Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus
2.2. Antibodies and Reagents
2.3. RNA Interference
2.4. Cell Viability Measurement
2.5. XBP1 mRNA Splicing Assay
2.6. Quantitative-PCR (qPCR) Analysis
2.7. Western Blot
2.8. Virus Replication Assay
2.9. Statistical Analyses
3. Results
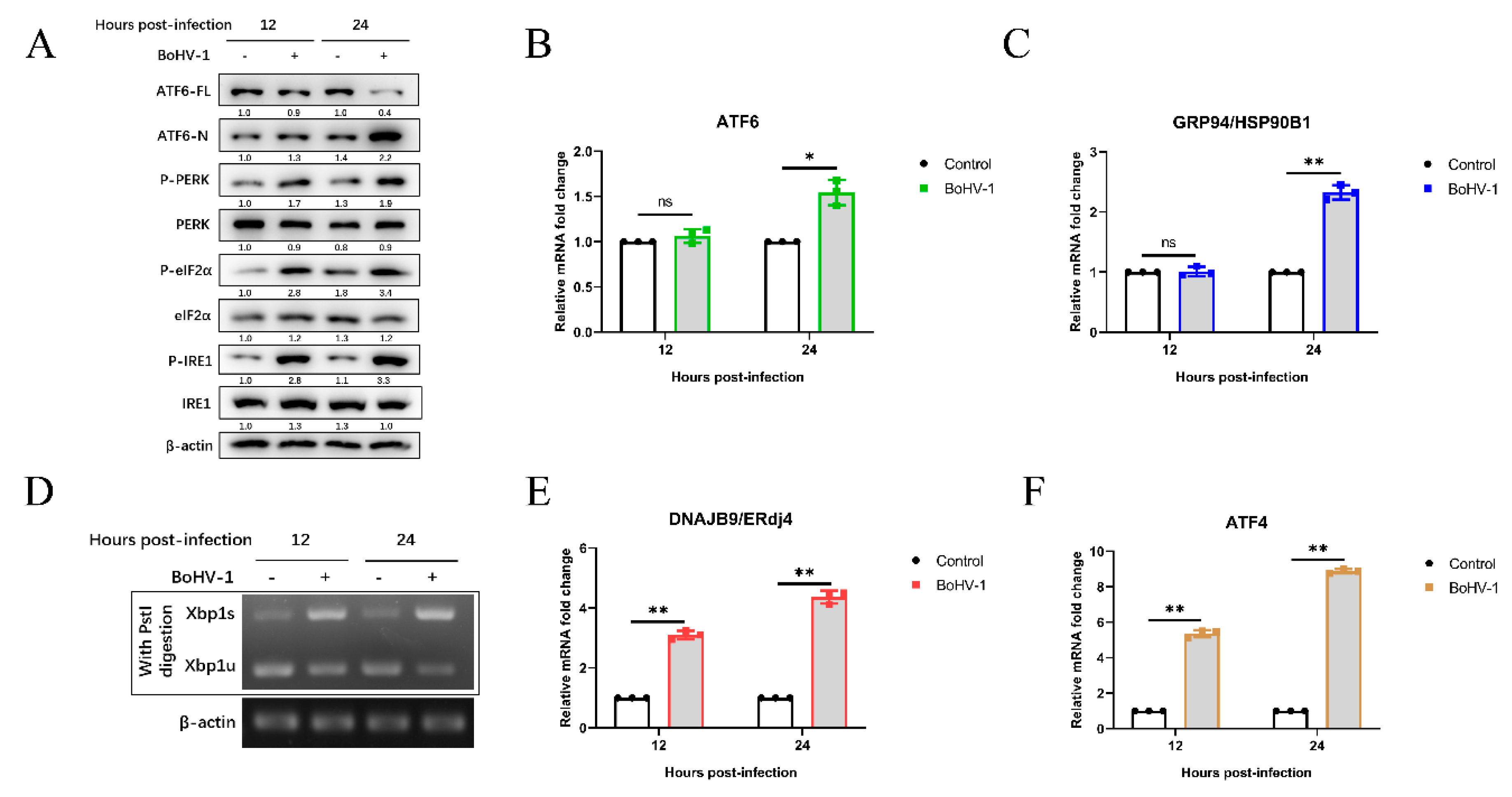
3.1. BoHV-1 Infection Triggers ER Stress in MDBK Cells
3.2. Bohv-1 Induces UPR Through All Three Signaling Branches
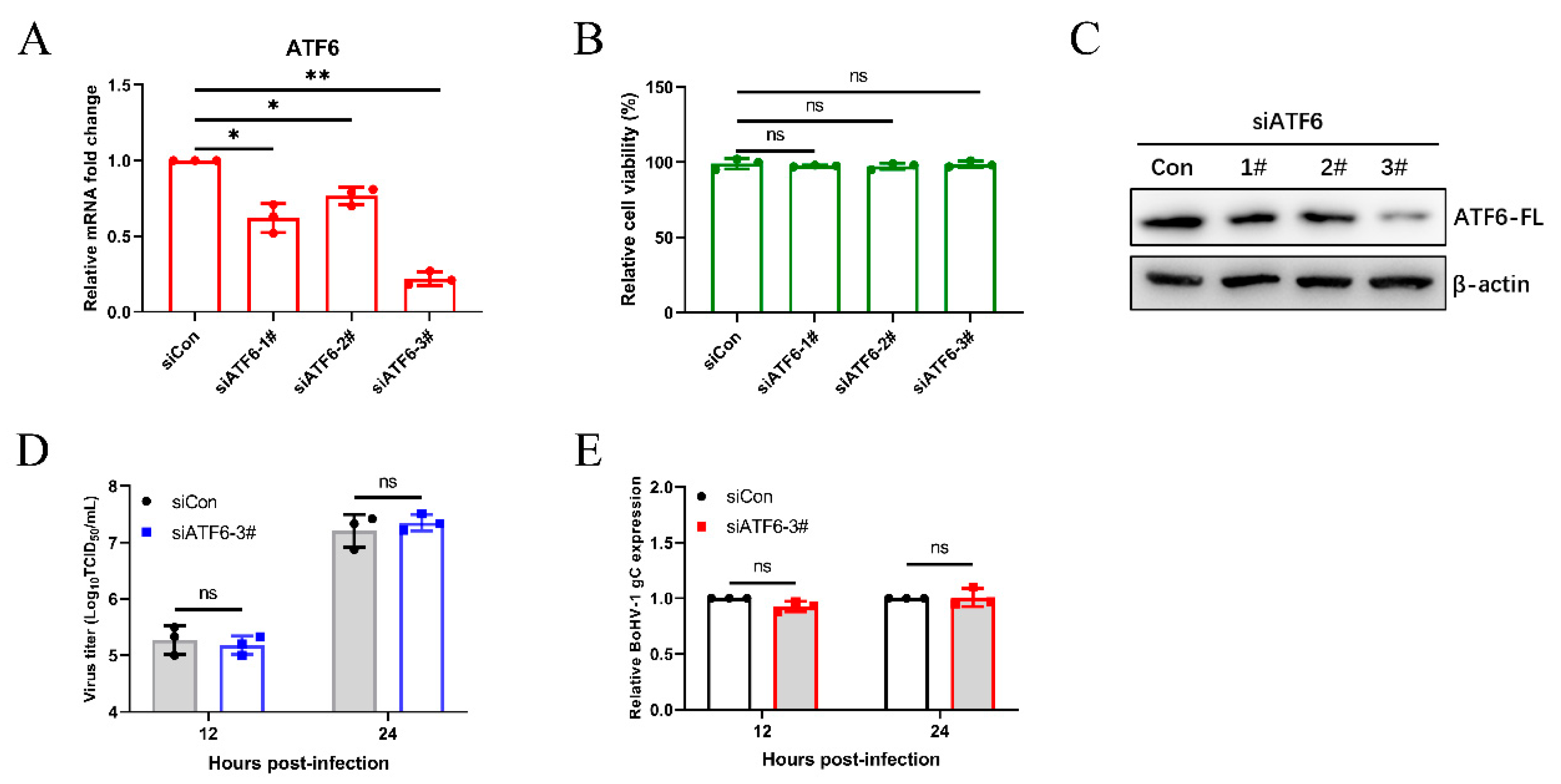
3.3. The Virus-Induced ATF6 Pathway Does Not Affect Bohv-1 Replication
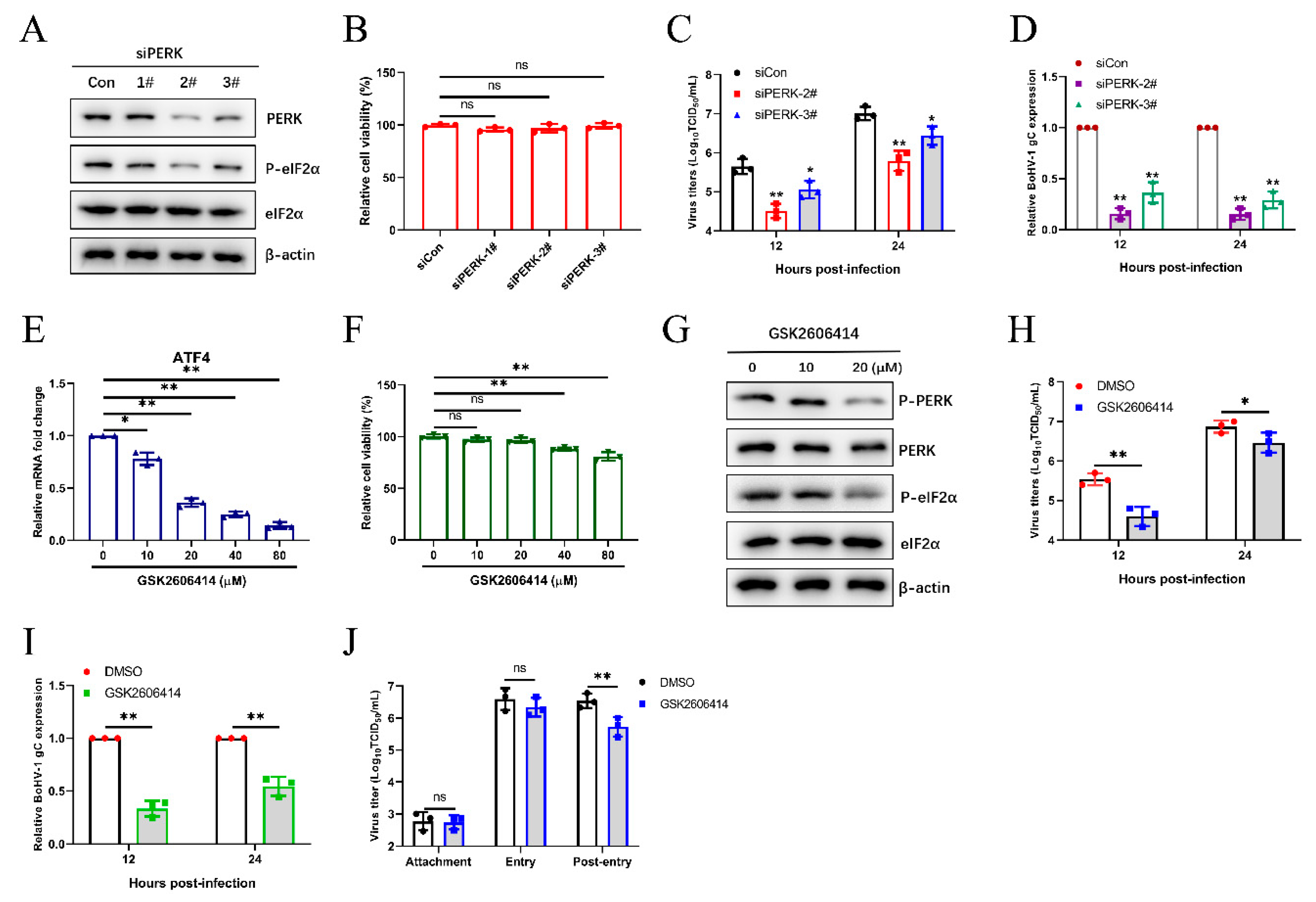
3.4. The BoHV1-Activated PERK Pathway Facilitates Viral Proliferation
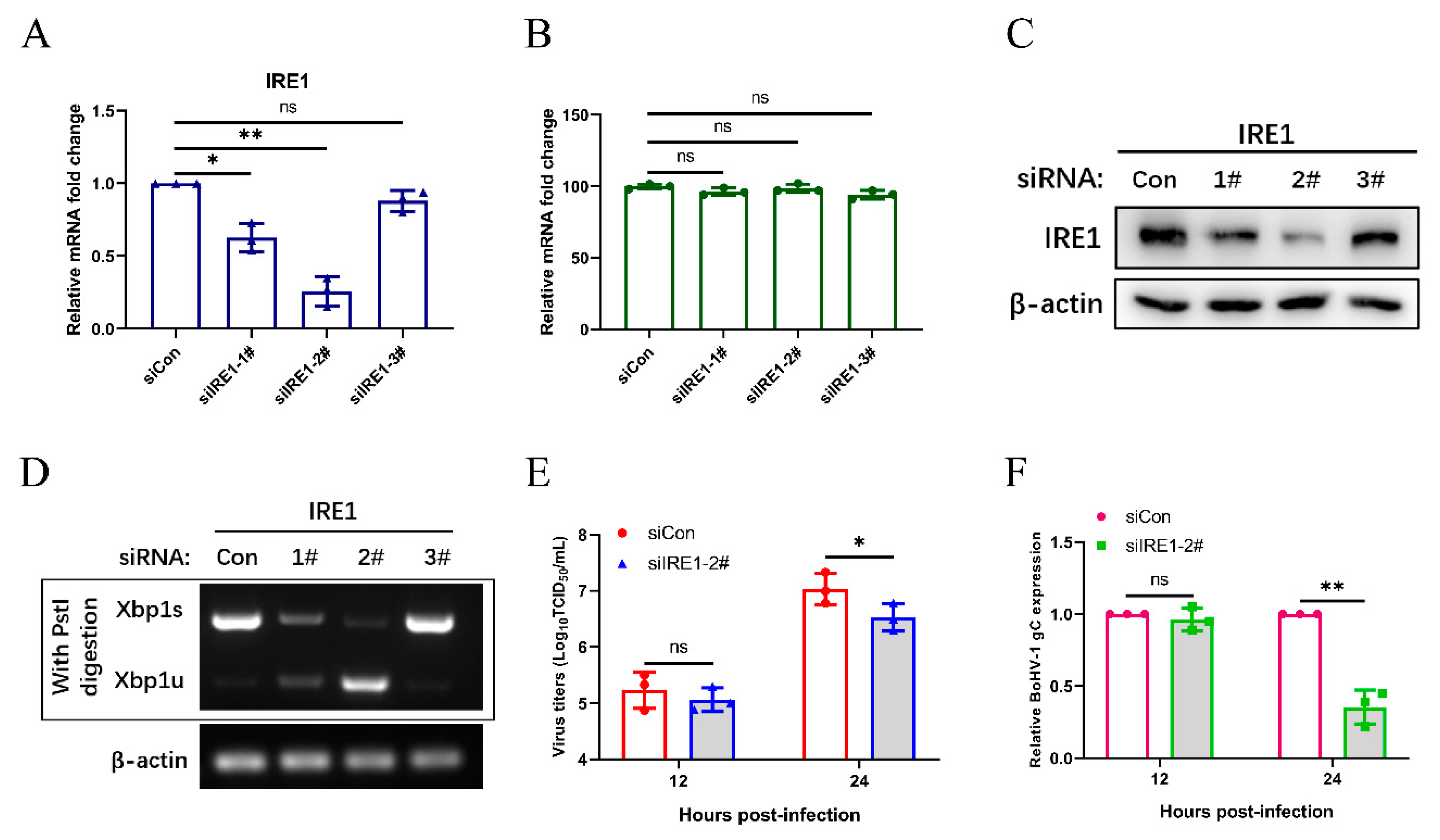
3.5. The IRE1 Pathway Induced by Bohv-1 Infection Promotes Viral Replication in MDBK Cells
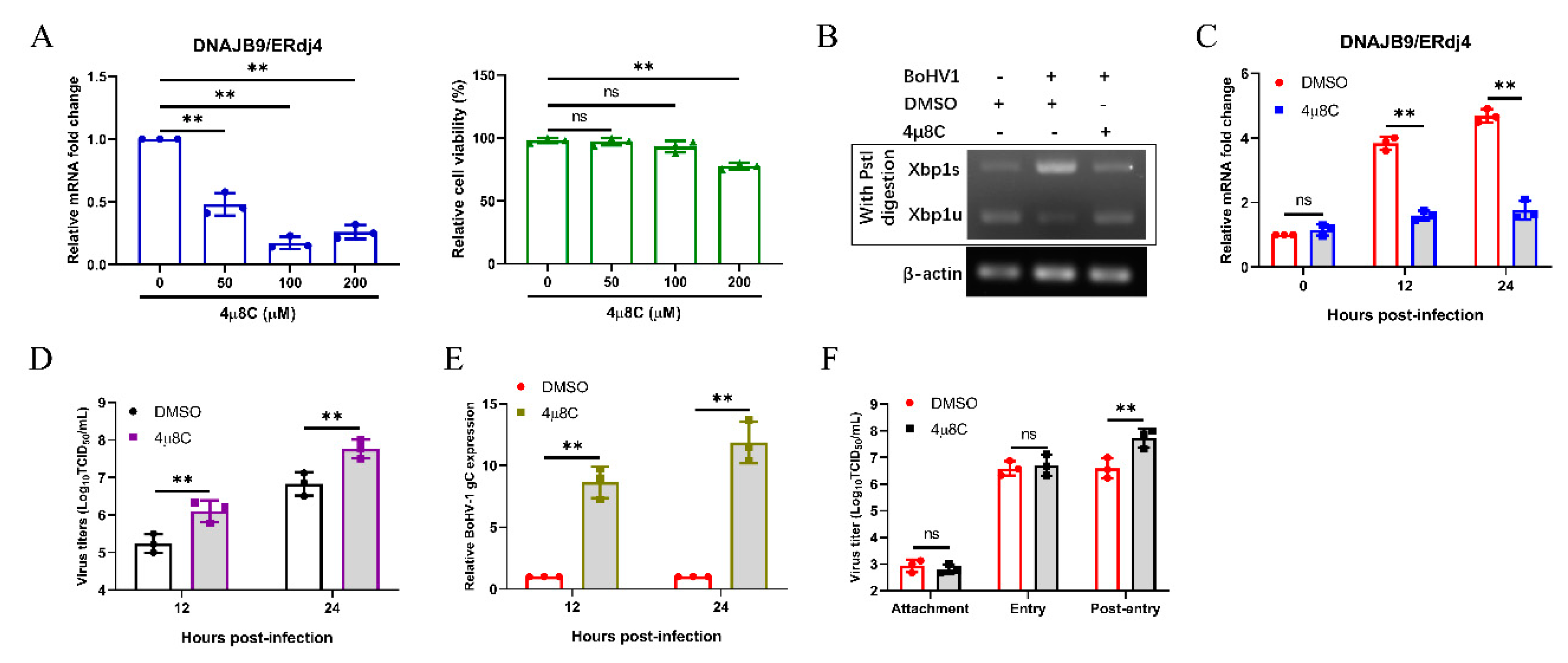
3.6. Bohv-1 Replication is Enhanced by Inhibiting IRE1 Rnase Activity in MDBK Cells
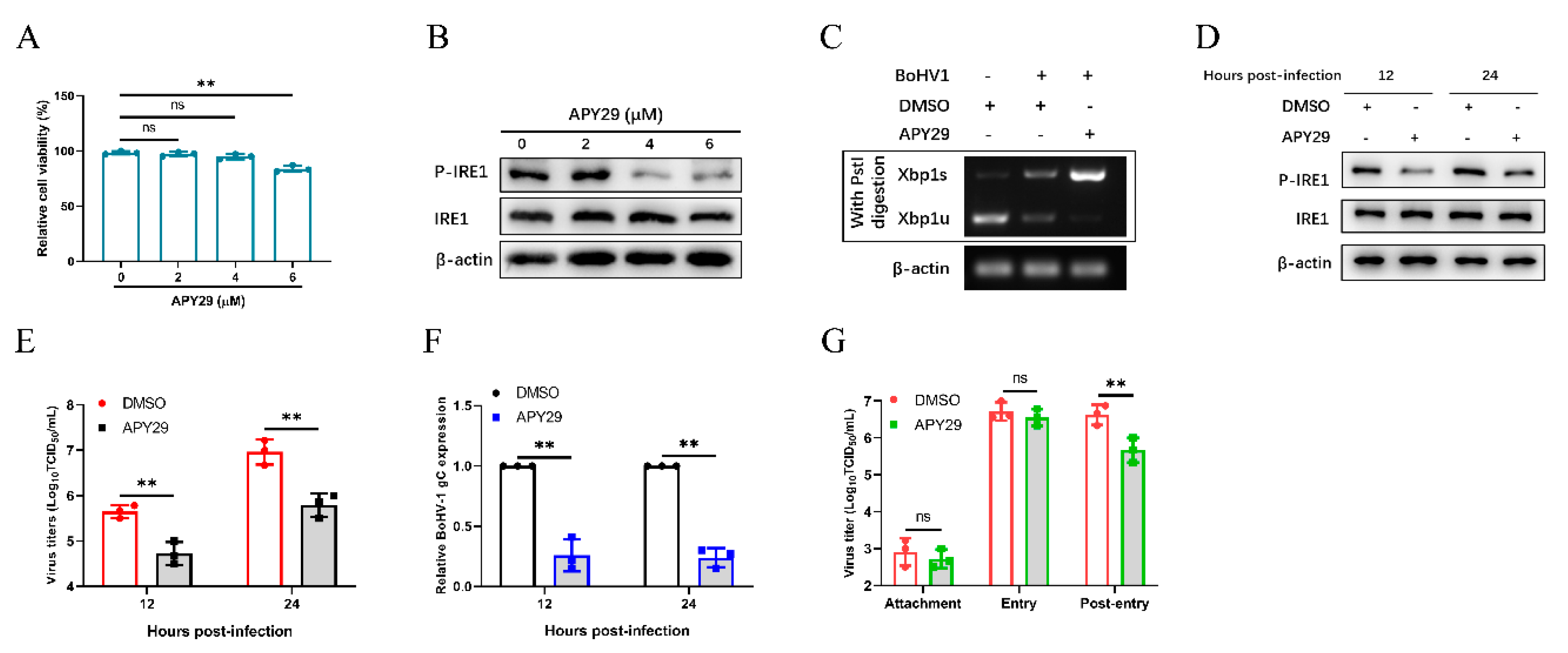
3.7. Inhibition of IRE1 Kinase Activity and Promotion of Rnase Activity Decrease Bohv-1 Replication
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Engels, M.J.V. Pro and contra IBR-eradication. Vet. Microbiol. 2006, 113, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Glazov, E.A.; Horwood, P.F.; Assavalapsakul, W.; Kongsuwan, K.; Mitchell, R.W.; Mitter, N.; Mahony, T.J. Characterization of microRNAs encoded by the bovine herpesvirus 1 genome. J. Gen. Virol. 2010, 91 Pt 1, 32–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Ren, Y.; Hou, X.; Liu, Y.; Wei, S.; Dai, G.; Meng, Y.; Hu, L.; Liu, Z.; et al. Phylogenetic analysis and characterization of bovine herpesvirus-1 in cattle of China, 2016–2019. Infect. Genet. Evol. 2020, 85, 104416. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhao, M.; He, W.; He, H.; Wang, H. Cellular microRNA bta-miR-2361 inhibits bovine herpesvirus 1 replication by directly targeting EGR1 gene. Vet. Microbiol. 2019, 233, 174–183. [Google Scholar] [CrossRef]
- Hou, P.; Wang, H.; Zhao, G.; He, C.; He, H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017, 13, 386. [Google Scholar] [CrossRef]
- Zhao, G.; Hou, P.; Huan, Y.; He, C.; Wang, H.; He, H. Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis. BMC Vet. Res. 2018, 14, 412. [Google Scholar] [CrossRef]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Hou, P.; He, C.; He, H. Rapid visual detection of Mycobacterium avium subsp. paratuberculosis by recombinase polymerase amplification combined with a lateral flow dipstick. J. Vet. Sci. 2018, 19, 242–250. [Google Scholar] [CrossRef]
- Salimena, A.P.; Lange, C.C.; Camussone, C.; Signorini, M.; Calvinho, L.F.; Brito, M.A.; Borges, C.A.; Guimaraes, A.S.; Ribeiro, J.B.; Mendonca, L.C.; et al. Genotypic and phenotypic detection of capsular polysaccharide and biofilm formation in Staphylococcus aureus isolated from bovine milk collected from Brazilian dairy farms. Vet. Res. Commun. 2016, 40, 97–106. [Google Scholar] [CrossRef]
- Miller, J.M.; Whetstone, C.A.; Van der Maaten, M.J. Abortifacient property of bovine herpesvirus type 1 isolates that represent three subtypes determined by restriction endonuclease analysis of viral DNA. Am. J. Vet. Res. 1991, 52, 458–461. [Google Scholar] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar]
- Kania, E.; Pajak, B.; Orzechowski, A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. BioMed Res. Int. 2015, 2015, 352794. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef]
- Shen, J.; Prywes, R. ER stress signaling by regulated proteolysis of ATF6. Methods 2005, 35, 382–389. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Reimold, A.M.; Etkin, A.; Clauss, I.; Perkins, A.; Friend, D.S.; Zhang, J.; Horton, H.F.; Scott, A.; Orkin, S.H.; Byrne, M.C.; et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000, 14, 152–157. [Google Scholar]
- Shi, Y.; Vattem, K.M.; Sood, R.; An, J.; Liang, J.; Stramm, L.; Wek, R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998, 18, 7499–7509. [Google Scholar] [CrossRef] [PubMed]
- Wek, R.C.; Jiang, H.Y.; Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006, 34 Pt 1, 7–11. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A. Virus-induced ER stress and the unfolded protein response. Front. Plant Sci. 2012, 3, 293. [Google Scholar] [CrossRef]
- Chan, S.W. The unfolded protein response in virus infections. Front. Microbiol. 2014, 5, 518. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; He, H.; Wang, H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Limjindaporn, T.; Wongwiwat, W.; Noisakran, S.; Srisawat, C.; Netsawang, J.; Puttikhunt, C.; Kasinrerk, W.; Avirutnan, P.; Thiemmeca, S.; Sriburi, R.; et al. Interaction of dengue virus envelope protein with endoplasmic reticulum-resident chaperones facilitates dengue virus production. Biochem. Biophys. Res. Commun. 2009, 379, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chai, Y.; Song, J.; Liu, T.; Chen, P.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Reprogramming the unfolded protein response for replication by porcine reproductive and respiratory syndrome virus. PLoS Pathog. 2019, 15, e1008169. [Google Scholar] [CrossRef]
- Isler, J.A.; Skalet, A.H.; Alwine, J.C. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 2005, 79, 6890–6899. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.P.; McCormick, C. Herpesviruses and the Unfolded Protein Response. Viruses 2019, 12, 17. [Google Scholar] [CrossRef]
- Cheng, G.; Feng, Z.; He, B. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein. J. Virol. 2005, 79, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Burnett, H.F.; Audas, T.E.; Liang, G.; Lu, R.R. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones 2012, 17, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Wang, H.; Li, Y.; Wang, X.; Chen, D.; Wu, Z. Opposite Roles of RNase and Kinase Activities of Inositol-Requiring Enzyme 1 (IRE1) on HSV-1 Replication. Viruses 2017, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.E.; Grose, C. Varicella-zoster virus glycoprotein expression differentially induces the unfolded protein response in infected cells. Front. Microbiol. 2014, 5, 322. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Magalhaes-Junior, M.J.; Baracat-Pereira, M.C.; Pereira, L.K.J.; Vital, C.E.; Santos, M.R.; Cunha, P.S.; Fernandes, K.M.; Bressan, G.C.; Fietto, J.L.R.; Silva-Junior, A.; et al. Proteomic and phosphoproteomic analyses reveal several events involved in the early stages of bovine herpesvirus 1 infection. Arch. Virol. 2020, 165, 69–85. [Google Scholar] [CrossRef]
- Ma, W.; Wang, H.; He, H. Bovine herpesvirus 1 tegument protein UL41 suppresses antiviral innate immune response via directly targeting STAT1. Vet. Microbiol. 2019, 239, 108494. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, C.; Li, D.; Yu, Z.; Li, F.; Li, F.; An, Q.; Bai, H.; Zhang, X.; Duan, Z.; et al. Cyclin-dependent kinase 11(p110) (CDK11(p110)) is crucial for human breast cancer cell proliferation and growth. Sci. Rep. 2015, 5, 10433. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, S.; Chen, Z.; Yu, J.; Tang, R.; Qiu, W.; Zhao, L.; Liu, Y.; Guo, X.; He, H.; et al. Successive Passage In Vitro Led to Lower Virulence and Higher Titer of A Variant Porcine Epidemic Diarrhea Virus. Viruses 2020, 12, 391. [Google Scholar] [CrossRef]
- Hou, P.; Xu, Y.; Wang, H.; He, H. Detection of bovine viral diarrhea virus genotype 1 in aerosol by a real time RT-PCR assay. BMC Vet. Res. 2020, 16, 114. [Google Scholar] [CrossRef]
- Wang, H.M.; Zhao, G.M.; Hou, P.L.; Yu, L.; He, C.Q.; He, H.B. Rapid detection of foot-and-mouth disease virus using reverse transcription recombinase polymerase amplification combined with a lateral flow dipstick. J. Virol. Methods 2018, 261, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Hsu, Y.W.; Liao, C.L.; Lin, Y.L. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J. Virol. 2006, 80, 11868–11880. [Google Scholar] [CrossRef]
- Xue, M.; Fu, F.; Ma, Y.; Zhang, X.; Li, L.; Feng, L.; Liu, P. The PERK Arm of the Unfolded Protein Response Negatively Regulates Transmissible Gastroenteritis Virus Replication by Suppressing Protein Translation and Promoting Type I Interferon Production. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; He, W.; He, H.; Wang, H. Beta-catenin inhibits bovine parainfluenza virus type 3 replication via innate immunity pathway. BMC Vet. Res. 2020, 16, 72. [Google Scholar] [CrossRef]
- Zhao, G.; He, H.; Wang, H. Use of a recombinase polymerase amplification commercial kit for rapid visual detection of Pasteurella multocida. BMC Vet. Res. 2019, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhao, G.; He, C.; Wang, H.; He, H. Biopanning of polypeptides binding to bovine ephemeral fever virus G1 protein from phage display peptide library. BMC Vet. Res. 2018, 14, 3. [Google Scholar] [CrossRef]
- Kanokudom, S.; Mahony, T.J.; Smith, D.R.; Assavalapsakul, W. Modulation of bovine herpesvirus 1 infection by virally encoded microRNAs. Virus Res. 2018, 257, 1–6. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, P.; He, H.; Wang, H. Cell apoptosis regulated by interaction between viral gene alpha 3 and host heterogeneous nuclear ribonucleoprotein K facilitates bovine ephemeral fever virus replication. Vet. Microbiol. 2020, 240, 108510. [Google Scholar] [CrossRef]
- Lv, L.; Zhao, G.; Wang, H.; He, H. Cholesterol 25-Hydroxylase inhibits bovine parainfluenza virus type 3 replication through enzyme activity-dependent and -independent ways. Vet. Microbiol. 2019, 239, 108456. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Wang, H.; Hou, P.; He, H. Annexin A2 gene interacting with viral matrix protein to promote bovine ephemeral fever virus release. J. Vet. Sci. 2020, 21, e33. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, C.; Ding, X.; Jones, C.; Zhu, G. The role of phospholipase C signaling in bovine herpesvirus 1 infection. Vet. Res. 2017, 48, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, X.; Tao, J.; Wang, J.; Zhao, X.; Zhu, G. Critical role of cholesterol in bovine herpesvirus type 1 infection of MDBK cells. Vet. Microbiol. 2010, 144, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Guo, H.C.; Sun, S.Q.; Jin, Y.; Wei, Y.Q.; Feng, X.; Yao, X.P.; Cao, S.Z.; Xiang Liu, D.; Liu, X.T. Productive Entry of Foot-and-Mouth Disease Virus via Macropinocytosis Independent of Phosphatidylinositol 3-Kinase. Sci. Rep. 2016, 6, 19294. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chu, Y.R.; Ye, Y.; Chen, X. Role of HERP and a HERP-related protein in HRD1-dependent protein degradation at the endoplasmic reticulum. J. Biol. Chem. 2014, 289, 4444–4454. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Yang, L.; Liu, P.; Zhang, Y.; Wang, X. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol. Lett. 2020, 222, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Katneni, U.K.; Bott, M.; Golovan, S.P.; Parcells, M.S. Induction of the unfolded protein response (UPR) during Marek’s disease virus (MDV) infection. Virology 2018, 522, 1–12. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef]
- Dey, S.; Baird, T.D.; Zhou, D.; Palam, L.R.; Spandau, D.F.; Wek, R.C. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J. Biol. Chem. 2010, 285, 33165–33174. [Google Scholar] [CrossRef]
- Augusto, L.; Amin, P.H.; Wek, R.C.; Sullivan, W.J., Jr. Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2019, 15, e1007746. [Google Scholar] [CrossRef]
- Cao, L.; Xue, M.; Chen, J.; Shi, H.; Zhang, X.; Shi, D.; Liu, J.; Huang, L.; Wei, Y.; Liu, C.; et al. Porcine parvovirus replication is suppressed by activation of the PERK signaling pathway and endoplasmic reticulum stress-mediated apoptosis. Virology 2020, 539, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prischi, F.; Nowak, P.R.; Carrara, M.; Ali, M.M. Phosphoregulation of Ire1 RNase splicing activity. Nat. Commun. 2014, 5, 3554. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.C.; Bond, P.J.; Sadowski, P.G.; Jha, B.K.; Zak, J.; Goodman, J.M.; Silverman, R.H.; Neubert, T.A.; Baxendale, I.R.; Ron, D.; et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA 2012, 109, E869–E878. [Google Scholar] [CrossRef] [PubMed]
- Lubamba, B.A.; Jones, L.C.; O’Neal, W.K.; Boucher, R.C.; Ribeiro, C.M. X-Box-Binding Protein 1 and Innate Immune Responses of Human Cystic Fibrosis Alveolar Macrophages. Am. J. Respir. Crit. Care Med. 2015, 192, 1449–1461. [Google Scholar] [CrossRef]
- Kemp, K.L.; Lin, Z.; Zhao, F.; Gao, B.; Song, J.; Zhang, K.; Fang, D. The serine-threonine kinase inositol-requiring enzyme 1alpha (IRE1alpha) promotes IL-4 production in T helper cells. J. Biol. Chem. 2013, 288, 33272–33282. [Google Scholar] [CrossRef]
- Korennykh, A.V.; Egea, P.F.; Korostelev, A.A.; Finer-Moore, J.; Zhang, C.; Shokat, K.M.; Stroud, R.M.; Walter, P. The unfolded protein response signals through high-order assembly of Ire1. Nature 2009, 457, 687–693. [Google Scholar] [CrossRef]
- Wang, L.; Perera, B.G.; Hari, S.B.; Bhhatarai, B.; Backes, B.J.; Seeliger, M.A.; Schurer, S.C.; Oakes, S.A.; Papa, F.R.; Maly, D.J. Divergent allosteric control of the IRE1alpha endoribonuclease using kinase inhibitors. Nat. Chem. Biol. 2012, 8, 982–989. [Google Scholar] [CrossRef]
- Korennykh, A.V.; Korostelev, A.A.; Egea, P.F.; Finer-Moore, J.; Stroud, R.M.; Zhang, C.; Shokat, K.M.; Walter, P. Structural and functional basis for RNA cleavage by Ire1. BMC Biol. 2011, 9, 47. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, W.; Niu, Q.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; Ding, C. eIF2alpha-CHOP-BCl-2/JNK and IRE1alpha-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 2019, 10, 891. [Google Scholar] [CrossRef]
- Turpin, J.; Frumence, E.; Harrabi, W.; Haddad, J.G.; El Kalamouni, C.; Despres, P.; Krejbich-Trotot, P.; Viranaicken, W. Zika virus subversion of chaperone GRP78/BiP expression in A549 cells during UPR activation. Biochimie 2020, 175, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Suomalainen, M.; Jasiqi, Y.; Hemmi, S.; Hearing, P.; Hosie, L.; Burgert, H.G.; Greber, U.F. The UPR sensor IRE1alpha and the adenovirus E3–19K glycoprotein sustain persistent and lytic infections. Nat. Commun. 2020, 11, 1997. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, H.; He, H. Bta-miR-2890 up-regulates JAK-STAT pathway to inhibit BoHV-1 replication by targeting viral gene UL41. Vet. Microbiol. 2020, 245, 108709. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Zhou, X.; Wang, H.; Li, X.; Zhao, A. Induction of the unfolded protein response (UPR) during pseudorabies virus infection. Vet. Microbiol. 2019, 239, 108485. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.P.; Pringle, E.S.; McCormick, C. KSHV activates unfolded protein response sensors but suppresses downstream transcriptional responses to support lytic replication. PLoS Pathog. 2019, 15, e1008185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, Y.; Yap, P.L.; Png, K.J.; Tam, J.P.; Liu, D.X. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J. Virol. 2009, 83, 12462–12472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.R.; Sun, M.X.; Ni, B.; Huan, C.; Huang, L.; Li, C.; Fan, H.J.; Ren, X.F.; Mao, X. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antivir. Res. 2014, 106, 33–41. [Google Scholar] [CrossRef]
- Datan, E.; Roy, S.G.; Germain, G.; Zali, N.; McLean, J.E.; Golshan, G.; Harbajan, S.; Lockshin, R.A.; Zakeri, Z. Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 2016, 7, e2127. [Google Scholar] [CrossRef]
- Ranjitha, H.B.; Ammanathan, V.; Guleria, N.; Hosamani, M.; Sreenivasa, B.P.; Dhanesh, V.V.; Santhoshkumar, R.; Sagar, B.K.C.; Mishra, B.P.; Singh, R.K.; et al. Foot-and-mouth disease virus induces PERK mediated autophagy to suppress antiviral interferon response. J. Cell Sci. 2020, 134, jcs240622. [Google Scholar] [CrossRef]
- Ambrose, R.L.; Mackenzie, J.M. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J. Virol. 2011, 85, 2723–2732. [Google Scholar] [CrossRef]
- Yu, Y.; Pierciey, F.J., Jr.; Maguire, T.G.; Alwine, J.C. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- Hassan, I.H.; Zhang, M.S.; Powers, L.S.; Shao, J.Q.; Baltrusaitis, J.; Rutkowski, D.T.; Legge, K.; Monick, M.M. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 2012, 287, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, H.; Gou, H.; Yuan, J.; Liao, J.; Chen, Y.; Fan, S.; Xie, B.; Deng, S.; Zhang, Y.; et al. CSFV Infection Up-Regulates the Unfolded Protein Response to Promote Its Replication. Front. Microbiol. 2017, 8, 2129. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Hampton, R.Y. Membrane biogenesis and the unfolded protein response. J. Cell Biol. 2004, 167, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Kolpikova, E.P.; Tronco, A.R.; Hartigh, A.B.D.; Jackson, K.J.; Iwawaki, T.; Fink, S.L. IRE1alpha Promotes Zika Virus Infection via XBP1. Viruses 2020, 12, 278. [Google Scholar] [CrossRef] [PubMed]

| Name | Sequences (5′ to 3′) |
|---|---|
| siATF6-1# | ACAGAAACCACTAGTATCA |
| siATF6-2# | CTCATCAACTCAGCATGTT |
| siATF6-3# | CAAGCCTTTATTACTTCCA |
| siPERK-1# | GATCCTAACTGATGTAAGA |
| siPERK-2# | GGTTGATGACTGCAATTAT |
| siPERK-3# | GCTGTATCTGCAATCATCA |
| siIRE1-1# | GCTTTGAGGAGGTCATTGA |
| siIRE1-2# | CTTCTACTACGTGATATCT |
| siIRE1-3# | GGAAATTCAGAACCTATAA |
| Name | Sequences (5′ to 3′) |
|---|---|
| GRP78-F | CGGAGGAGGAGGACAAGAAGGAG C |
| GRP78-R | ATAAGACGGCGTGATGCGGTTG |
| HERPUD1-F | ATCAGAACGCTGCTCCACAAGTG |
| HERPUD1-R | TAGCGGCTGAGTAGGTCCAATCC |
| ATF6-F | GAGGAGCAAGACACATCGGATGAC |
| ATF6-R | TGACAGGGAGGCGGAGGAATATAG |
| GRP94/HSP90B1-F | CAAGATCGAGAAGGCTGTGGTGTC |
| GRP94/HSP90B1-F | GATGTCCTTGCCTGTCTGGTATGC |
| ATF4-F | CCCAAACCCTACGACCCTCCTG |
| ATF4-R | TCCTGTTCCGCCCTCTTCTTCTG |
| DNAJB9/ERdj4-F | GGAGCGCCAAGTCAAGAAGG |
| DNAJB9/ERdj4-R | GCTTCAGCATCAGGGCTCTT |
| BoHV-1 gC-F | ATGTTAGCGCTCTGGAACC |
| BoHV-1 gC-R | CTTTACGGTCGACGACTCC [47] |
| β-actin-F | CCATCGGCAATGAGCGGTTCC |
| β-actin-R | CGTGTTGGCGTAGAGGTCCTTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ma, X.; Wang, H.; He, H. Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection. Viruses 2020, 12, 974. https://doi.org/10.3390/v12090974
Wang S, Ma X, Wang H, He H. Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection. Viruses. 2020; 12(9):974. https://doi.org/10.3390/v12090974
Chicago/Turabian StyleWang, Song, Xiaomei Ma, Hongmei Wang, and Hongbin He. 2020. "Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection" Viruses 12, no. 9: 974. https://doi.org/10.3390/v12090974
APA StyleWang, S., Ma, X., Wang, H., & He, H. (2020). Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection. Viruses, 12(9), 974. https://doi.org/10.3390/v12090974





