, Q.; Higdon, M.M.; Baggett, H.C.; Brooks, W.A.; Deloria Knoll, M.; Hammitt, L.L.; Howie, S.; et al. Is Higher Viral Load in the Upper Respiratory Tract Associated with Severe Pneumonia? Findings From the PERCH Study. Clin. Infect. Dis. 2017, 64, S337–S346. [Google Scholar] [CrossRef] [PubMed]
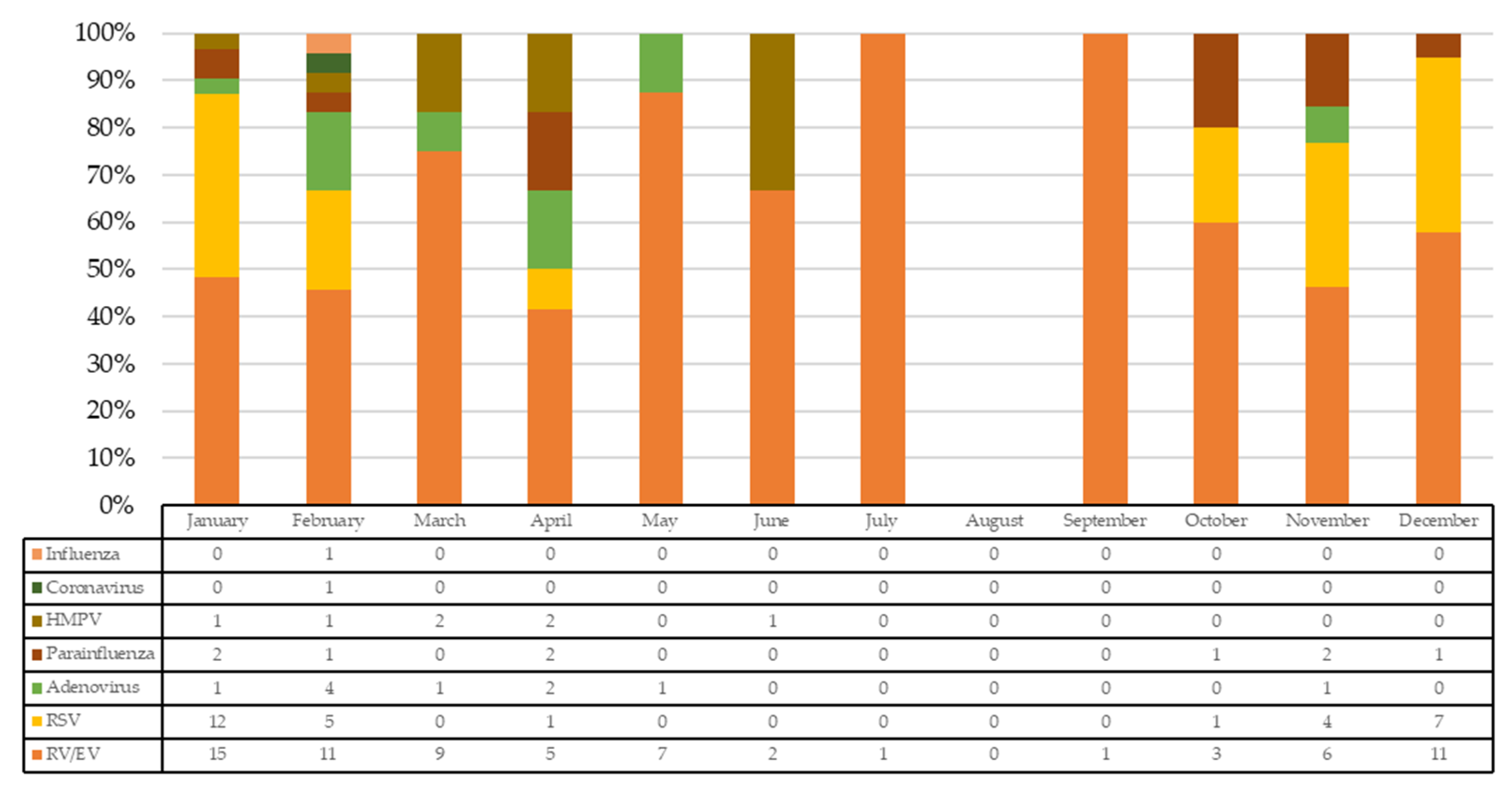
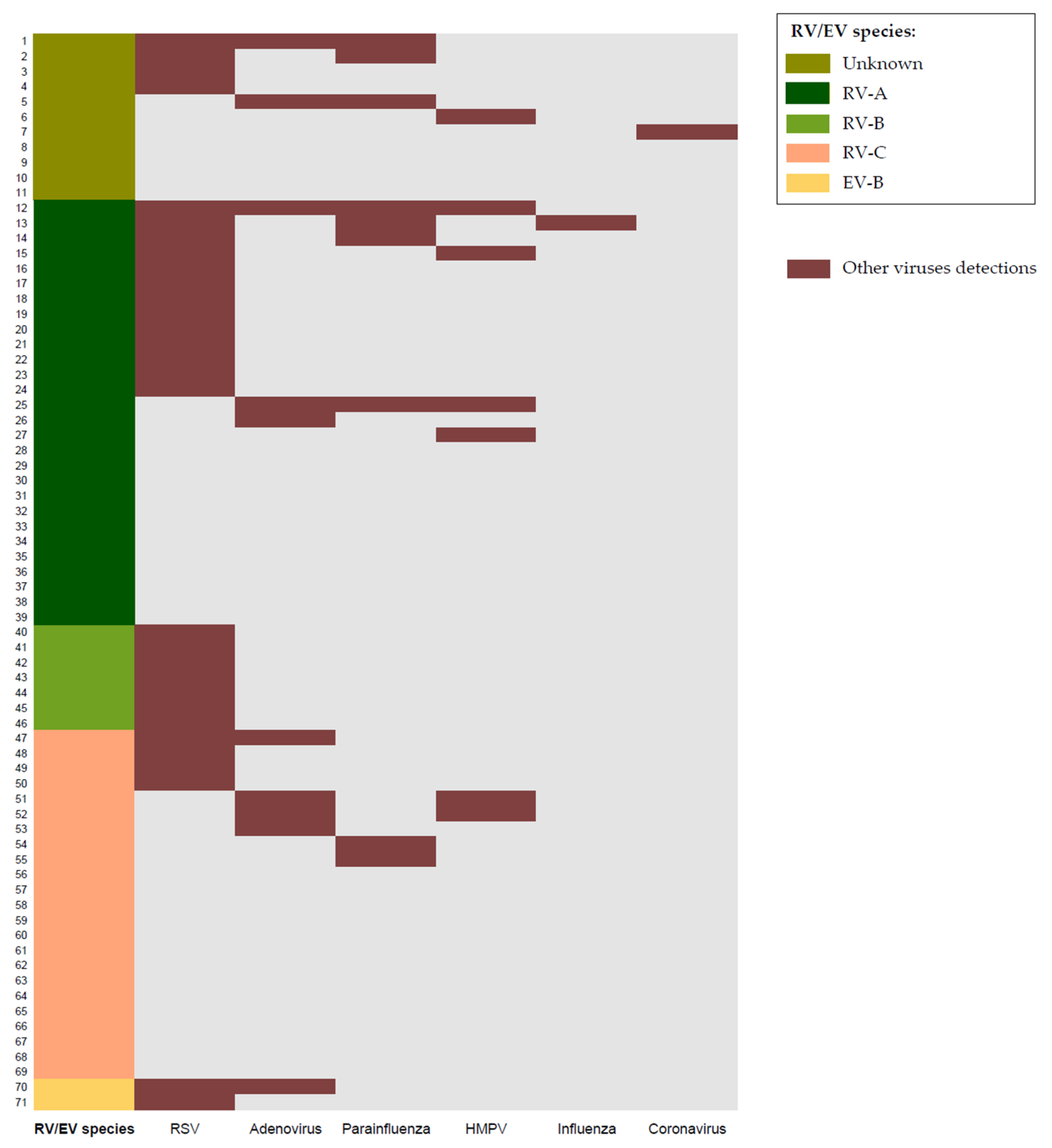
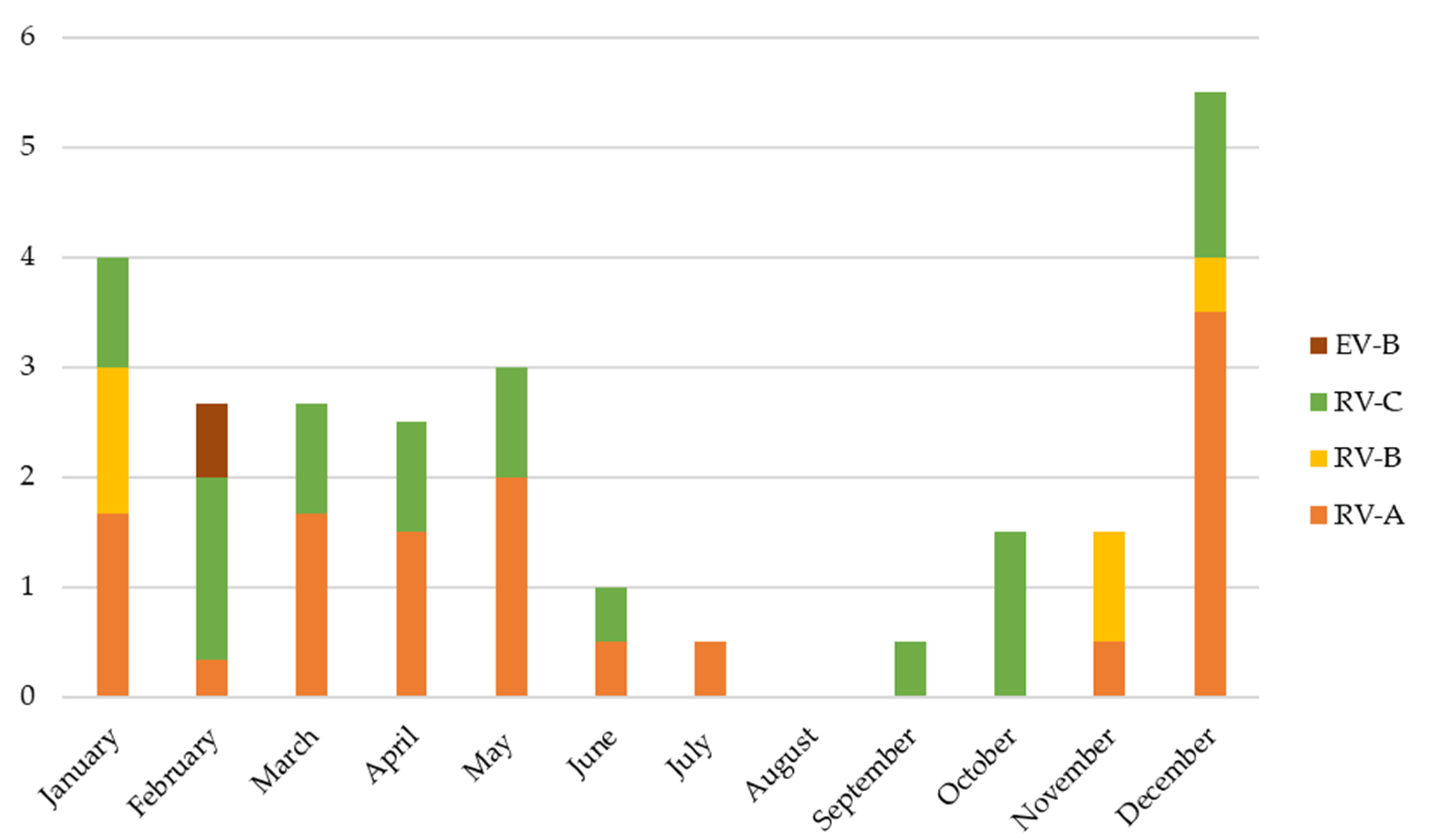
| Variables | Total n = 71 | RV/EV n = 31 | RV/EV + RSV Codetection n = 22 | RV/EV + Multiple Viral Codetection n = 18 | p-Value * |
|---|---|---|---|---|---|
| Sex (male), n (%) | 40 (56%) | 17 (55%) | 16 (73%) | 7 (39%) | 0.097 |
| Age (months), median (IQR) | 2.1 (1.2–9.3) | 1.7 (0.7–8.1) | 1.8 (1.0–2.7) | 7.9 (2.2–22.7) | 0.018 |
| Race (Caucasian), n (%) | 51 (78%) | 20 (64%) | 19 (86%) | 12 (67%) | 0.187 |
| Breastfeeding, n (%) | 57 (80%) | 27 (87%) | 17 (77%) | 13 (72%) | 0.412 |
| Parental smoking, n (%) | 23 (32%) | 12 (39%) | 7 (33%) | 4 (21%) | 0.492 |
| Household contacts, n (%) | 4 (4–4) | 4 (4–4) | 4 (3–5) | 4 (4–4) | 0.926 |
| Recurrent wheezing, n (%) | 28 (40%) | 11 (37%) | 4 (18%) | 13 (72%) | 0.002 |
| Fever, n (%) | 30 (42%) | 9 (29%) | 9 (41%) | 12 (67%) | 0.036 |
| Days with symptoms before PICU admission, median (IQR) | 3 (1–5) | 2 (1–4) | 3 (2–5) | 5 (3–6) | 0.003 |
| Length of PICU stay (days), median (IQR) | 5 (3–9) | 6 (3–9) | 8 (4–12) | 3 (2–5) | 0.004 |
| Hospital stay (days), median (IQR) | 11 (9–18) | 12 (9–20) | 13 (10–17) | 9 (6–11) | 0.028 |
| Chest X-ray (total n = 60) | 0.572 | ||||
| Normal, n (%) | 17 (28%) | 8 (32%) | 5 (29%) | 4 (22%) | |
| Chest X-ray opacities 1 quadrant, n (%) | 18 (30%) | 10 (40%) | 4 (23%) | 5 (28%) | |
| Chest X-ray opacities > 1 quadrant, n (%) | 24 (40%) | 7 (28%) | 8 (47%) | 9 (50%) | |
| NIVM, n (%) | 67 (94%) | 29 (93%) | 21 (95%) | 17 (94%) | 0.957 |
| IMV, n (%) | 17 (24%) | 9 (29%) | 8 (36%) | 0 (0%) | 0.019 |
| Days of MV, median (IQR) | 4 (3–8) | 4 (2–7) | 8 (3–11) | 3 (1–4) | 0.002 |
| Total white blood cell count (cells × 109/L), median (IQR) | 11.6 (8.2–16.4) | 11.8 (8.2–17.1) | 9.4 (6.8–13.4) | 12.9 (9.0–19.3) | 0.265 |
| Neutrophils (cells × 109/L), median (IQR) | 5.2 (2.7–8.3) | 5.2 (2.5–8.1) | 4.4 (1.9–8.5) | 5.4 (3.8–8.0) | 0.516 |
| C-RP (mg/L), median (IQR) | 33.9 (13.2–66.9) | 37 (6.9–73) | 30 (15–52) | 34 (18–71) | 0.962 |
| PCT (ng/mL), median (IQR) | 0.33 (0.14–1.67) | 0.40 (0.12–1.08) | 0.22 (0.19–2.78) | 0.54 (0.13–2.13) | 0.770 |
| Suspected bacterial pneumonia criteria, n (%) | 14 (23%) | 5(20%) | 3 (18%) | 6 (33%) | 0.480 |
| RV/EV species: | |||||
| -RV-A | 28 | 12 (39%) | 9 (41%) | 7 (39%) | 0.986 |
| -RV-B | 7 | 0 (0%) | 7 (32%) | 0 (0%) | <0.001 |
| -RV-C | 23 | 14 (45%) | 3 (14%) | 6 (33%) | 0.054 |
| -EV-B | 2 | 0 (0%) | 1 (4%) | 1 (6%) | 0.442 |
| -Unknown | 11 | 5 (16%) | 2 (9%) | 4 (22%) | 0.517 |
| Variables | RV+ RSV– (n = 41) | RV+ RSV+ (n = 30) | p-Value * | RV + RSV as the Sole Codetection (n = 22) | RV + RSV in Codetection with Other Viruses (n = 8) | p-Value * |
|---|---|---|---|---|---|---|
| Age (months), median (IQR) | 2.9 (1.2–13.1) | 1.6 (1.1–5-5) | 0.625 | 1.9 (1.0–2.7) | 8.7 (1.7–18.6) | 0.090 |
| Recurrent wheezing, n (%) | 9 (22.5%) | 6 (20%) | 0.801 | 2 (9%) | 4 (26%) | 0.029 |
| Fever, n (%) | 16 (39%) | 14 (47%) | 0.520 | 9 (41%) | 5 (62%) | 0.417 |
| Days with symptoms before PICU admission, median (IQR) | 2 (1–4) | 3 (2–5) | 0.006 | 3 (2–5) | 6 (4–11) | 0.008 |
| Length of PICU stay (days), median (IQR) | 5 (2–8) | 5 (3–9) | 0.512 | 7.5 (4.7–12.0) | 3 (2.2–3.7) | <0.001 |
| Hospital stay (days), median (IQR) | 11.5 (8–18) | 11 (9–15) | 0.863 | 13 (10.0–17.2) | 9.5 (6.7–10.7) | 0.060 |
| IMV, n (%) | 9 (22%) | 8 (27%) | 0.646 | 8 (36%) | 0 (0%) | 0.046 |
| Days of MV, median (IQR) | 4 (2–6) | 5 (3–9) | 0.267 | 8 (3–11) | 3 (1–3) | 0.002 |
| Univariate Analysis | Multivariable Analysis * | Univariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | PICU Stay > 5 d (n = 33) | PICU Stay ≤ 5 d (n = 38) | p-Value ** | Adjusted Odds-Ratio | p-Value | IMV (n = 17) | NIMV (n = 54) | p-Value ** |
| Sex (male), n (%) | 20 (61%) | 20 (53%) | 0.499 | 10 (56%) | 30 (56%) | 0.813 | ||
| Age (months), median (IQR) | 2.2 (1.2–5.6) | 2.1 (0.9–14.8) | 0.836 | 1.8 (1.0–9.9) | 2.1 (1.2–10.3) | 0.652 | ||
| Recurrent wheezing, n (%) | 10 (30%) | 18 (47%) | 0.170 | 6 (35%) | 22 (41%) | 0.649 | ||
| Race: | ||||||||
| Caucasian, n (%) | 25 (76%) | 26 (68%) | 0.493 | 13 (76%) | 38 (70%) | 0.625 | ||
| Others, n (%) | 8 (24%) | 8 (32%) | 4 (24%) | 16 (30%) | ||||
| Fever, n (%) | 15 (45%) | 15 (39%) | 0.611 | 8 (47%) | 22 (41%) | 0.646 | ||
| Chest X-ray at hospital admission (total n = 60) | ||||||||
| Normal, n (%) | 4 (15%) | 13 (38%) | 0.110 | 1 (8%) | 16 (33%) | 0.183 | ||
| Chest X-ray opacities 1 quadrant, n (%) | 11 (42%) | 8 (23%) | 4 (33%) | 15 (31%) | ||||
| Chest X-ray opacities > 1 quadrant, n (%) | 11 (42%) | 13 (38%) | 7 (58%) | 17 (35%) | ||||
| Viral detections: | ||||||||
| RV/EV, n (%) | 16 (48%) | 15 (39%) | 0.445 | - | - | 9 (53%) | 22 (41%) | 0.376 |
| RV/EV + RSV, n (%) | 14 (42%) | 8 (21%) | 0.052 | 1.64 (0.54–5.02) | 0.386 | 8 (47%) | 14 (26%) | 0.101 |
| RV/EV + Multiple viral codetection, n (%) | 3 (9%) | 15 (40%) | 0.003 | 0.19 (0.05–0.78) | 0.021 | 0 (0%) | 18 (33%) | 0.004 |
| RV/EV species | ||||||||
| RV A, n (%) | 14 (42%) | 14 (37%) | 0.631 | 6 (35%) | 22 (41%) | 0.689 | ||
| RV B, n (%) | 4 (12%) | 3 (8%) | 0.697 | 1 (6%) | 6 (11%) | 0 | ||
| RV C, n (%) | 8 (24%) | 15 (39%) | 0.171 | 5 (29%) | 18 (33%) | 0.763 | ||
| EV B, n (%) | 1 (3%) | 1 (3%) | 1 | 1 (6%) | 1 (2%) | 0.424 | ||
| Unknown, n (%) | 6 (18%) | 5 (13%) | 0.560 | 4 (24%) | 7 (13%) | 0.441 | ||
| Total white blood cell count (cells × 109/L), median (IQR) | 10,500 (7425–15,825) | 12,000 (8600–17,350) | 0.296 | 9300 (6600–15,400) | 11,750 (8925–17,000) | 0.316 | ||
| Neutrophils (cells × 109 /L), median (IQR) | 4490 (2675–8375) | 5450 (3850–7850) | 0.494 | 4500 (1800–9000) | 5200 (3800–7700) | 0.794 | ||
| C-RP (mg/L), median (IQR) | 38 (21–64) | 30 (11–72) | 0.302 | 32 (12–63) | 35 (13–66) | 0.946 | ||
| PCT (ng/mL), median (IQR) | 0.35 (0.19–1.49) | 0.20 (0.08–2.08) | 0.328 | 0.22 (0.18–2.78) | 0.35 (0.12–1.34) | 0.872 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penela-Sánchez, D.; González-de-Audicana, J.; Armero, G.; Henares, D.; Esteva, C.; de-Sevilla, M.-F.; Ricart, S.; Jordan, I.; Brotons, P.; Cabrerizo, M.; et al. Lower Respiratory Tract Infection and Genus Enterovirus in Children Requiring Intensive Care: Clinical Manifestations and Impact of Viral Co-Infections. Viruses 2021, 13, 2059. https://doi.org/10.3390/v13102059
Penela-Sánchez D, González-de-Audicana J, Armero G, Henares D, Esteva C, de-Sevilla M-F, Ricart S, Jordan I, Brotons P, Cabrerizo M, et al. Lower Respiratory Tract Infection and Genus Enterovirus in Children Requiring Intensive Care: Clinical Manifestations and Impact of Viral Co-Infections. Viruses. 2021; 13(10):2059. https://doi.org/10.3390/v13102059
Chicago/Turabian StylePenela-Sánchez, Daniel, Jon González-de-Audicana, Georgina Armero, Desiree Henares, Cristina Esteva, Mariona-Fernández de-Sevilla, Silvia Ricart, Iolanda Jordan, Pedro Brotons, María Cabrerizo, and et al. 2021. "Lower Respiratory Tract Infection and Genus Enterovirus in Children Requiring Intensive Care: Clinical Manifestations and Impact of Viral Co-Infections" Viruses 13, no. 10: 2059. https://doi.org/10.3390/v13102059
APA StylePenela-Sánchez, D., González-de-Audicana, J., Armero, G., Henares, D., Esteva, C., de-Sevilla, M.-F., Ricart, S., Jordan, I., Brotons, P., Cabrerizo, M., Muñoz-Almagro, C., & Launes, C. (2021). Lower Respiratory Tract Infection and Genus Enterovirus in Children Requiring Intensive Care: Clinical Manifestations and Impact of Viral Co-Infections. Viruses, 13(10), 2059. https://doi.org/10.3390/v13102059






