Identification and Prevalence of Phascolarctid Gammaherpesvirus Types 1 and 2 in South Australian Koala Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Sample Collection
2.3. Wild-Caught Koalas
2.4. Euthanased Cohort
2.5. DNA Extraction
2.6. Quality Control
2.7. Molecular Diagnostics (Conventional PCR)
2.8. Statistical Analyses
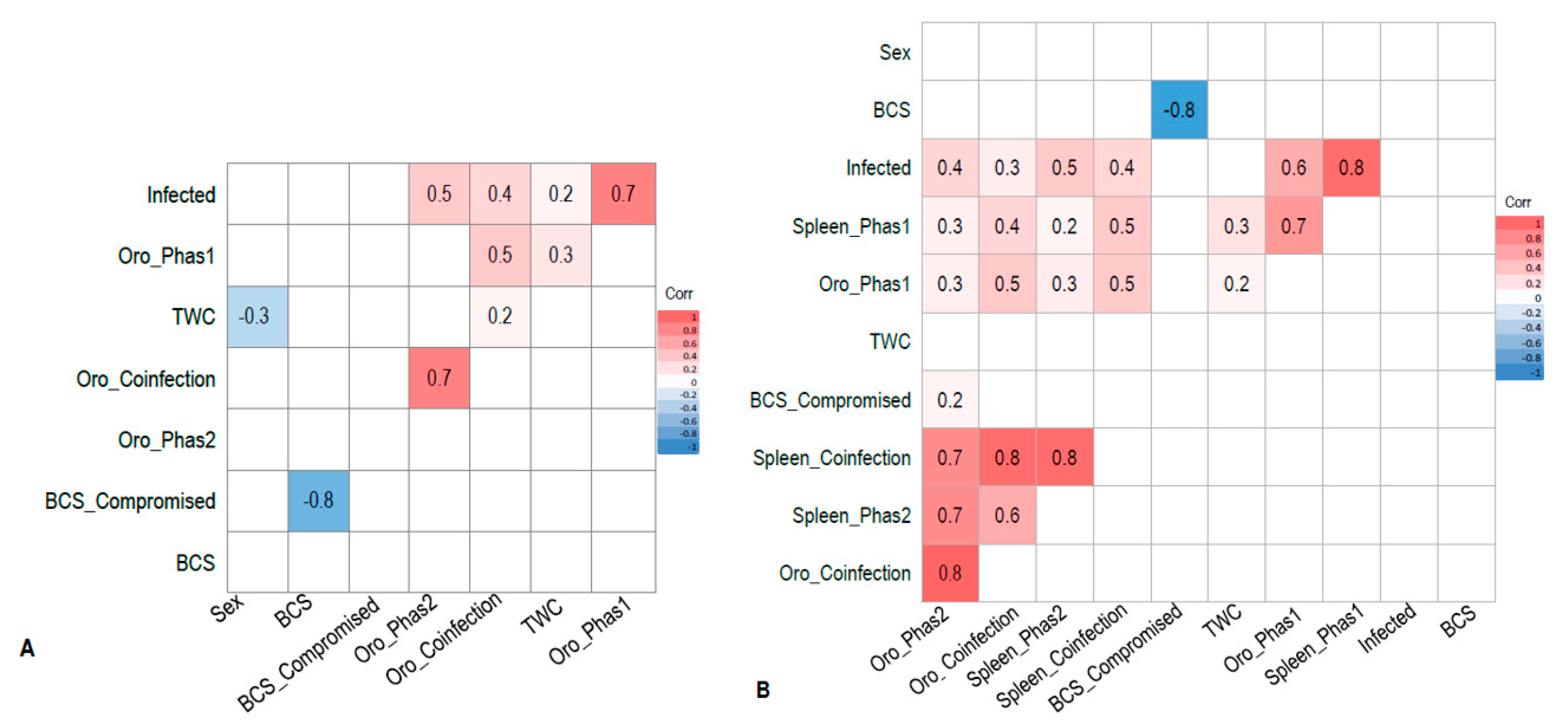
3. Results
3.1. PhaHV-1 and PhaHV-2 Specific PCR Test
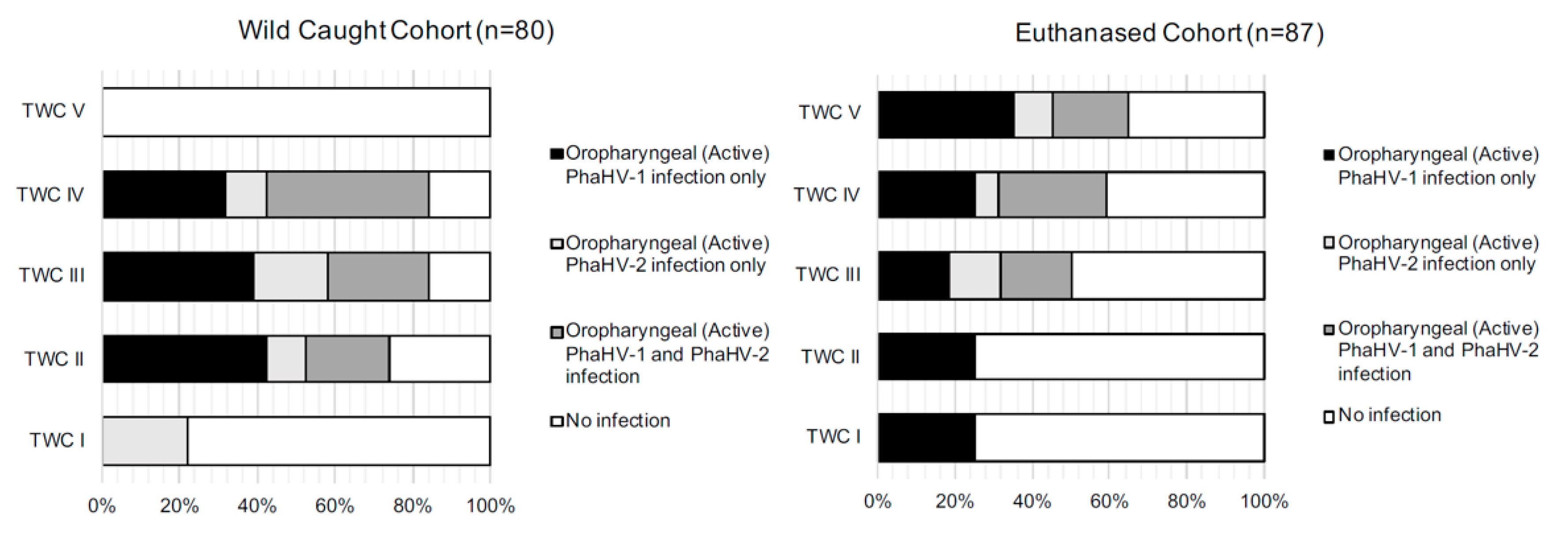
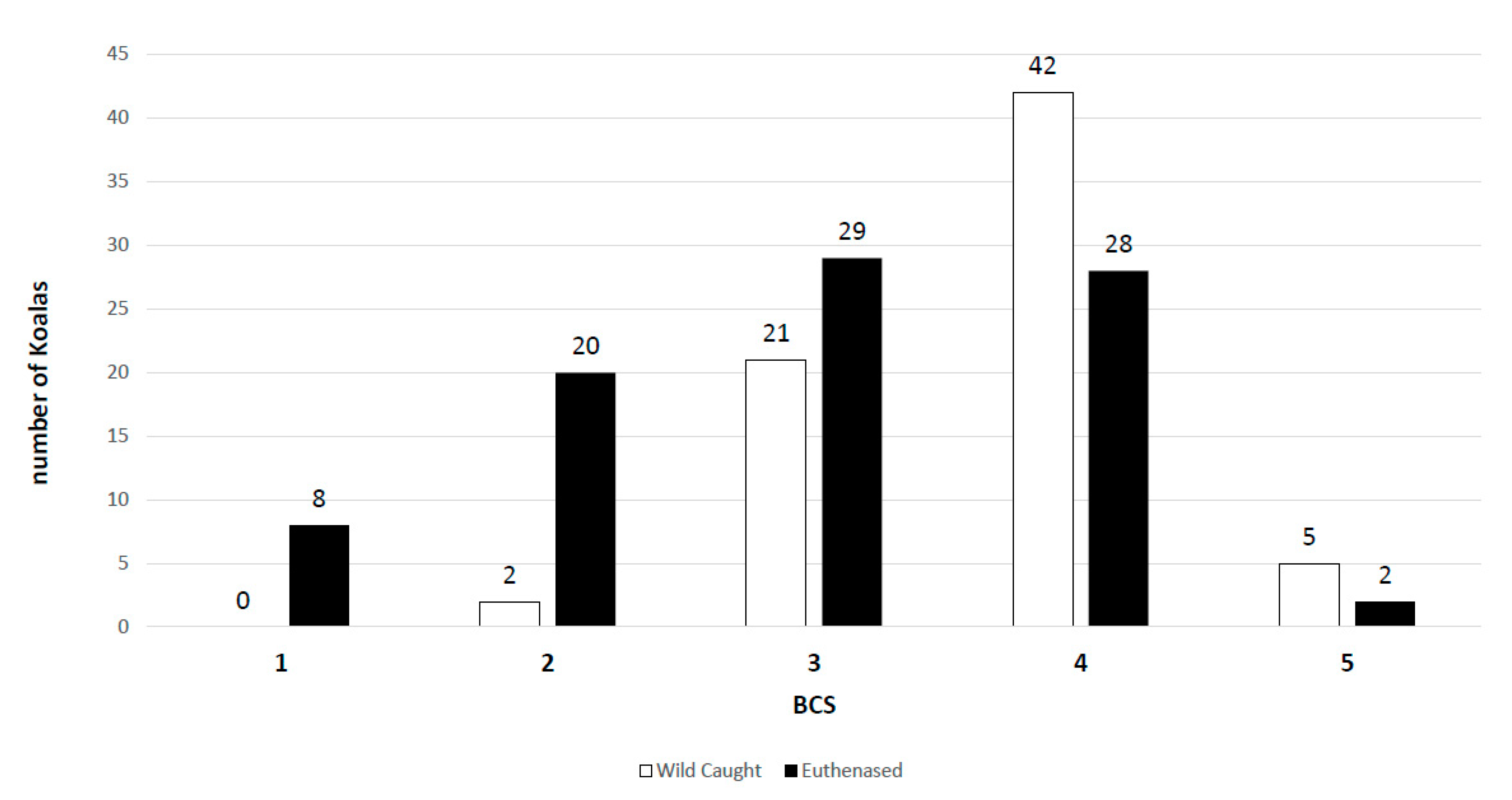
3.2. Wild-Caught Cohort of Koalas
3.3. Euthanased Cohort of Koalas
4. Discussion
4.1. Active Shedding
4.2. Systemic Infection and Active Shedding
4.3. Sites of Active Viral Shedding
4.4. Potential Areas for Further Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapter 9 - Herpesvirales. In Fenner’s Veterinary Virology (Fifth Edition); MacLachlan, N.J., Dubovi, E.J., Eds.; Academic Press: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Finnie, E.P.; Littlejohns, I.R.; Acland, H.M. Letter: Mortalities in parma wallabies (Macropus parma) associated with probable herpesvirus. Aust. Vet. J. 1976, 52, 294. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Whalley, J.M.; Littlejohns, I.R.; Dickson, J.; Smith, V.W.; Wilks, C.R.; Reisner, A.H. Macropodid herpesviruses 1 and 2: Two herpesviruses from Australian marsupials differentiated by restriction endonucleases, DNA composition and hybridization. Brief Rep. Arch. Virol. 1985, 85, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Wellehan, J.F., Jr.; Pogranichniy, R.M.; Childress, A.L.; Landolfi, J.A.; Terio, K.A. Identification and isolation of a novel herpesvirus in a captive mob of eastern grey kangaroos (Macropus giganteus). Vet. Microbiol. 2008, 129, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Vaz, P.; Whiteley, P.L.; Wilks, C.R.; Duignan, P.J.; Ficorilli, N.; Gilkerson, J.R.; Browning, G.F.; Devlin, J.M. Detection of a novel gammaherpesvirus in koalas (Phascolarctos cinereus). J. Wildl. Dis. 2011, 47, 787–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaz, P.; Whiteley, P.L.; Wilks, C.R.; Browning, G.F.; Gilkerson, J.R.; Ficorilli, N.; Devlin, J.M. Detection of a second novel gammaherpesvirus in a free-ranging koala (Phascolarctos cinereus). Wildl. Dis. 2012, 48, 226–229. [Google Scholar] [CrossRef]
- Vaz, P.K.; Legione, A.R.; Hartley, C.A.; Devlin, J.M. Detection and Differentiation of Two Koala Gammaherpesviruses by Use of High-Resolution Melt (HRM) Analysis Reveals Differences in Viral Prevalence and Clinical Associations in a Large Study of Free-Ranging Koalas. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Stalder, K.; Vaz, P.K.; Gilkerson, J.R.; Baker, R.; Whiteley, P.; Ficorilli, N.; Tatarczuch, L.; Portas, T.; Skogvold, K.; Anderson, G.A.; et al. Prevalence and Clinical Significance of Herpesvirus Infection in Populations of Australian Marsupials. PLoS ONE 2015, 10, e0133807. [Google Scholar] [CrossRef]
- Sugden, B.; Kintner, C.R.; Mark, W. The molecular biology of lymphotropic herpesviruses. Adv. Cancer Res. 1979, 30, 239–278. [Google Scholar] [CrossRef]
- Li, H.; Taus, N.S.; Lewis, G.S.; Kim, O.; Traul, D.L.; Crawford, T.B. Shedding of ovine herpesvirus 2 in sheep nasal secretions: The predominant mode for transmission. J. Clin. Microbiol. 2004, 42, 5558–5564. [Google Scholar] [CrossRef]
- Ackermann, M. Pathogenesis of gammaherpesvirus infections. Vet. Microbiol. 2006, 113, 211–222. [Google Scholar] [CrossRef]
- Hussy, D.; Janett, F.; Albini, S.; Stauber, N.; Thun, R.; Ackermann, M. Analysis of the pathogenetic basis for shedding and transmission of ovine gamma herpesvirus 2. Clin. Microbiol. 2002, 40, 4700–4704. [Google Scholar] [CrossRef] [PubMed]
- Fabijan, J.; Sarker, N.; Speight, N.; Owen, H.; Meers, J.; Simmons, G.; Seddon, J.; Emes, R.D.; Tarlinton, R.; Hemmatzadeh, F.; et al. Pathological Findings in Koala Retrovirus-positive Koalas (Phascolarctos cinereus) from Northern and Southern Australia. J. Comp. Pathol. 2020, 176, 50–66. [Google Scholar] [CrossRef]
- Hemsley, S.; Canfield, P.J. Histopathological and immunohistochemical investigation of naturally occurring chlamydial conjunctivitis and urogenital inflammation in koalas (Phascolarctos cinereus). J. Comp. Pathol. 1997, 116, 273–290. [Google Scholar] [CrossRef]
- Flano, E.; Kim, I.J.; Woodland, D.L.; Blackman, M.A. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J. Exp. Med. 2002, 196, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Gerow, C.M.; Rapin, N.; Voordouw, M.J.; Elliot, M.; Misra, V.; Subudhi, S. Arousal from hibernation and reactivation of Eptesicus fuscus gammaherpesvirus (EfHV) in big brown bats. Transbound. Emerg. Dis. 2019, 66, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Weck, K.E.; Barkon, M.L.; Yoo, L.I.; Speck, S.H. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus. Virology 1996. [Google Scholar] [CrossRef]
- Feldman, E.R.; Kara, M.; Coleman, C.B.; Grau, K.R.; Oko, L.M.; Krueger, B.J.; Renne, R.; van Dyk, L.F.; Tibbetts, S.A. Virus-encoded microRNAs facilitate gammaherpesvirus latency and pathogenesis in vivo. mBio 2014, 5, e00914–e00981. [Google Scholar] [CrossRef]
- Sharma, V.; Mobeen, F.; Prakash, T. Comparative Genomics of Herpesviridae Family to Look for Potential Signatures of Human Infecting Strains. Int. J. Genom. 2016, 2016, 9543274. [Google Scholar] [CrossRef]
- van Dyk, L.F.; Virgin, H.W.t.; Speck, S.H. Maintenance of gammaherpesvirus latency requires viral cyclin in the absence of B lymphocytes. Virology 2003, 77, 5118–5126. [Google Scholar] [CrossRef][Green Version]
- Martin, R.W. Age-Specific Fertility in Three Populations of the Koala, Phascolarctos cinereus Goldfuss, in Victoria. Wildl. Res. 1981, 8, 275–283. [Google Scholar] [CrossRef]
- Blanshard, W.H.; Bodley, K. Chapter 8: Koalas. In Medicine of Australian Mammals, 1st ed.; Vogelnest, L., Woods, R., Eds.; CSIRO Publishing: Collingwood, Clayton, Australia, 2008; pp. 227–328. [Google Scholar]
- Lieberman, P.M. Keeping it quiet: Chromatin control of gammaherpesvirus latency. Nat. Rev. Microbiol. 2013, 11, 863–875. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier 2012, 99–107. [Google Scholar] [CrossRef]
- Shojima, T.; Yoshikawa, R.; Hoshino, S.; Shimode, S.; Nakagawa, S.; Ohata, T.; Nakaoka, R.; Miyazawa, T. Identification of a novel subgroup of Koala retrovirus from Koalas in Japanese zoos. Virology 2013, 87, 9943–9948. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.; Blanshard, W.H.; Ocallaghan, P.G. Microbiological Studies of the Intestinal Microflora of the Koala, Phascolarctos cinereus. Pap, a Special Maternal Feces Consumed by Juvenile Koalas. Aust. J. Zool. 1993, 41, 527–536. [Google Scholar] [CrossRef]
- Kent, A.; Ehlers, B.; Mendum, T.; Newman, C.; Macdonald, D.W.; Chambers, M.; Buesching, C.D. Genital tract screening finds widespread infection with mustelid gammaherpesvirus 1 in the uropean badger (meles meles). J. Wildl. Dis. 2018, 133–137. [Google Scholar] [CrossRef]
- Meier-Trummer, C.S.; Rehrauer, H.; Franchini, M.; Patrignani, A.; Wagner, U.; Ackermann, M. Malignant catarrhal fever of cattle is associated with low abundance of IL-2 transcript and a predominantly latent profile of ovine herpesvirus 2 gene expression. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Razonable, R.R.; Brown, R.A.; Humar, A.; Covington, E.; Alecock, E.; Paya, C.V. Herpesvirus infections in solid organ transplant patients at high risk of primary cytomegalovirus disease. J. Infect. Dis. 2005, 192, 1331–1339. [Google Scholar] [CrossRef]
- Blazquez-Navarro, A.; Dang-Heine, C.; Wittenbrink, N.; Bauer, C.; Wolk, K.; Sabat, R.; Westhoff, T.; Sawitzki, B.; Reinke, P.; Thomusch, O.; et al. BKV, CMV, and EBV Interactions and their Effect on Graft Function One Year Post-Renal Transplantation: Results from a Large Multi-Centre Study. EBioMedicine 2018, 34, 113–121. [Google Scholar] [CrossRef]
- Aalto, S.M.; Linnavuori, K.; Peltola, H.; Vuori, E.; Weissbrich, B.; Schubert, J.; Hedman, K. Immunoreactivation of Epstein-Barr virus due to cytomegalovirus primary infection. J. Med. Virol. 1998, 56, 186–191. [Google Scholar] [CrossRef]
- Jain, R.; Trehan, A.; Mishra, B.; Singh, R.; Saud, B.; Bansal, D. Cytomegalovirus disease in children with acute lymphoblastic leukemia. J. Pediatric Hematol. /Oncol. 2016, 33, 239–247. [Google Scholar] [CrossRef]
- Handous, I.; Achour, B.; Marzouk, M.; Rouis, S.; Hazgui, O.; Brini, I.; Khelif, A.; Hannachi, N.; Boukadida, J. Co-infections of human herpesviruses (CMV, HHV-6, HHV-7 and EBV) in non-transplant acute leukemia patients undergoing chemotherapy. Virology 2020, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.; Hanger, J.; Jelocnik, M.; Quigley, B.L.; Timms, P. Longitudinal study of wild koalas (Phascolarctos cinereus) reveals chlamydial disease progression in two thirds of infected animals. Sci. Rep. 2019, 9, 13194. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Boss, J.M.; Speck, S.H. Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. Virology 2009, 83, 6484–6493. [Google Scholar] [CrossRef] [PubMed]
- Rekow, M.M.; Darrah, E.J.; Mboko, W.P.; Lange, P.T.; Tarakanova, V.L. Gammaherpesvirus targets peritoneal B-1 B cells for long-term latency. Virology 2016, 492, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Usherwood, E.J.; Roy, D.J.; Ward, K.; Surman, S.L.; Dutia, B.M.; Blackman, M.A.; Stewart, J.P.; Woodland, D.L. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 2000, 192, 943–952. [Google Scholar] [CrossRef]
- Higgins, D.; Lau, Q.; Maher, I. Koala immunology and the koala retrovirus (KoRV). Tech. Rep. Aust. Mus. Online 2014, 35–38. [Google Scholar] [CrossRef][Green Version]
- Wilkinson, R.; Kotlarski, I.; Barton, M. Koala lymphoid cells: Analysis of antigen-specific responses. Vet. Immunol. Immunopathol. 1992, 33, 237–247. [Google Scholar] [CrossRef]

| Target | Primer Name | Primer Sequence | Product Bp | Annealing Temp | Reference |
|---|---|---|---|---|---|
| β-actin (QC) | β-actin-Fwd | 5′ GAGACCTTCAACACCCCAGC 3′ | 111 | 60 °C | Shojima et al. (2013) [25] |
| β-actin-Rev | 5′ GTGGGTCACACCATCACCAG 3′ | ||||
| PhaHV-1 | VK-PhaHV-1-Fwd | 5′ CGGCATCCTCCCCTGTTTAA 3′ | 220 | 61 °C | Current study |
| VK-PhaHV-1-Rev | 5′ GCCCCTACATTCAACGAACA 3′ | ||||
| PhaHV-2 | VK-PhaHV-2 Fwd | 5′ CGCACTCTAAGCTGTCCCTT 3′ | 330 | 64 °C | Current study |
| VK-PhaHV-2 Rev | 5′ TTTCGAGCATCATGCGTCCT 3′ |
| Primers | Sample | PhaHV-1 (JN585829.1) | PhaHV-2 (JQ996387.1) | Source | Query Cover | Per Ident |
|---|---|---|---|---|---|---|
| VK-PhaHV-1 | K18-051 | + | Oro | 100% | 100% | |
| K18-051 | + | Spleen | 100% | 100% | ||
| K18-064 | + | Oro | 100% | 100% | ||
| K18-064 | + | Spleen | 100% | 100% | ||
| VK-PhaHV-2 | K18-043 | + | Oro | 100% | 100% | |
| K18-043 | + | Spleen | 100% | 100% | ||
| K18-044 | + | Oro | 100% | 100% | ||
| K18-044 | + | Spleen | 100% | 100% |
| Type of Infection | Wild-Caught | Euthanased | ||
|---|---|---|---|---|
| n | % | n | % | |
| Active Shedding | 58 | 73% | 47 | 54% |
| Active Shedding Only PhaHV-1 | 26 | 33% | 23 | 26% |
| Active Shedding Only PhaHV-2 | 12 | 15% | 7 | 8% |
| Coinfected Shedding | 20 | 25% | 17 | 20% |
| No active shedding | 22 | 28% | 40 | 46% |
| TOTAL | 80 | 87 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimov, V.; Stephenson, T.; Speight, N.; Chaber, A.-L.; Boardman, W.; Easther, R.; Hemmatzadeh, F. Identification and Prevalence of Phascolarctid Gammaherpesvirus Types 1 and 2 in South Australian Koala Populations. Viruses 2020, 12, 948. https://doi.org/10.3390/v12090948
Kasimov V, Stephenson T, Speight N, Chaber A-L, Boardman W, Easther R, Hemmatzadeh F. Identification and Prevalence of Phascolarctid Gammaherpesvirus Types 1 and 2 in South Australian Koala Populations. Viruses. 2020; 12(9):948. https://doi.org/10.3390/v12090948
Chicago/Turabian StyleKasimov, Vasilli, Tamsyn Stephenson, Natasha Speight, Anne-Lise Chaber, Wayne Boardman, Ruby Easther, and Farhid Hemmatzadeh. 2020. "Identification and Prevalence of Phascolarctid Gammaherpesvirus Types 1 and 2 in South Australian Koala Populations" Viruses 12, no. 9: 948. https://doi.org/10.3390/v12090948
APA StyleKasimov, V., Stephenson, T., Speight, N., Chaber, A.-L., Boardman, W., Easther, R., & Hemmatzadeh, F. (2020). Identification and Prevalence of Phascolarctid Gammaherpesvirus Types 1 and 2 in South Australian Koala Populations. Viruses, 12(9), 948. https://doi.org/10.3390/v12090948







