Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Virus Quantification
2.4. Antiviral Compounds
2.5. Determination of Effective Concentration 50 (EC50) for Fluoroquinolones against SARS-CoV-2 and MERS-CoV
2.6. Anti-SARS-CoV-2 Activity of Fluoroquinolones in Human Lung Cells that Overexpress ACE2 Receptor
2.7. Cellular Toxicity of Fluoroquinolones in Vero and A549 Cells
3. Results
3.1. High Micromolar Concentrations of Enoxacin Suppress SARS-CoV-2 and MERS-CoV Replication in Vero and A549/ACE2 Cells
3.2. High Micromolar Concentrations of Ciprofloxacin Suppress SARS-CoV-2 Replication
3.3. High Micromolar Concentrations of Levofloxacin Suppress SARS-CoV-2 Replication
3.4. High Micromolar Concentrations of Moxifloxacin Suppresses SARS-CoV-2 Replication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536. [CrossRef] [PubMed]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef]
- Zaki, A.; van Boheemen, S.; Bestebroer, T.; Osterhaus, A.; Fouchier, R. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020. [CrossRef]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; van Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; López, J.R.A.; Cattelan, A.M.; Viladomiu, A.S.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A. Faculty Opinions recommendation of Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. Fac. Opin. Post-Public. Peer Rev. Biomed. Lit. 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 1 November 2020).
- Wolfson, J.S.; Hooper, D.C. The fluoroquinolones: Structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob. Agents Chemother. 1985, 28, 581–586. [Google Scholar] [CrossRef]
- Scroggs, S.L.; Andrade, C.C.; Chinnasamy, R.; Azar, S.R.; Schirtzinger, E.E.; Garcia, E.I.; Arterburn, J.B.; Hanley, K.A.; Rossi, S.L. Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses. Viruses 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Scroggs, S.L.; Gass, J.T.; Chinnasamy, R.; Widen, S.G.; Azar, S.R.; Rossi, S.L.; Arterburn, J.B.; Vasilakis, N.; Hanley, K.A. Evolution of resistance to fluoroquinolones by dengue virus serotype 4 provides insight into mechanism of action and consequences for viral fitness. Virology 2021, 552, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.-R.; et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Siddiqui, S.; Rehmani, S.; Kazmi, S.U.; Ali, S.H. Fluoroquinolones inhibit HCV by targeting its helicase. Antivir. Ther. 2012, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, M.; Nishimura, H.; Hatachi, Y.; Yasuda, H.; Deng, X.; Sasaki, T.; Mizuta, K.; Kubo, H.; Nagatomi, R. Levofloxacin Inhibits Rhinovirus Infection in Primary Cultures of Human Tracheal Epithelial Cells. Antimicrob. Agents Chemother. 2012, 56, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Gilles, M.; Barral, K.; Nougairède, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Kumar, D.; Chandel, V.; Raj, S.; Rathi, B. In silico identification of potent FDA approved drugs against Coronavirus COVID-19 main protease: A drug repurposing approach. Chem. Biol. Lett. 2020, 7, 166–175. [Google Scholar]
- Marciniec, K.; Beberok, A.; Pęcak, P.; Boryczka, S.; Wrześniok, D. Ciprofloxacin and moxifloxacin could interact with SARS-CoV-2 protease: Preliminary in silico analysis. Pharmacol. Rep. 2020, 1–9. [Google Scholar] [CrossRef]
- Karampela, I.; Dalamaga, M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch. Med. Res. 2020, 57, 741–742. [Google Scholar] [CrossRef]
- Mercorelli, B.; Palù, G.; Loregian, A. Drug Repurposing for Viral Infectious Diseases: How Far Are We? Trends Microbiol. 2018, 26, 865–876. [Google Scholar] [CrossRef]
- Lewy, T.G.; Offerdahl, D.K.; Grabowski, J.M.; Kellman, E.M.; Mlera, L.; Chiramel, A.I.; Bloom, M.E. PERK-Mediated Unfolded Protein Response Signaling Restricts Replication of the Tick-Borne Flavivirus Langat Virus. Viruses 2020, 12, 328. [Google Scholar] [CrossRef]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Emanuel, J.; Callison, J.; McNally, K.L.; Arndt, N.; Chadinha, S.; Martellaro, C.; Rosenke, R.; Scott, D.P.; Safronetz, D.; et al. Lethal Zika Virus Disease Models in Young and Older Interferon α/β Receptor Knock Out Mice. Front. Cell. Infect. Microbiol. 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Jan, J.-T.; Chen, C.-M.; Hsieh, H.-P.; Hwang, D.-R.; Liu, H.-W.; Liu, C.-Y.; Huang, H.-W.; Chen, S.-C.; Hong, C.-F.; et al. Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication by Niclosamide. Antimicrob. Agents Chemother. 2004, 48, 2693–2696. [Google Scholar] [CrossRef]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mösbauer, K.; Zellner, A.; et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Desmyter, J.; Melnick, J.L.; Rawls, W.E. Defectiveness of Interferon Production and of Rubella Virus Interference in a Line of African Green Monkey Kidney Cells (Vero). J. Virol. 1968, 2, 955–961. [Google Scholar] [CrossRef]
- Emeny, J.M.; Morgan, M.J. Regulation of the Interferon System: Evidence that Vero Cells have a Genetic Defect in Interferon Production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef]
- Owens, R.C.; Ambrose, P.G. Antimicrobial safety: Focus on fluoroquinolones. Clin. Infect. Dis. 2005, 41, S144–S157. [Google Scholar] [CrossRef]
- Hooper, D.C.; Wolfson, J.S. The fluoroquinolones: Pharmacology, clinical uses, and toxicities in humans. Antimicrob. Agents Chemother. 1985, 28, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Keynan, O.; Feldbrin, Z.; Poch, F. Concentrations of moxifloxacin in serum and synovial fluid, and ex vivo bactericidal activity against arthritis-causing pathogens. Diagn. Microbiol. Infect. Dis. 2004, 48, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Soman, A.; Honeybourne, D.; Andrews, J.; Jevons, G.; Wise, R. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 1999, 44, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Bartosik-Wich, B.; Sörgel, F.; Gutzler, F. In vitro activity, pharmacokinetics, clinical safety and therapeutic efficacy of enoxacin in the treatment of patients with complicated urinary tract infections. Infection 1985, 13, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bergan, T.; Thorsteinsson, S.B.; Solberg, R.; Bjornskau, L.; Kolstad, I.M.; Johnsen, S. Pharmacokinetics of ciprofloxacin: Intravenous and increasing oral doses. Am. J. Med. 1987, 82, 97–102. [Google Scholar] [PubMed]
- Davis, R.L.; Koup, J.R.; Williams-Warren, J.; Weber, A.; Smith, A.L. Pharmacokinetics of three oral formulations of ciprofloxacin. Antimicrob. Agents Chemother. 1985, 28, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A. Immunomodulatory Activities of Fluoroquinolones. Infection 2005, 33, 55–70. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.K.; Wu, A.K.L.; Ip, W.K.; Lee, N.L.S.; Chan, I.H.S.; Lit, L.C.W.; Hui, D.S.C.; Chan, M.H.M.; Chung, S.S.C.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Khabour, O.F.; Zhang, Q.; Makhdoum, H.M.; Suliman, B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018, 104, 8–13. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
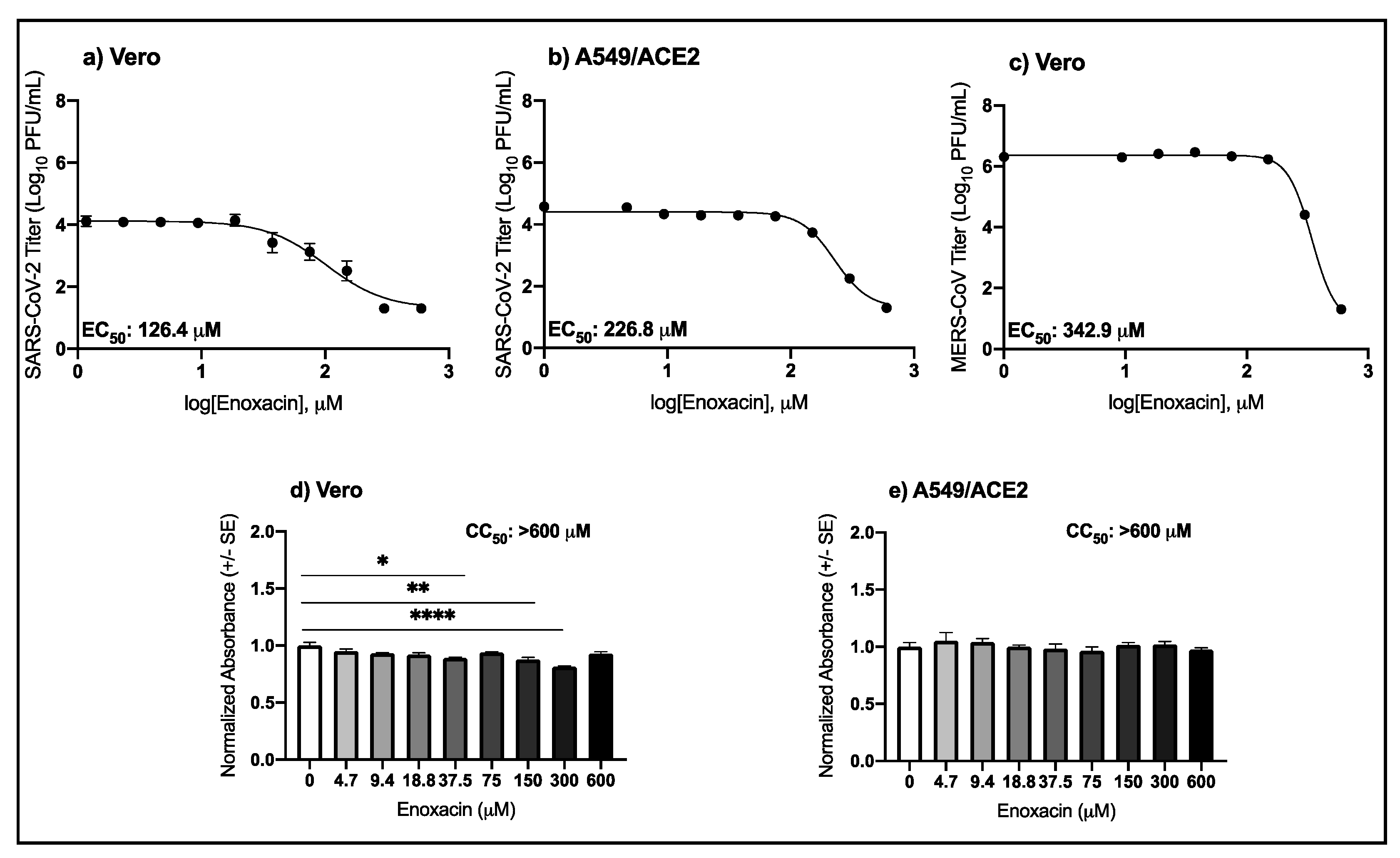
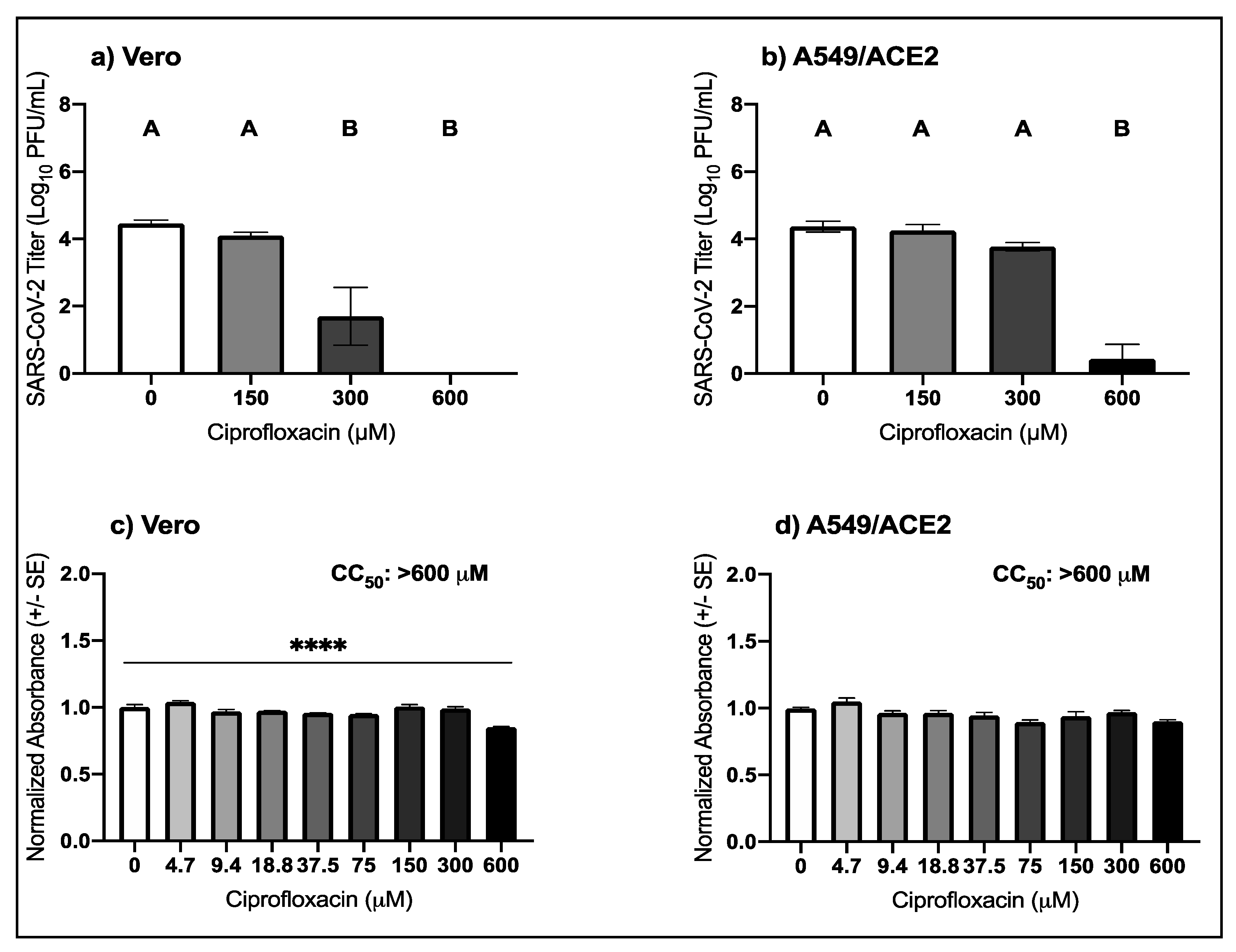
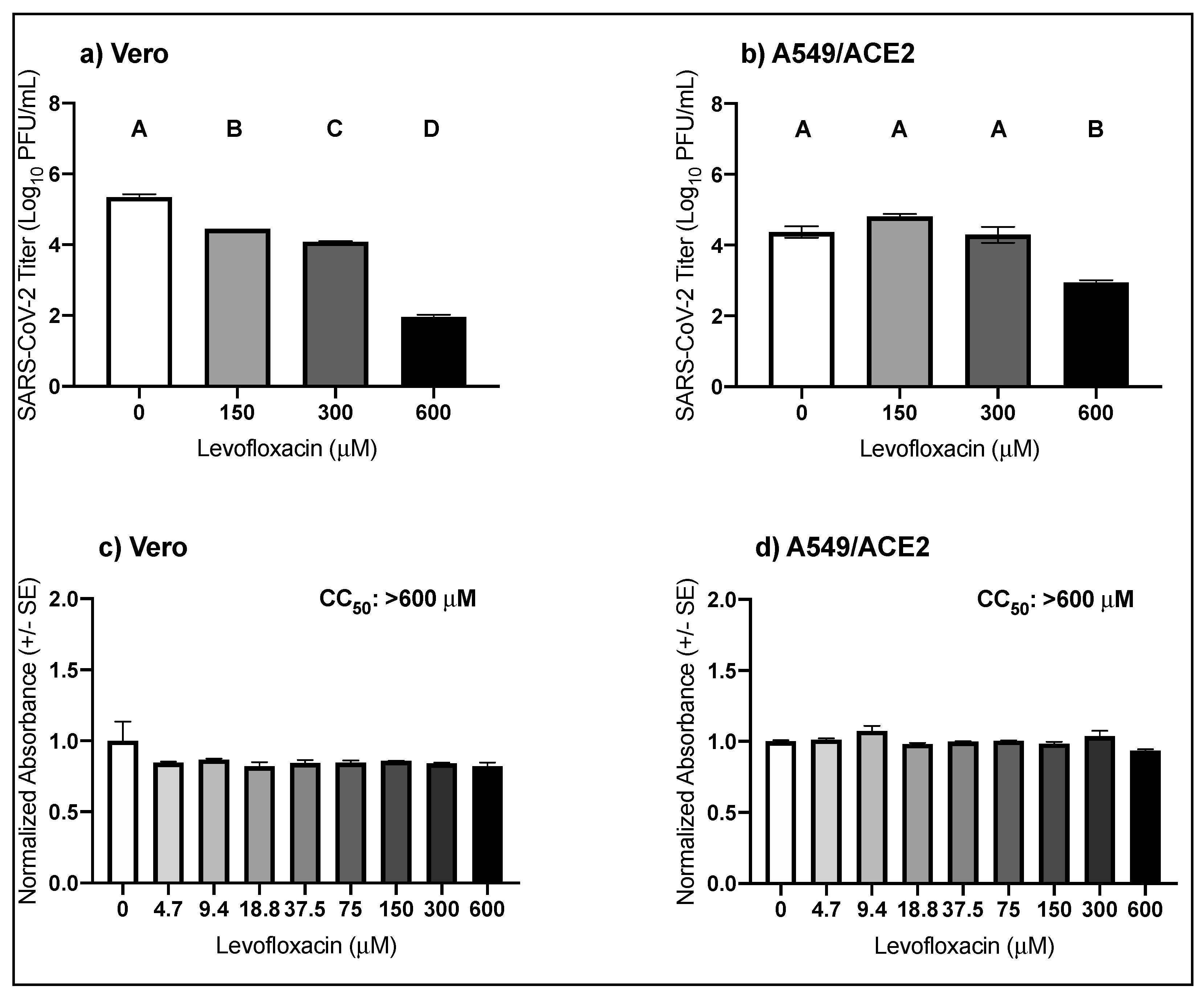
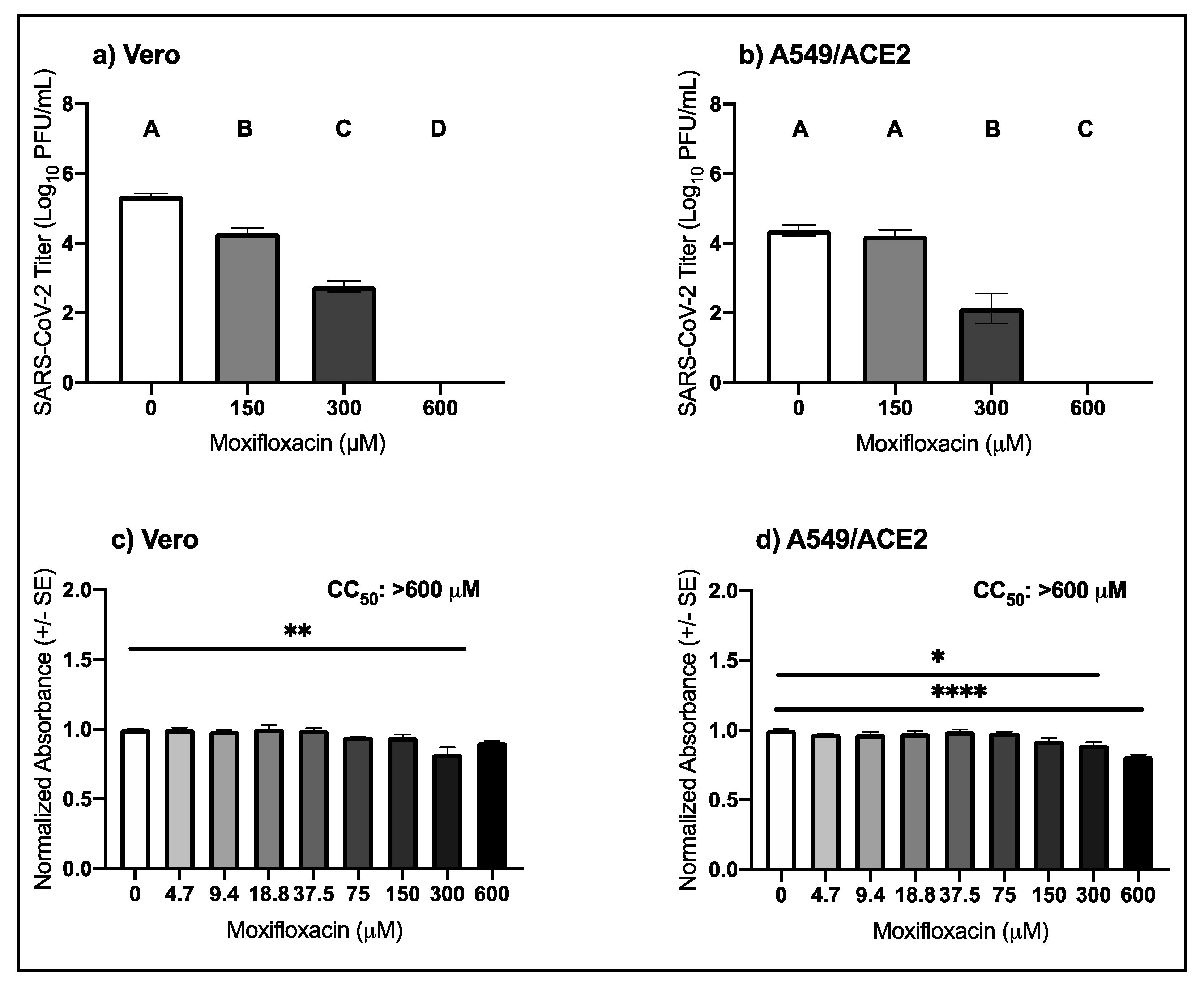
| Virus | Drug | Cell Type | EC50 μM (95% CI) |
|---|---|---|---|
| SARS-CoV-2 | Enoxacin | Vero | 126.4 (88.3–256.4) |
| Enoxacin | A549/ACE2 | 226.8 (210.1–244.6) | |
| Ciprofloxacin | Vero | 246.9 (197.6–313.3) | |
| Levofloxacin | Vero | 418.6 (365.5–480.6) | |
| Moxifloxacin | Vero | 239.7 (213.7–267.4) | |
| MERS-CoV | Enoxacin | Vero | 342.9 (324.0–368.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scroggs, S.L.P.; Offerdahl, D.K.; Flather, D.P.; Morris, C.N.; Kendall, B.L.; Broeckel, R.M.; Beare, P.A.; Bloom, M.E. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses 2021, 13, 8. https://doi.org/10.3390/v13010008
Scroggs SLP, Offerdahl DK, Flather DP, Morris CN, Kendall BL, Broeckel RM, Beare PA, Bloom ME. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses. 2021; 13(1):8. https://doi.org/10.3390/v13010008
Chicago/Turabian StyleScroggs, Stacey L. P., Danielle K. Offerdahl, Dylan P. Flather, Ciera N. Morris, Benjamin L. Kendall, Rebecca M. Broeckel, Paul A. Beare, and Marshall E. Bloom. 2021. "Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV" Viruses 13, no. 1: 8. https://doi.org/10.3390/v13010008
APA StyleScroggs, S. L. P., Offerdahl, D. K., Flather, D. P., Morris, C. N., Kendall, B. L., Broeckel, R. M., Beare, P. A., & Bloom, M. E. (2021). Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses, 13(1), 8. https://doi.org/10.3390/v13010008






