Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Rabies Tissue Culture Isolation Test (RTCIT)
2.2. In Vivo Studies
2.3. Next Generation Sequencing (NGS) and Phylogenetic Analysis
2.4. Plasmids and cDNA Cloning
2.5. G-Deleted Virus and Generation of Pseudotyped RABV
2.6. Cross-Neutralization of KBLV by Human Sera
3. Results
3.1. Virus Isolation from Tissue Samples Failed
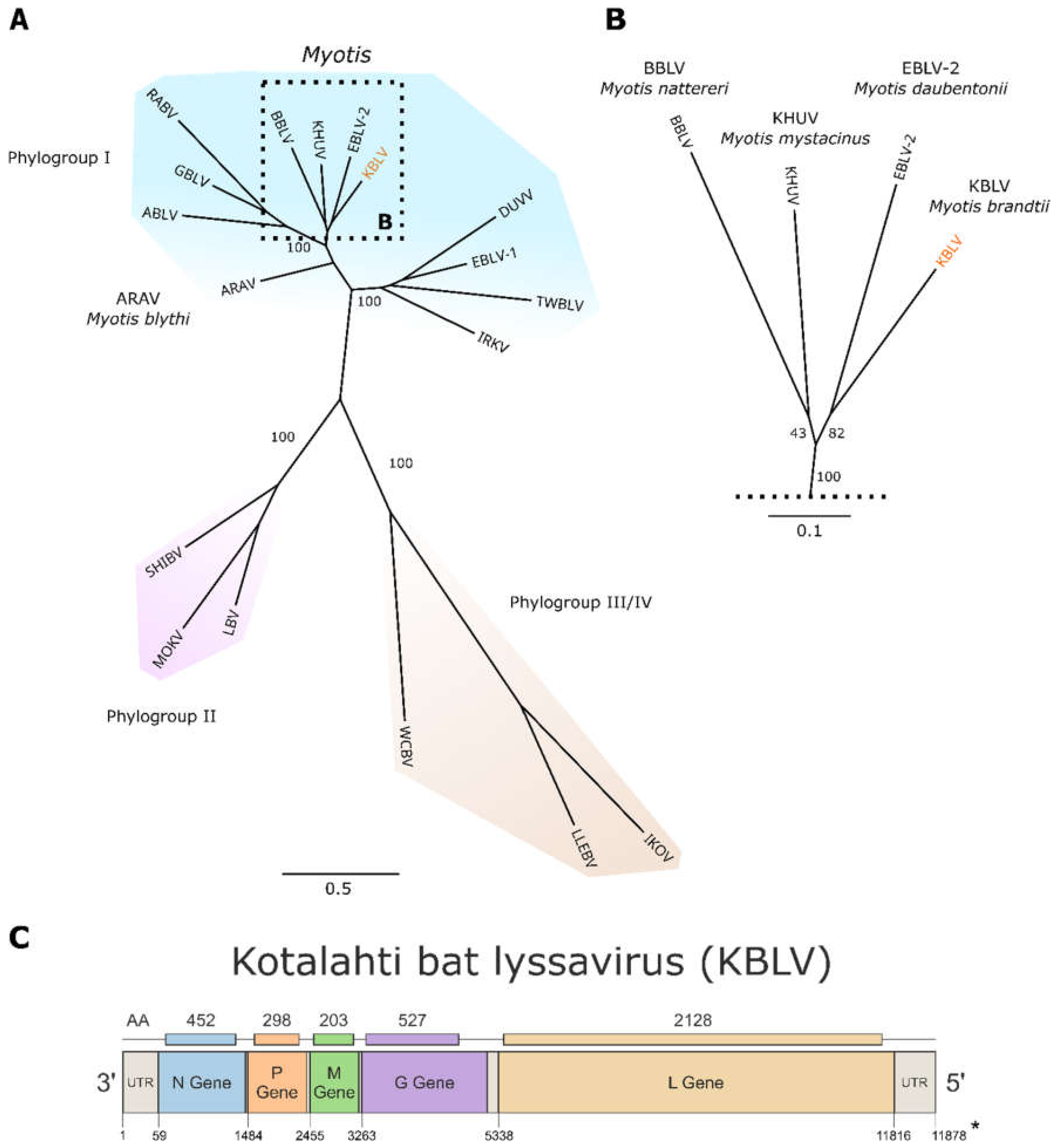
3.2. NGS, Sequence Analysis and Phylogeny
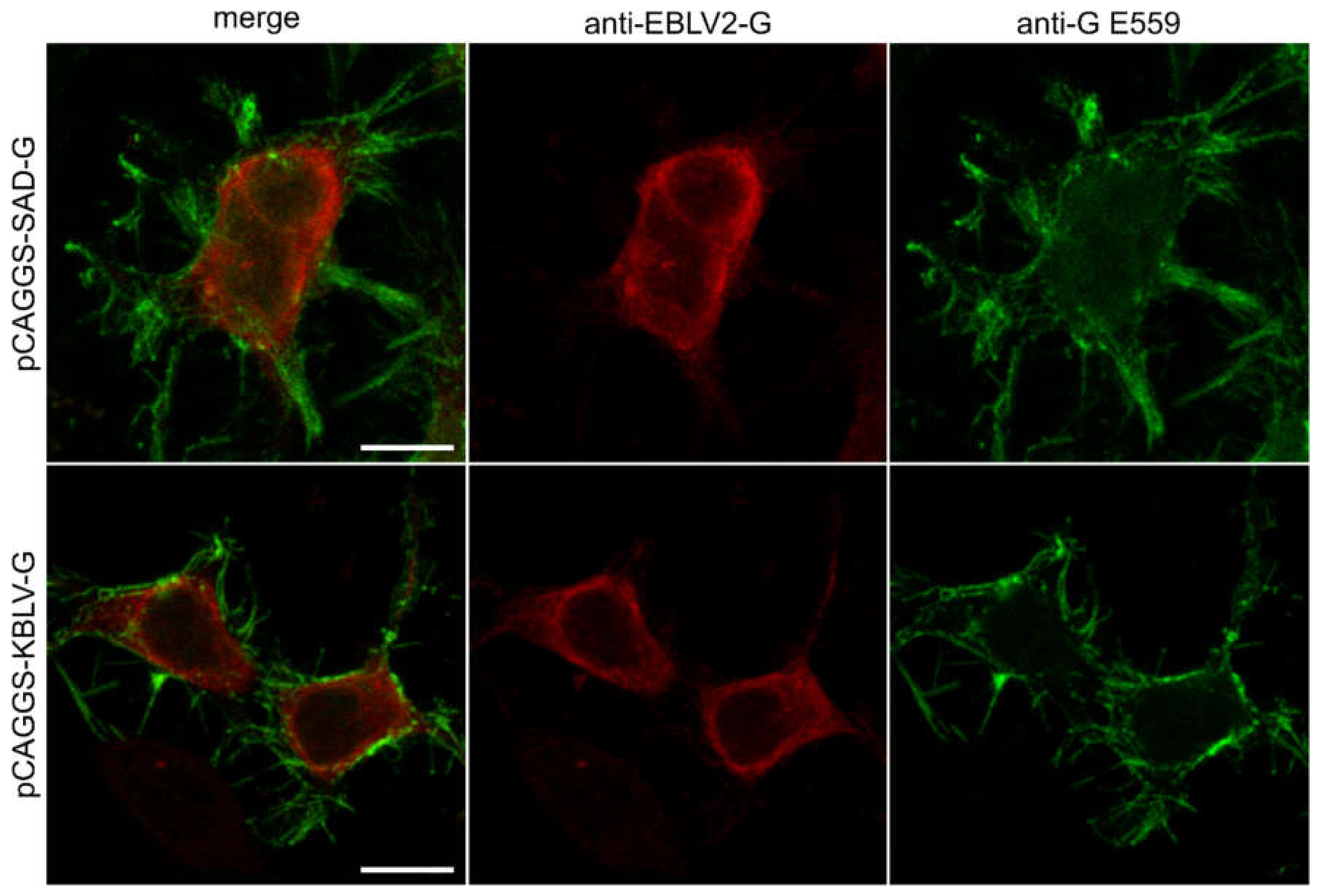
3.3. Expression of KBLV Proteins in Transfected Cells
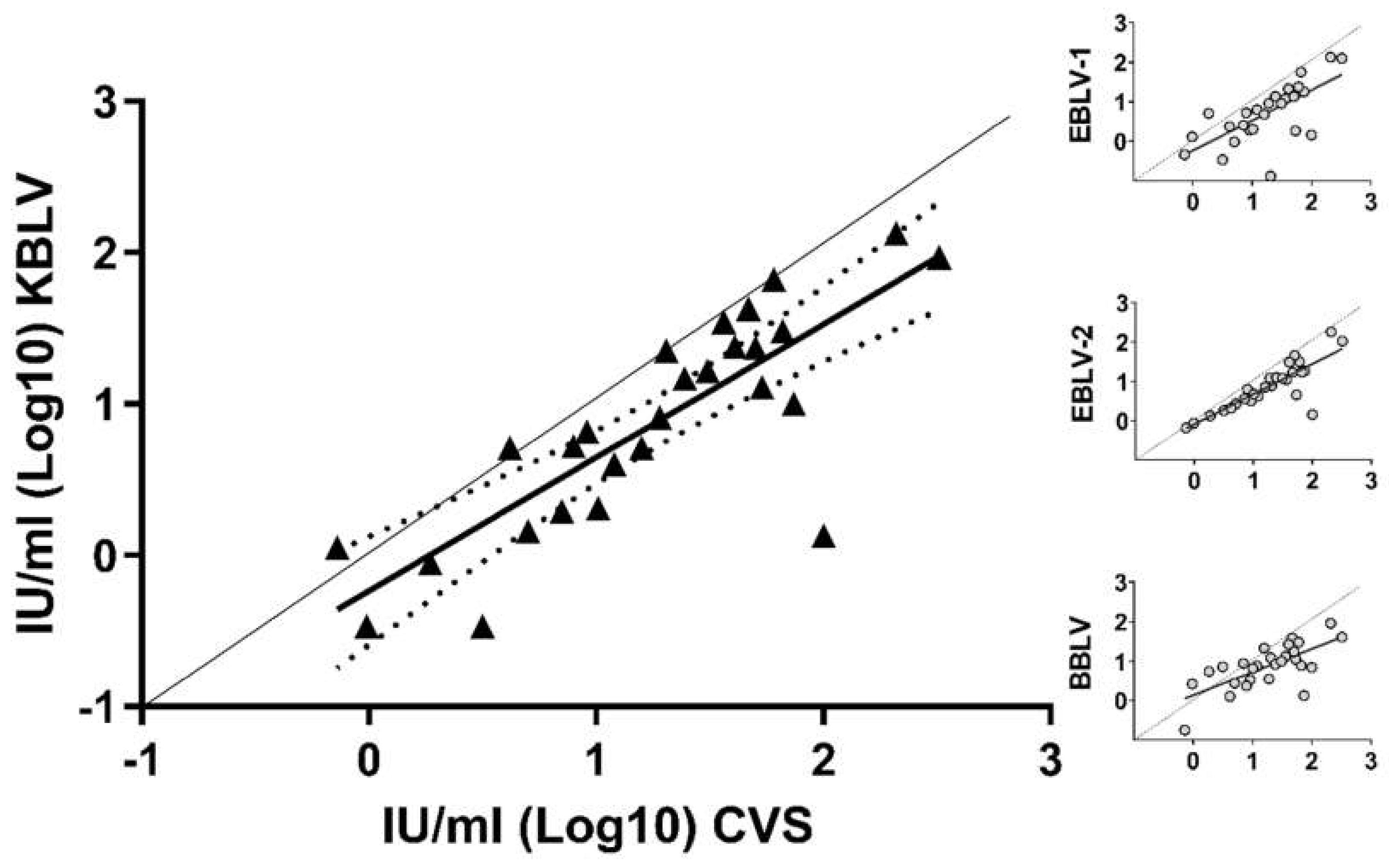
3.4. Cross-Neutralization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnarsson, I.; Zambrana-Torrelio, C.M.; Flores-Saldana, N.P.; May-Collado, L.J. A time-calibrated species-level phylogeny of bats (Chiroptera, Mammalia). PLoS Curr. 2011, 3. [Google Scholar] [CrossRef]
- Calisher, C.H.; Child, J.E.; Field, H.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Wibbelt, G.; Moore, M.S.; Schountz, T.; Voigt, C.C. Emerging diseases in Chiroptera: Why bats? Biol. Lett. 2010, 6, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, V.; Feldmann, H.; Grard, G.; Becker, S.; Leroy, E.M. Ebola and Marburg haemorrhagic fever. J. Clin. Virol. 2015, 64, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Streicker, D.G.; Gilbert, A.T. Contextualizing bats as viral reservoirs. Science 2020, 370, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Davis, A.; Gilbert, A.T.; Markotter, W. Bat rabies. In Rabies—Scientific Basis of the Disease and Its Management, 4th ed.; Fooks, A.R., Jackson, A.C., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-818705-0. [Google Scholar]
- Amarasinghe, G.K.; Arechiga Ceballos, N.G.; Banyard, A.C.; Basler, C.F.; Bavari, S.; Bennett, A.J.; Blasdell, K.R.; Briese, T.; Bukreyev, A.; Cai, Y.; et al. Taxonomy of the order Mononegavirales: Update 2018. Arch. Virol. 2018. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses. Virus Taxonomy: 2019 Release. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/w/rhabdoviridae/795/genus-lyssavirus (accessed on 11 November 2020).
- Badrane, H.; Bahloul, C.; Perrin, P.; Tordo, N. Evidence of Two Lyssavirus Phylogroups with Distinct Pathogenicity and Immunogenicity. J. Virol. 2001, 75, 3268–3276. [Google Scholar] [CrossRef]
- Banyard, A.C.; Selden, D.; Wu, G.; Thorne, L.; Jennings, D.; Marston, D.; Finke, S.; Freuling, C.M.; Müller, T.; Echevarria, J.E.; et al. Isolation, antigenicity and immunogenicity of Lleida bat lyssavirus. J. Gen. Virol. 2018. [Google Scholar] [CrossRef]
- Horton, D.L.; Banyard, A.; Marston, D.A.; Wise, E.; Selden, D.; Nunez, A.; Hicks, D.; Lembo, T.; Cleaveland, S.; Peel, A.J.; et al. Antigenic and genetic characterisation of a divergent African virus, Ikoma lyssavirus. J. Gen. Virol. 2014. [Google Scholar] [CrossRef]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Vos, A.; Freuling, C.; Tordo, N.; Fooks, A.R.; Muller, T. Human rabies due to lyssavirus infection of bat origin. Vet. Microbiol. 2010, 142, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Selimov, M.A.; Tatarov, A.G.; Botvinkin, A.D.; Klueva, E.V.; Kulikova, L.G.; Khismatullina, N.A. Rabies-related Yuli virus; identification with a panel of monoclonal antibodies. Acta Virol 1989, 33, 542–546. [Google Scholar]
- Lumio, J.; Hillbom, M.; Roine, R.; Ketonen, L.; Haltia, M.; Valle, M.; Neuvonen, E.; Lähdevirta, J. Human rabies of bat origin in Europe. Lancet 1986, 1, 378. [Google Scholar] [CrossRef]
- Fooks, A.R.; McElhinney, L.M.; Pounder, D.J.; Finnegan, C.J.; Mansfield, K.; Johnson, N.; Brookes, S.M.; Parsons, G.; White, K.; McIntyre, P.G.; et al. Case report: Isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J. Med. Virol. 2003, 71, 281–289. [Google Scholar] [CrossRef]
- Müller, T.; Cox, J.; Peter, W.; Schäfer, R.; Johnson, N.; McElhinney, L.M.; Geue, J.L.; Tjornehoj, K.; Fooks, A.R. Spill-over of European bat lyssavirus type 1 into a stone marten (Martes foina) in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 49–54. [Google Scholar] [CrossRef]
- Tjornehoj, K.; Fooks, A.R.; Agerholm, J.S.; Ronsholt, L. Natural and Experimental Infection of Sheep with European Bat Lyssavirus Type-1 of Danish Bat Origin. J. Comp. Pathol. 2006, 134, 190–201. [Google Scholar] [CrossRef]
- Dacheux, L.; Larrous, F.; Mailles, A.; Boisseleau, D.; Delmas, O.; Biron, C.; Bourchier, C.; Ilari, F.; Lefranc, T.; Raffi, F.; et al. European bat lyssavirus transmission among cats, Europe. Emerg. Infect. Dis. 2009, 15, 280–284. [Google Scholar] [CrossRef]
- Bourhy, H.; Kissi, B.; Tordo, N. Molecular diversity of the Lyssavirus genus. Virology 1993, 194, 70–81. [Google Scholar] [CrossRef]
- Schatz, J.; Fooks, A.R.; McElhinney, L.; Horton, D.; Echevarria, J.; Vazquez-Moron, S.; Kooi, E.A.; Rasmussen, T.B.; Müller, T.; Freuling, C.M. Bat Rabies Surveillance in Europe. Zoonoses Public Health 2013, 60, 22–34. [Google Scholar] [CrossRef]
- Vazquez-Moron, S.; Juste, J.; Ibanez, C.; Ruiz-Villamor, E.; Avellon, A.; Vera, M.; Echevarria, J.E. Endemic Circulation of European Bat Lyssavirus Type 1 in Serotine Bats, Spain. Emerg. Infect. Dis. 2008, 14, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, L.M.; Marston, D.A.; Wise, E.L.; Freuling, C.M.; Bourhy, H.; Zanoni, R.; Moldal, T.; Kooi, E.A.; Neubauer-Juric, A.; Nokireki, T.; et al. Molecular Epidemiology and Evolution of European Bat Lyssavirus 2. Int. J. Mol. Sci. 2018, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Hughes, G.J.; Botvinkin, A.D.; Orciari, L.A.; Rupprecht, C.E. Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for lyssavirus genotype definition. Virus Res. 2005, 111, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Arechiga Ceballos, N.; Moron, S.V.; Berciano, J.M.; Nicolas, O.; Lopez, C.A.; Juste, J.; Nevado, C.R.; Setien, A.A.; Echevarria, J.E. Novel lyssavirus in bat, Spain. Emerg. Infect. Dis. 2013, 19, 793–795. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Beven, V.; Hirchaud, E.; Guillaume, C.; Larcher, G.; Robardet, E.; Servat, A.; Blanchard, Y.; Cliquet, F. Lleida Bat Lyssavirus isolation in Miniopterus schreibersii in France. Zoonoses Public Health 2018. [Google Scholar] [CrossRef]
- Freuling, C.M.; Beer, M.; Conraths, F.J.; Finke, S.; Hoffmann, B.; Keller, B.; Kliemt, J.; Mettenleiter, T.C.; Muhlbach, E.; Teifke, J.P.; et al. Novel Lyssavirus in Natterer’s Bat, Germany. Emerg. Infect. Dis. 2011, 17, 1519–1522. [Google Scholar] [CrossRef]
- Eggerbauer, E.; Troupin, C.; Passior, K.; Pfaff, F.; Höper, D.; Neubauer-Juric, A.; Haberl, S.; Bouchier, C.; Mettenleiter, T.C.; Bourhy, H.; et al. The Recently Discovered Bokeloh Bat Lyssavirus: Insights Into Its Genetic Heterogeneity and Spatial Distribution in Europe and the Population Genetics of Its Primary Host. Adv. Virus Res. 2017, 99, 199–232. [Google Scholar] [CrossRef]
- Smreczak, M.; Orłowska, A.; Marzec, A.; Trębas, P.; Müller, T.; Freuling, C.M.; Żmudziński, J.F. Bokeloh bat lyssavirus isolation in a Natterer’s bat, Poland. Zoonoses Public Health 2018, 65, 1015–1019. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Servat, A.; Robardet, E.; Moinet, M.; Borel, C.; Cliquet, F. Isolation of Bokeloh bat lyssavirus in Myotis nattereri in France. Arch. Virol. 2013, 158, 2333–2340. [Google Scholar] [CrossRef]
- Nokireki, T.; Tammiranta, N.; Kokkonen, U.-M.; Kantala, T.; Gadd, T. Tentative novel lyssavirus in a bat in Finland. Transbound. Emerg. Dis. 2018, 1–4. [Google Scholar] [CrossRef]
- Rupprecht, C.; Fooks, A.; Abela-Ridder, B. (Eds.) Virus isolation in cell culture: The rabies tissue culture infection test (RTCIT). In Laboratory Techniques in Rabies, 5th ed.; World Health Organization: Geneva, Switzerland, 2018; pp. 85–95. [Google Scholar]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. (Eds.) The direct fluorescent antibody test. In Laboratory Techniques in Rabies, 5th ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Wylezich, C.; Papa, A.; Beer, M.; Höper, D. A Versatile Sample Processing Workflow for Metagenomic Pathogen Detection. Sci. Rep. 2018, 8, 13108. [Google Scholar] [CrossRef]
- Wylezich, C.; Calvelage, S.; Schlottau, K.; Ziegler, U.; Pohlmann, A.; Höper, D.; Beer, M. Next-generation diagnostics: Virus capture facilitates a sensitive viral diagnosis for epizootic and zoonotic pathogens including SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, K.H.; Cox, J.H.; Schneider, L.G.; Thiel, H.J. Molecular Cloning and Complete Nucleotide Sequence of the Attenuated Rabies Virus SAD B19. Virology 1990, 175, 485–499. [Google Scholar] [CrossRef]
- Hitoshi, N.; Ken-ichi, Y.; Jun-ichi, M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef]
- Fu, C.; Donovan, W.P.; Shikapwashya-Hasser, O.; Ye, X.; Cole, R.H. Hot Fusion: An efficient method to clone multiple DNA fragments as well as inverted repeats without ligase. PLoS ONE 2014, 9, e115318. [Google Scholar] [CrossRef] [PubMed]
- Wickersham, I.R.; Finke, S.; Conzelmann, K.K.; Callaway, E.M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 2007, 4, 47–49. [Google Scholar] [CrossRef]
- Müller, T.; Dietzschold, B.; Ertl, H.; Fooks, A.R.; Freuling, C.; Fehlner-Gardiner, C.; Kliemt, J.; Meslin, F.X.; Franka, R.; Rupprecht, C.E.; et al. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Neglect. Trop. Dis. 2009, 3, e542. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Malerczyk, C.; Freuling, C.; Gniel, D.; Giesen, A.; Selhorst, T.; Müller, T. Cross-neutralization of antibodies induced by vaccination with Purified Chick Embryo Cell Vaccine (PCECV) against different Lyssavirus species. Hum. Vaccin. Immunother. 2014, 10, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.L.; McElhinney, L.M.; Marston, D.A.; Wood, J.L.N.; Russell, C.A.; Lewis, N.; Kuzmin, I.V.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Fooks, A.R.; et al. Quantifying Antigenic Relationships among the Lyssaviruses. J. Virol. 2010, 84, 11841–11848. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.B. Order Chiroptera. In Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 513. ISBN 978-0-8018-8221-0. [Google Scholar]
- Ruedi, M.; Stadelmann, B.; Gager, Y.; Douzery, E.J.P.; Francis, C.M.; Lin, L.-K.; Guillén-Servent, A.; Cibois, A. Molecular phylogenetic reconstructions identify East Asia as the cradle for the evolution of the cosmopolitan genus Myotis (Mammalia, Chiroptera). Mol. Phylogenet. Evol. 2013, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Malerczyk, C.; Selhorst, T.; Tordo, N.; Moore, S.A.; Müller, T. Antibodies induced by vaccination with purified chick embryo cell culture vaccine (PCECV) cross-neutralize non-classical bat lyssavirus strains. Vaccine 2009, 27, 5320–5325. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Calisher, C.H.; Kurath, G.; Kuzmin, I.V.; Rodriguez, L.L.; Stone, D.M. Family Rhabdoviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses—Ninth Report of the International Committee on Taxonomy of Viruses; King, A.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 686–713. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvelage, S.; Tammiranta, N.; Nokireki, T.; Gadd, T.; Eggerbauer, E.; Zaeck, L.M.; Potratz, M.; Wylezich, C.; Höper, D.; Müller, T.; et al. Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV). Viruses 2021, 13, 69. https://doi.org/10.3390/v13010069
Calvelage S, Tammiranta N, Nokireki T, Gadd T, Eggerbauer E, Zaeck LM, Potratz M, Wylezich C, Höper D, Müller T, et al. Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV). Viruses. 2021; 13(1):69. https://doi.org/10.3390/v13010069
Chicago/Turabian StyleCalvelage, Sten, Niina Tammiranta, Tiina Nokireki, Tuija Gadd, Elisa Eggerbauer, Luca M. Zaeck, Madlin Potratz, Claudia Wylezich, Dirk Höper, Thomas Müller, and et al. 2021. "Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV)" Viruses 13, no. 1: 69. https://doi.org/10.3390/v13010069
APA StyleCalvelage, S., Tammiranta, N., Nokireki, T., Gadd, T., Eggerbauer, E., Zaeck, L. M., Potratz, M., Wylezich, C., Höper, D., Müller, T., Finke, S., & Freuling, C. M. (2021). Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV). Viruses, 13(1), 69. https://doi.org/10.3390/v13010069





