Inter-Laboratory Reproducibility of Inducible HIV-1 Reservoir Quantification by TILDA
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sample Collection
2.2. Ethics Statement
2.3. Quantitative Plasma HIV-1 RNA Measurements
2.4. TILDA
2.5. Tat/rev RNA Standard and Cell Lines Used for Assay Validation
2.6. Statistical Analysis
3. Results
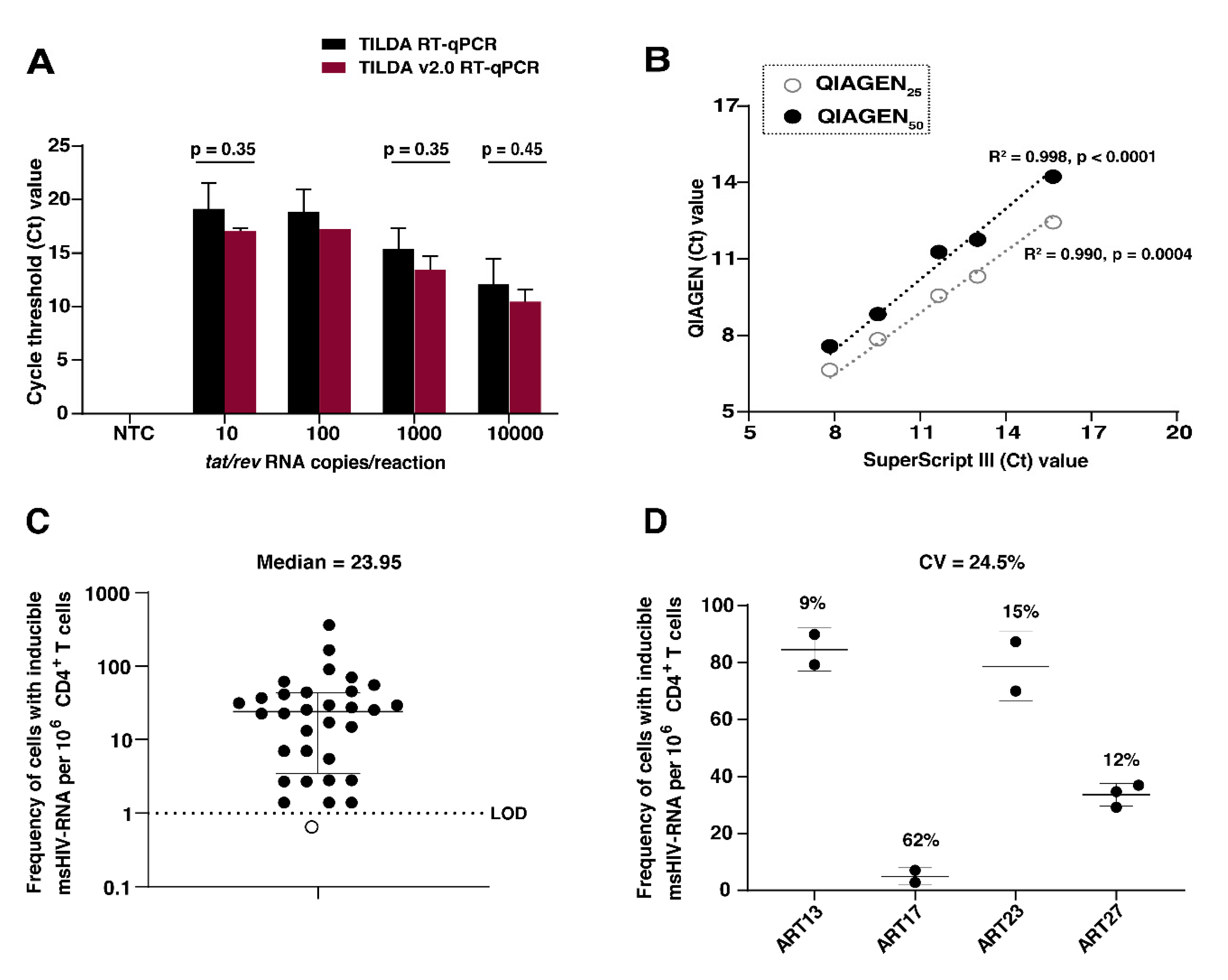
3.1. TILDA Is Amenable to Limited Modifications without Compromising Analytical and Clinical Sensitivity
3.2. TILDA Shows High Inter-Laboratory Reproducibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitman, M.C.; Lau, J.S.Y.; McMahon, J.H.; Lewin, S.R. Barriers and strategies to achieve a cure for HIV. Lancet HIV 2018, 5, e317–e328. [Google Scholar] [CrossRef]
- Laird, G.M.; Rosenbloom, D.I.; Lai, J.; Siliciano, R.F.; Siliciano, J.D. Measuring the frequency of latent HIV-1 in resting CD4(+) T cells using a limiting dilution coculture assay. Methods Mol. Biol. 2016, 1354, 239–253. [Google Scholar]
- Siliciano, J.D.; Siliciano, R.F. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 2005, 304, 3–15. [Google Scholar]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Massanella, M.; Yek, C.; Lada, S.M.; Nakazawa, M.; Shefa, N.; Huang, K.; Richman, D.D. Improved assays to measure and characterize the inducible HIV reservoir. EBioMedicine 2018, 36, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, D.I.S.; Bacchetti, P.; Stone, M.; Deng, X.; Bosch, R.J.; Richman, D.D.; Siliciano, J.D.; Mellors, J.W.; Deeks, S.G.; Ptak, R.G.; et al. Assessing intra-lab precision and inter-lab repeatability of outgrowth assays of HIV-1 latent reservoir size. PLoS Comput. Biol. 2019, 15, e1006849. [Google Scholar] [CrossRef]
- Norton, N.J.; Fun, A.; Bandara, M.; Wills, M.R.; Mok, H.P.; Lever, A.M.L. Innovations in the quantitative virus outgrowth assay and its use in clinical trials. Retrovirology 2017, 14, 58. [Google Scholar] [CrossRef]
- Stone, M.; Rosenbloom, D.; Bacchetti, P.; Deng, X.; Dimapasoc, M.; Keating, S.; Bakkour, S.; Richman, D.; Mellors, J.; Deeks, S.; et al. Assessing suitability of next-generation viral outgrowth assays as proxies for classic QVOA to measure HIV-1 latent reservoir size. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Bocket, L.; Hober, D. Quantification of viral DNA during HIV-1 infection: A review of relevant clinical uses and laboratory methods. Pathol. Biol. 2015, 63, 53–59. [Google Scholar] [CrossRef]
- Plantin, J.; Massanella, M.; Chomont, N. Inducible HIV RNA transcription assays to measure HIV persistence: Pros and cons of a compromise. Retrovirology 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.E.; Niessl, J.; Fromentin, R.; Richard, J.; Porichis, F.; Massanella, M.; Brassard, N.; Alsahafi, N.; Routy, J.P.; Finzi, A.; et al. Multiparametric characterization of rare HIV-infected cells using an RNA-flow FISH technique. Nat. Protoc. 2017, 12, 2029–2049. [Google Scholar] [CrossRef]
- Baxter, A.E.; Niessl, J.; Morou, A.; Kaufmann, D.E. RNA flow cytometric FISH for investigations into HIV immunology, vaccination and cure strategies. Aids Res. 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.E.; O’Doherty, U.; Kaufmann, D.E. Beyond the replication-competent HIV reservoir: Transcription and translation-competent reservoirs. Retrovirology 2018, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Grau-Exposito, J.; Serra-Peinado, C.; Miguel, L.; Navarro, J.; Curran, A.; Burgos, J.; Ocana, I.; Ribera, E.; Torrella, A.; Planas, B.; et al. A novel single-cell fISH-flow assay identifies effector memory CD4(+) T cells as a major niche for HIV-1 transcription in HIV-infected patients. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Pardons, M.; Baxter, A.E.; Massanella, M.; Pagliuzza, A.; Fromentin, R.; Dufour, C.; Leyre, L.; Routy, J.P.; Kaufmann, D.E.; Chomont, N. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019, 15, e1007619. [Google Scholar] [CrossRef] [PubMed]
- Procopio, F.A.; Fromentin, R.; Kulpa, D.A.; Brehm, J.H.; Bebin, A.G.; Strain, M.C.; Richman, D.D.; O’Doherty, U.; Palmer, S.; Hecht, F.M.; et al. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine 2015, 2, 874–883. [Google Scholar] [CrossRef]
- Hu, Y.; Smyth, G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef]
- Clouse, K.A.; Powell, D.; Washington, I.; Poli, G.; Strebel, K.; Farrar, W.; Barstad, P.; Kovacs, J.; Fauci, A.S.; Folks, T.M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 1989, 142, 431–438. [Google Scholar] [PubMed]
- Folks, T.M.; Clouse, K.A.; Justement, J.; Rabson, A.; Duh, E.; Kehrl, J.H.; Fauci, A.S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. USA 1989, 86, 2365–2368. [Google Scholar] [CrossRef]
- Leyre, L.; Kroon, E.; Vandergeeten, C.; Sacdalan, C.; Colby, D.J.; Buranapraditkun, S.; Schuetz, A.; Chomchey, N.; de Souza, M.; Bakeman, W.; et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, A.; D’Urbano, V.; Bon, I.; Verbon, A.; Rokx, C.; Boucher, C.; van Kampen, J.J.A.; Gruters, R.A.; Gallinella, G.; Calza, L.; et al. Development of C-TILDA: A modified TILDA method for reservoir quantification in long term treated patients infected with subtype C HIV-1. J. Virol. Methods 2020, 276, 113778. [Google Scholar] [CrossRef] [PubMed]
- Chatel, L.; Yang, X.; Cholette, F.; Soudeyns, H.; Sandstrom, P.; Lavigne, C. Impact of pre-amplification conditions on sensitivity of the tat/rev induced limiting dilution assay. Arch. Virol. 2018, 163, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Pezzi, H.M.; Berry, S.M.; Beebe, D.J.; Striker, R. RNA-mediated TILDA for improved cell capacity and enhanced detection of multiply-spliced HIV RNA. Integr. Biol. 2017, 9, 876–884. [Google Scholar] [CrossRef]
- Frank, I.; Acharya, A.; Routhu, N.K.; Aravantinou, M.; Harper, J.L.; Maldonado, S.; Sole Cigoli, M.; Semova, S.; Mazel, S.; Paiardini, M.; et al. A Tat/Rev induced limiting dilution assay to measure viral reservoirs in non-human primate models of HIV infection. Sci. Rep. 2019, 9, 12078. [Google Scholar] [CrossRef]
- Dhummakupt, A.; Rubens, J.H.; Anderson, T.; Powell, L.; Nonyane, B.A.; Siems, L.V.; Collinson-Streng, A.; Nilles, T.; Jones, R.B.; Tepper, V.; et al. Differences in inducibility of the latent HIV reservoir in perinatal and adult infection. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Crooks, A.M.; Bateson, R.; Cope, A.B.; Dahl, N.P.; Griggs, M.K.; Kuruc, J.D.; Gay, C.L.; Eron, J.J.; Margolis, D.M.; Bosch, R.J.; et al. Precise quantitation of the latent HIV-1 reservoir: Implications for eradication strategies. J. Infect. Dis. 2015, 212, 1361–1365. [Google Scholar] [CrossRef]
- Henrich, T.J.; Deeks, S.G.; Pillai, S.K. Measuring the size of the latent human immunodeficiency virus reservoir: The present and future of evaluating eradication strategies. J. Infect. Dis. 2017, 215, S134–S141. [Google Scholar] [CrossRef][Green Version]
- Massanella, M.; Richman, D.D. Measuring the latent reservoir in vivo. J. Clin. Investig. 2016, 126, 464–472. [Google Scholar] [CrossRef]

| Characteristic | TILDA | TILDA v2.0 |
|---|---|---|
| Input volume of cells per reaction 1 | 1 µL | 10 µL |
| One-step RT-PCR reagents | Superscript III Platinum Taq (Life Technologies) & Buffer, RNase inhibitor (Life Technologies), Tris-EDTA (TE) buffer | HotStarTaq DNA Polymerase, Sensiscript and Omniscript Reverse Transcriptases & Buffer (QIAGEN), RNasin Ribonuclease Inhibitor (Promega), 0.1% solution of Triton X-100 |
| Final reaction volume | 11 µL | 50 µL |
| Cycling conditions | 50 °C for 15 min, 95 °C for 2 min, 24 cycles of amplification (95 °C 15 s, 60 °C 4 min) | 50 °C for 30 min, 95 °C for 15 min, 25 cycles of amplification (95 °C 30 s, 55 °C 1 min, 72 °C 2 min) final extension at 72 °C for 5 min |
| Input volume of pre-amplified product per reaction | 1 µL of 1:5 dilution | 2 µL |
| Real-time PCR reagent | LightCycler 480 Probe Master buffer (Roche Applied Sciences) | Custom TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific) |
| Final reaction volume | 10 µL | 20 µL |
| Cycling conditions | 95 °C for 10 min, 45 cycles of 95 °C 10 s, 60 °C 30 s, 72 °C 1 s and a cooling step at 40 °C for 30 s | 50 °C for 5 min (UNG step), 95 °C for 20 s, 45 cycles of 95 °C for 3 s and 60 °C for 30 s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.; Procopio, F.A.; Overmars, R.J.; Beerkens, R.J.J.; Voermans, J.J.C.; Rao, S.; Prins, H.A.B.; Rokx, C.; Pantaleo, G.; Vijver, D.A.M.C.v.d.; et al. Inter-Laboratory Reproducibility of Inducible HIV-1 Reservoir Quantification by TILDA. Viruses 2020, 12, 973. https://doi.org/10.3390/v12090973
Lungu C, Procopio FA, Overmars RJ, Beerkens RJJ, Voermans JJC, Rao S, Prins HAB, Rokx C, Pantaleo G, Vijver DAMCvd, et al. Inter-Laboratory Reproducibility of Inducible HIV-1 Reservoir Quantification by TILDA. Viruses. 2020; 12(9):973. https://doi.org/10.3390/v12090973
Chicago/Turabian StyleLungu, Cynthia, Francesco A. Procopio, Ronald J. Overmars, Rob J. J. Beerkens, Jolanda J. C. Voermans, Shringar Rao, Henrieke A. B. Prins, Casper Rokx, Giuseppe Pantaleo, David A. M. C. van de Vijver, and et al. 2020. "Inter-Laboratory Reproducibility of Inducible HIV-1 Reservoir Quantification by TILDA" Viruses 12, no. 9: 973. https://doi.org/10.3390/v12090973
APA StyleLungu, C., Procopio, F. A., Overmars, R. J., Beerkens, R. J. J., Voermans, J. J. C., Rao, S., Prins, H. A. B., Rokx, C., Pantaleo, G., Vijver, D. A. M. C. v. d., Mahmoudi, T., Boucher, C. A. B., Gruters, R. A., & Kampen, J. J. A. v. (2020). Inter-Laboratory Reproducibility of Inducible HIV-1 Reservoir Quantification by TILDA. Viruses, 12(9), 973. https://doi.org/10.3390/v12090973








