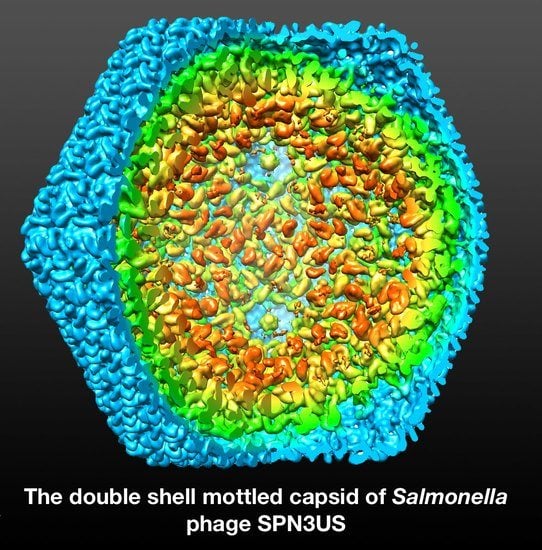
The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell
Abstract
1. Introduction
2. Materials and Methods
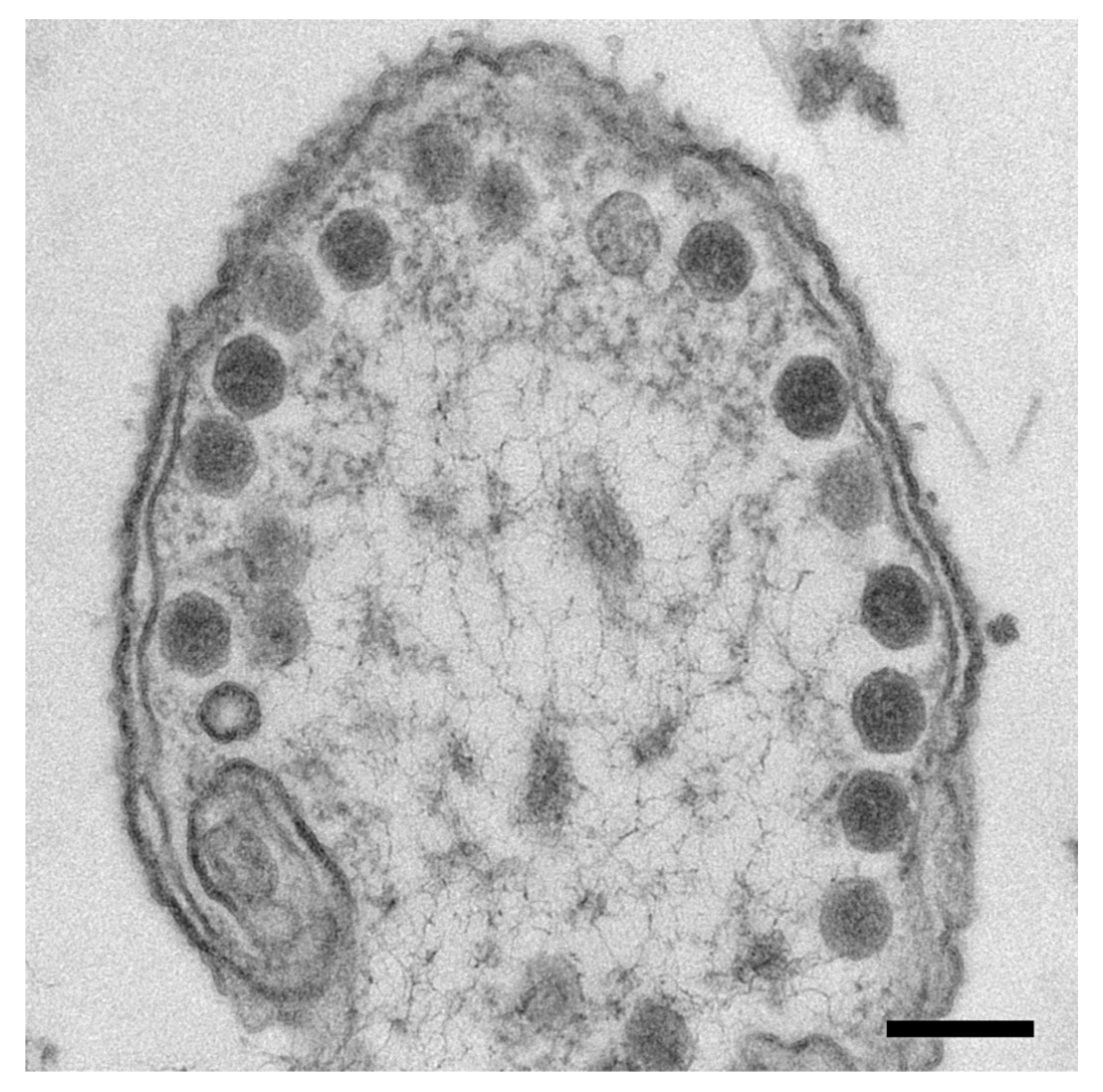
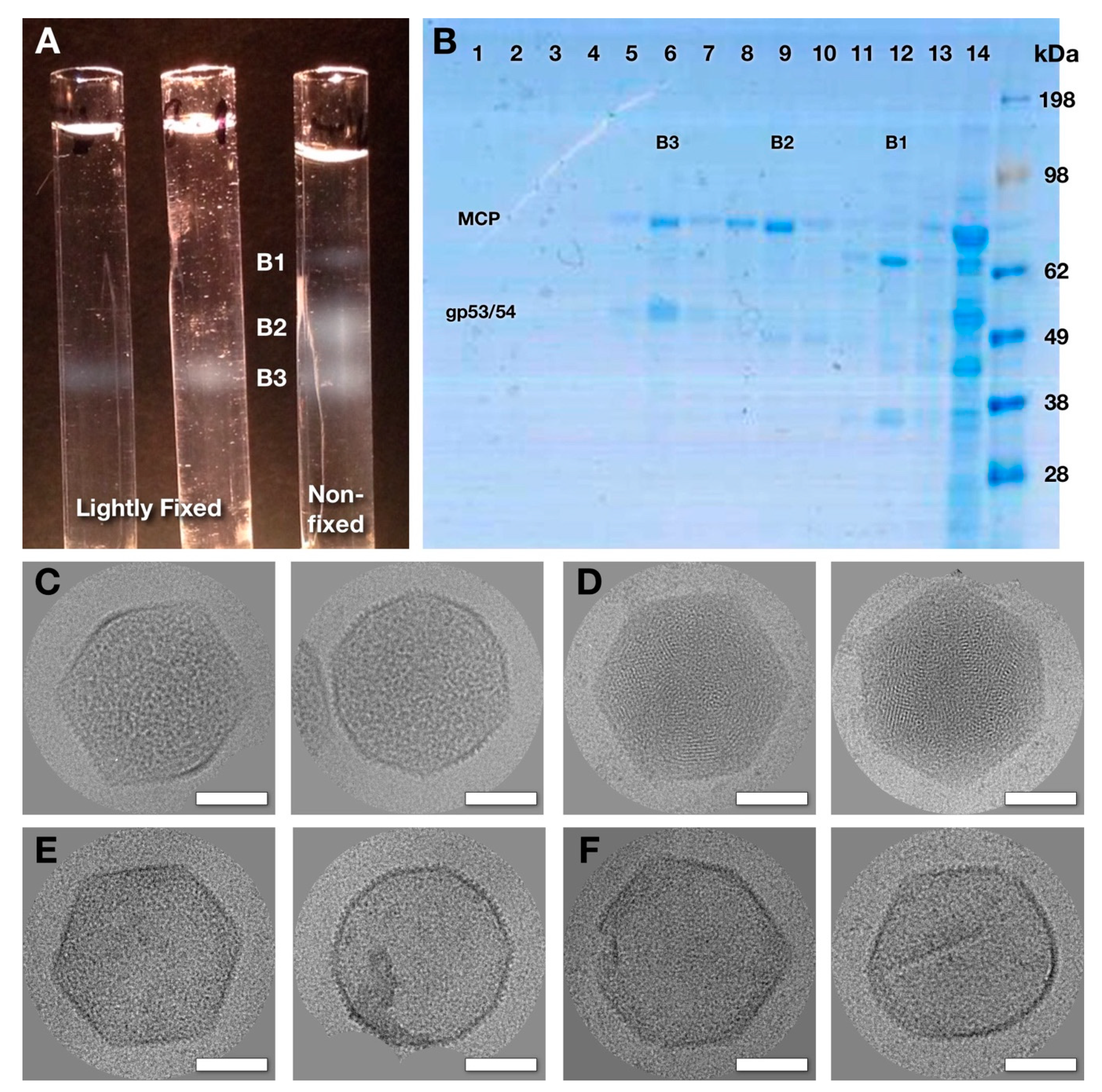
2.1. Purification of Wild-Type Virions and Mutant Capsids.
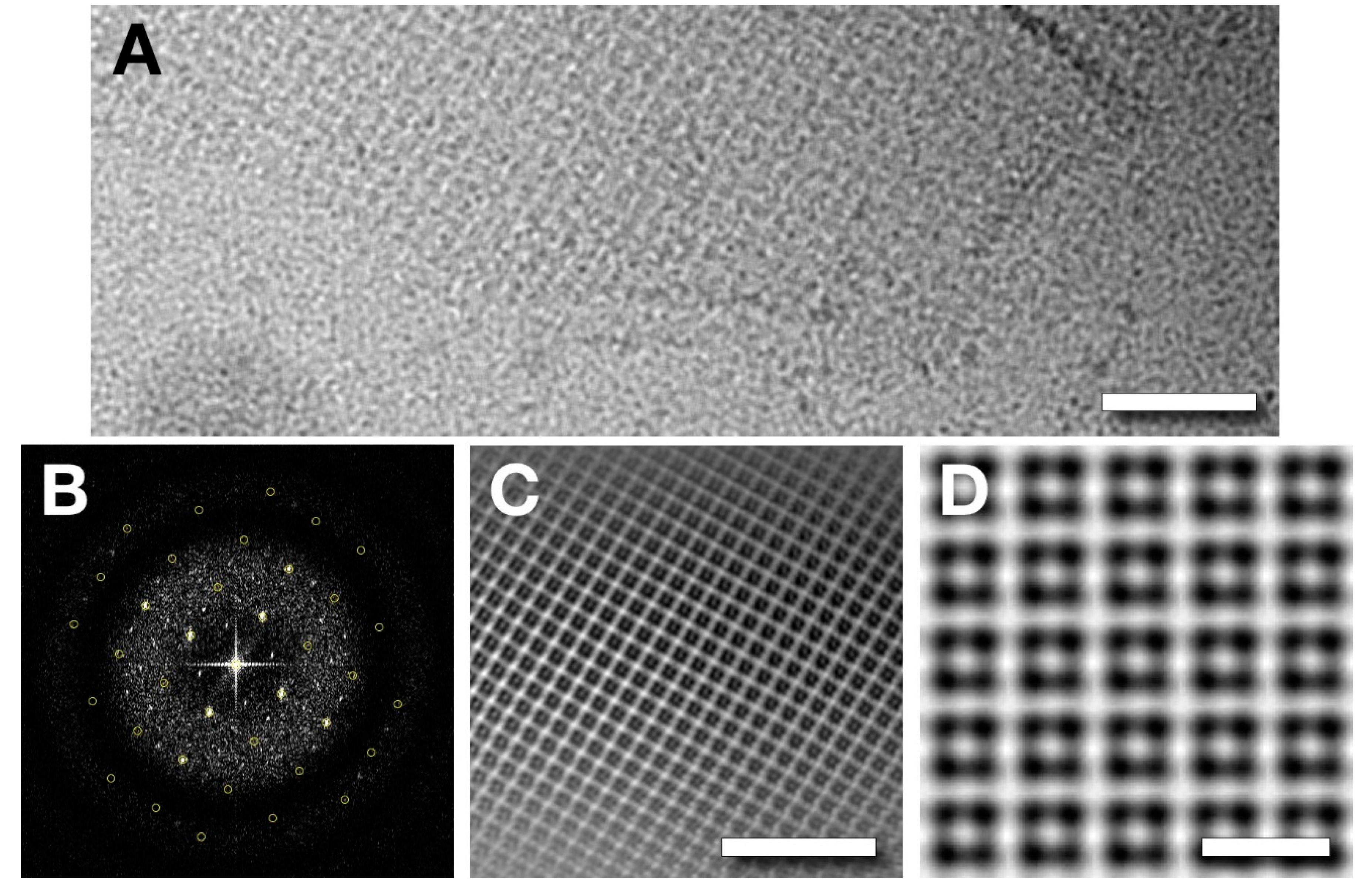
2.2. Electron Microscopy
2.3. Image Processing
3. Results
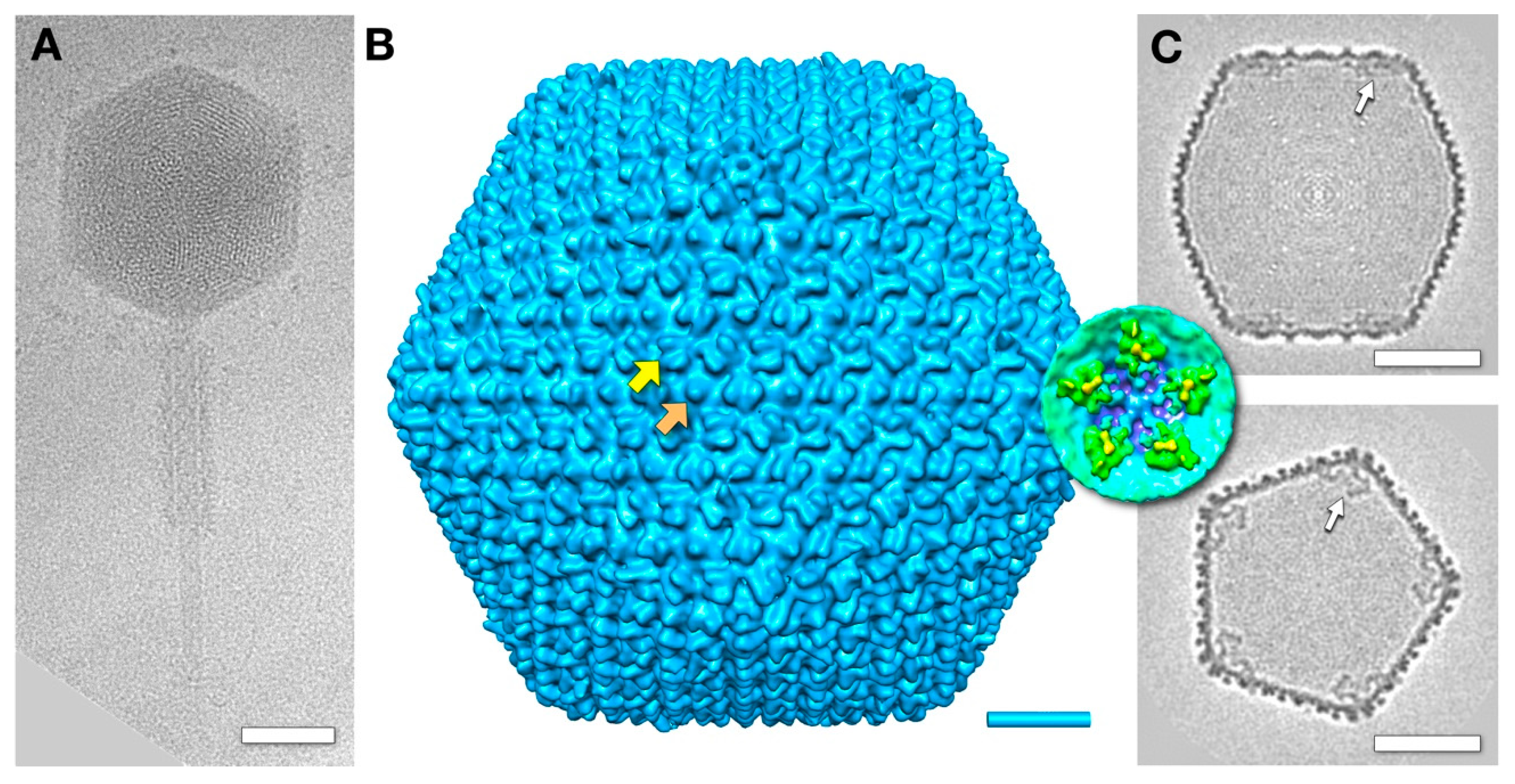
3.1. The Mature Virion Head
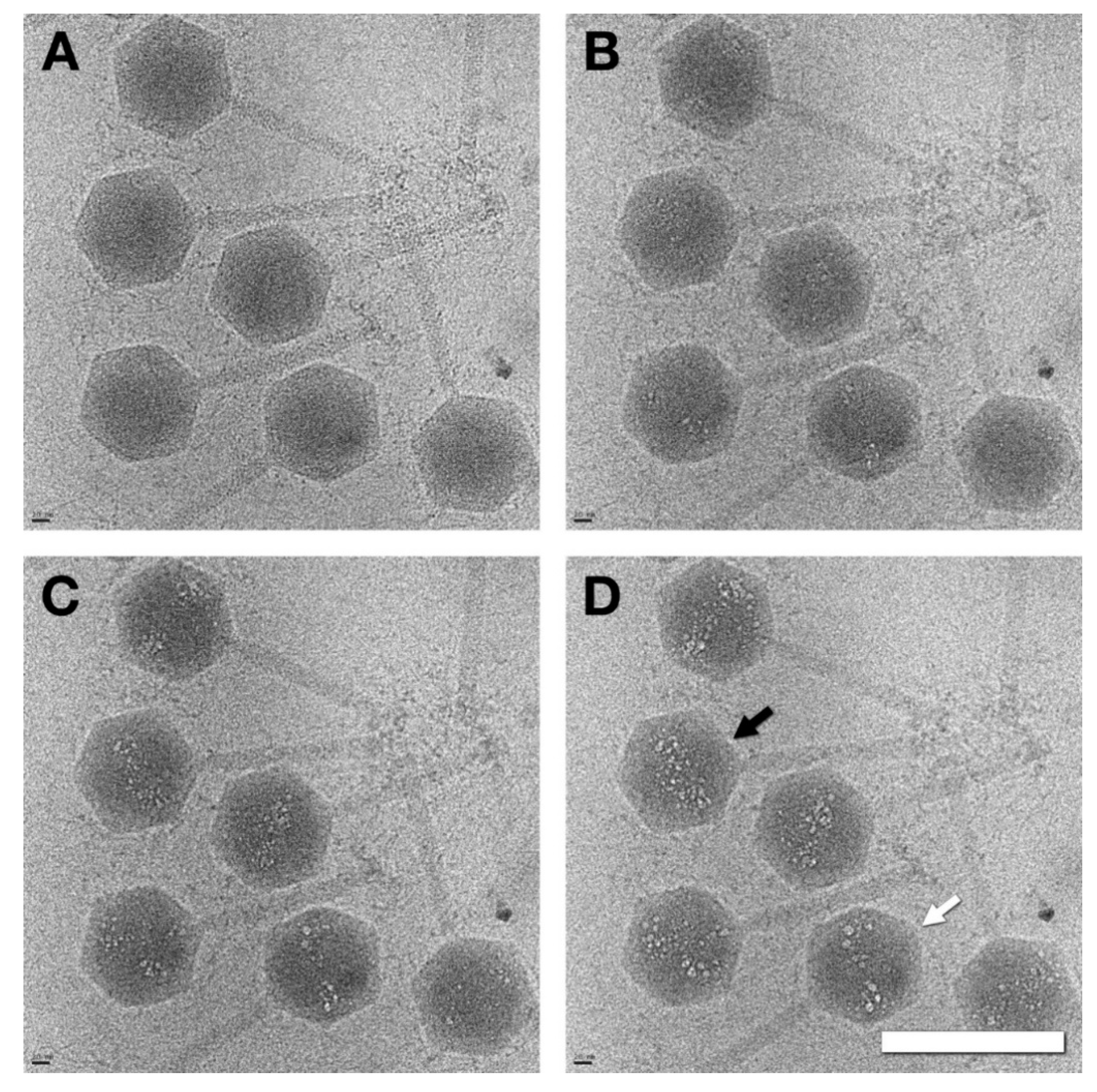
3.2. Capsids Produced with Impaired Protease Activity
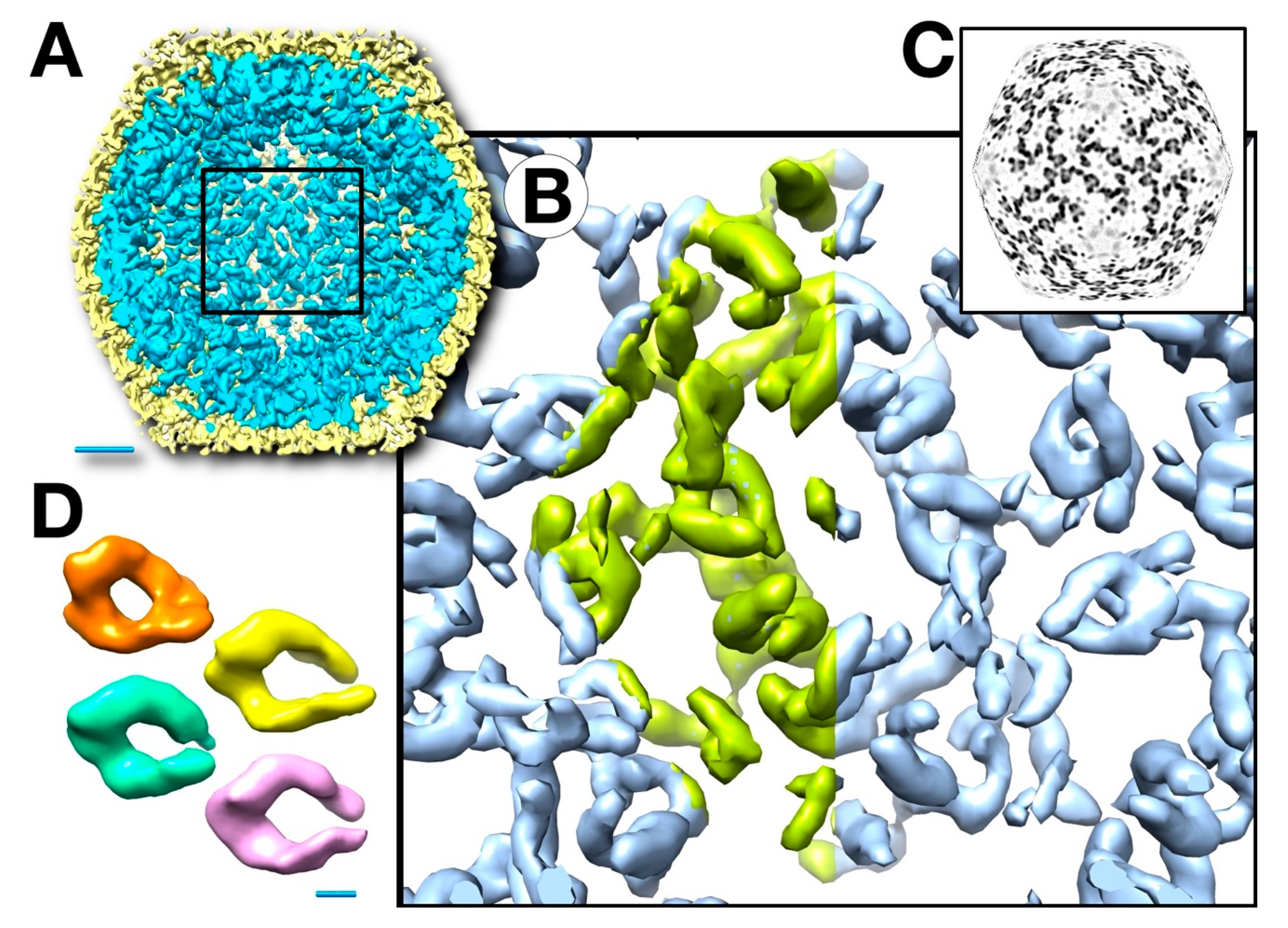
3.3. Three-Dimensional Reconstructions
3.4. Mass Distribution in the Capsid Maps
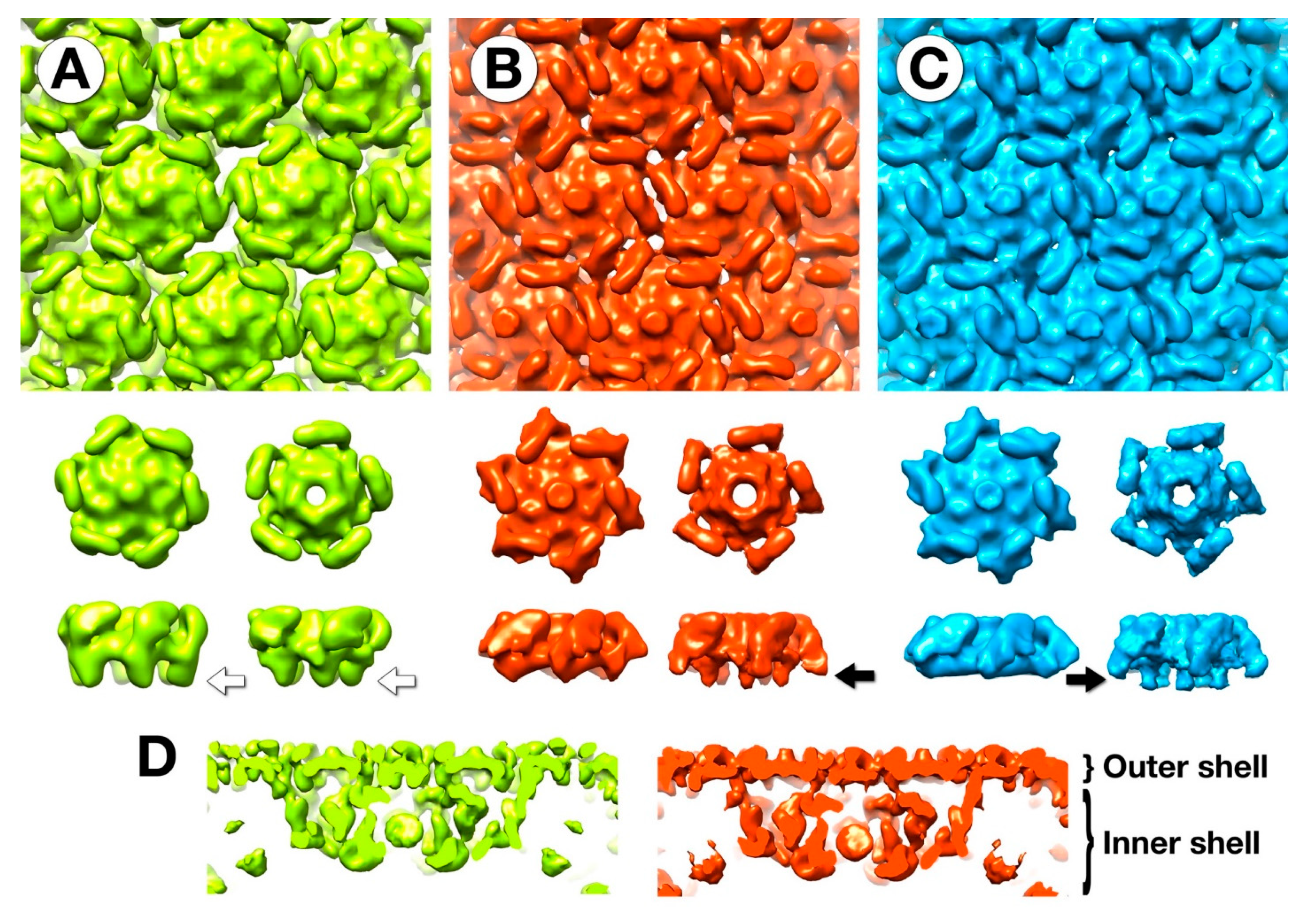
3.5. The Outer Shell
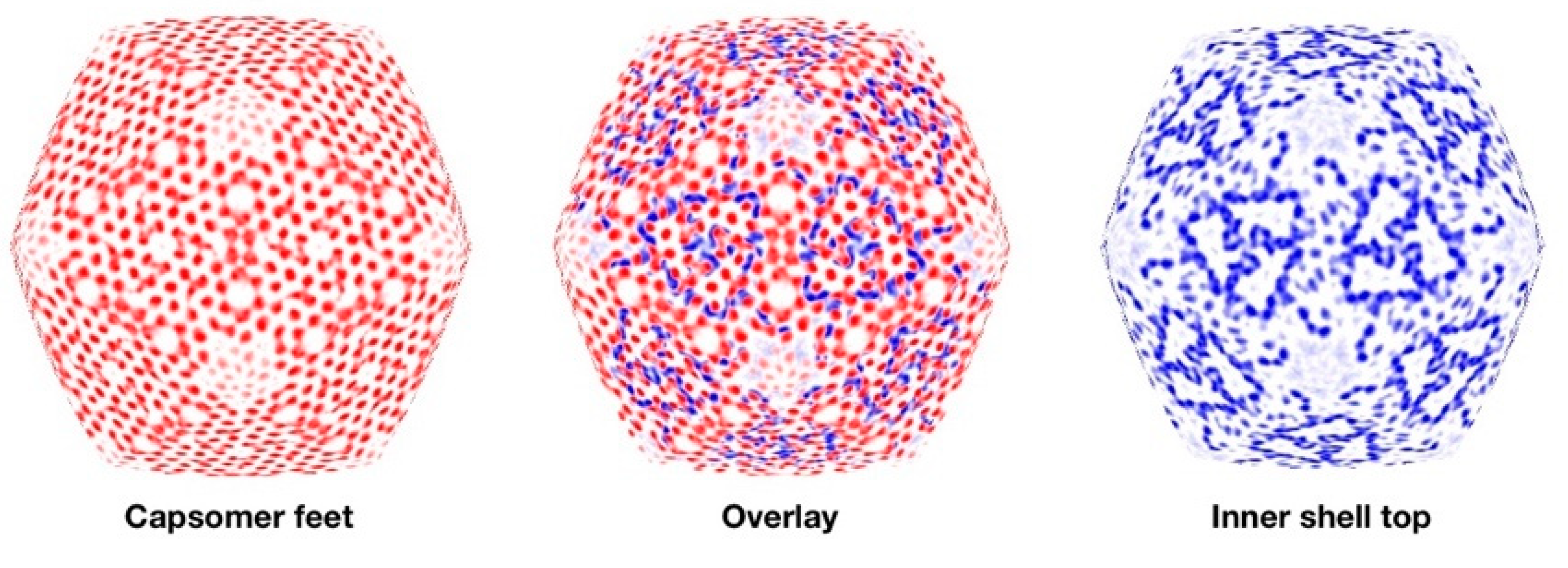
3.6. Segmentation and Analysis of the Inner Shell
3.7. Analysis of a Tetragonal Sheet Observed in the 8k Supe
4. Discussion
4.1. The Outer Capsid Shell
4.2. Major Internal Proteins
4.3. Minor Proteins
4.4. The Mottled Capsid—A Maturation Intermediate?
4.5. Future Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hendrix, R.W. Jumbo bacteriophages. Curr. Top. Microbiol. Immunol. 2009, 328, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Benitez Quintana, A.D.; Bosch, M.A.; Coll De Pena, A.; Aguilera, E.; Coulibaly, A.; Wu, W.; Osier, M.V.; Hudson, A.O.; Weintraub, S.T.; et al. Identification of Essential Genes in the Salmonella Phage SPN3US Reveals Novel Insights into Giant Phage Head Structure and Assembly. J. Virol. 2016, 90, 10284–10298. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Desmond, M.I.; Mallory, S.A.; Benitez, A.D.; Buckley, L.J.; Weintraub, S.T.; Osier, M.V.; Black, L.W.; Thomas, J.A. To Be or Not To Be T4: Evidence of a Complex Evolutionary Pathway of Head Structure and Assembly in Giant Salmonella Virus SPN3US. Front. Microbiol. 2017, 8, 2251. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Johnson, J.E. Bacteriophage HK97 capsid assembly and maturation. Adv. Exp. Med. Biol. 2012, 726, 351–363. [Google Scholar] [CrossRef]
- Dedeo, C.L.; Cingolani, G.; Teschke, C.M. Portal Protein: The Orchestrator of Capsid Assembly for the dsDNA Tailed Bacteriophages and Herpesviruses. Annu. Rev. Virol. 2019, 6, 141–160. [Google Scholar] [CrossRef]
- Baker, M.L.; Jiang, W.; Rixon, F.J.; Chiu, W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J. Virol. 2005, 79, 14967–14970. [Google Scholar] [CrossRef]
- Heymann, J.B.; Cheng, N.; Newcomb, W.W.; Trus, B.L.; Brown, J.C.; Steven, A.C. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat. Struct. Biol. 2003, 10, 334–341. [Google Scholar] [CrossRef]
- Hsiao, C.L.; Black, L.W. Head morphogenesis of bacteriophage T4. III. The role of gene 20 in DNA packaging. Virology 1978, 91, 26–38. [Google Scholar] [CrossRef]
- Hsiao, C.L.; Black, L.W. Head morphogenesis of bacteriophage T4. II. The role of gene 40 in initiating prehead assembly. Virology 1978, 91, 15–25. [Google Scholar] [CrossRef]
- Fokine, A.; Battisti, A.J.; Bowman, V.D.; Efimov, A.V.; Kurochkina, L.P.; Chipman, P.R.; Mesyanzhinov, V.V.; Rossmann, M.G. Cryo-EM study of the Pseudomonas bacteriophage phiKZ. Structure 2007, 15, 1099–1104. [Google Scholar] [CrossRef]
- Hua, J.; Huet, A.; Lopez, C.A.; Toropova, K.; Pope, W.H.; Duda, R.L.; Hendrix, R.W.; Conway, J.F. Capsids and Genomes of Jumbo-Sized Bacteriophages Reveal the Evolutionary Reach of the HK97 Fold. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Effantin, G.; Hamasaki, R.; Kawasaki, T.; Bacia, M.; Moriscot, C.; Weissenhorn, W.; Yamada, T.; Schoehn, G. Cryo-electron microscopy three-dimensional structure of the jumbo phage PhiRSL1 infecting the phytopathogen Ralstonia solanacearum. Structure 2013, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Peng, L.; Baldwin, P.R.; Mann, D.S.; Jiang, W.; Rees, I.; Ludtke, S.J. EMAN2: An extensible image processing suite for electron microscopy. J. Struct. Biol. 2007, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Heymann, J.B. Guidelines for using Bsoft for high resolution reconstruction and validation of biomolecular structures from electron micrographs. Protein Sci. 2018, 27, 159–171. [Google Scholar] [CrossRef]
- Leong, P.A.; Heymann, J.B.; Jensen, G.J. Peach: A simple Perl-based system for distributed computation and its application to cryo-EM data processing. Structure 2005, 13, 505–511. [Google Scholar] [CrossRef]
- Krylov, V.N.; Smirnova, T.A.; Minenkova, I.B.; Plotnikova, T.G.; Zhazikov, I.Z.; Khrenova, E.A. Pseudomonas bacteriophage phi KZ contains an inner body in its capsid. Can. J. Microbiol. 1984, 30, 758–762. [Google Scholar] [CrossRef]
- Wu, W.; Thomas, J.A.; Cheng, N.; Black, L.W.; Steven, A.C. Bubblegrams reveal the inner body of bacteriophage phiKZ. Science 2012, 335, 182. [Google Scholar] [CrossRef]
- Leapman, R.D.; Sun, S. Cryo-electron energy loss spectroscopy: Observations on vitrified hydrated specimens and radiation damage. Ultramicroscopy 1995, 59, 71–79. [Google Scholar] [CrossRef]
- Sokolova, O.S.; Shaburova, O.V.; Pechnikova, E.V.; Shaytan, A.K.; Krylov, S.V.; Kiselev, N.A.; Krylov, V.N. Genome packaging in EL and Lin68, two giant phiKZ-like bacteriophages of P. aeruginosa. Virology 2014, 468, 472–478. [Google Scholar] [CrossRef]
- Lata, R.; Conway, J.F.; Cheng, N.; Duda, R.L.; Hendrix, R.W.; Wikoff, W.R.; Johnson, J.E.; Tsuruta, H.; Steven, A.C. Maturation dynamics of a viral capsid: Visualization of transitional intermediate states. Cell 2000, 100, 253–263. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Liljas, L.; Duda, R.L.; Tsuruta, H.; Hendrix, R.W.; Johnson, J.E. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 2000, 289, 2129–2133. [Google Scholar] [CrossRef]
- Iwasaki, K.; Trus, B.L.; Wingfield, P.T.; Cheng, N.; Campusano, G.; Rao, V.B.; Steven, A.C. Molecular architecture of bacteriophage T4 capsid: Vertex structure and bimodal binding of the stabilizing accessory protein, Soc. Virology 2000, 271, 321–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newcomb, W.W.; Trus, B.L.; Cheng, N.; Steven, A.C.; Sheaffer, A.K.; Tenney, D.J.; Weller, S.K.; Brown, J.C. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 2000, 74, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Duda, R.L.; Martincic, K.; Hendrix, R.W. Genetic basis of bacteriophage HK97 prohead assembly. J. Mol. Biol. 1995, 247, 636–647. [Google Scholar] [CrossRef]
- Huet, A.; Duda, R.L.; Hendrix, R.W.; Boulanger, P.; Conway, J.F. Correct Assembly of the Bacteriophage T5 Procapsid Requires Both the Maturation Protease and the Portal Complex. J. Mol. Biol. 2016, 428, 165–181. [Google Scholar] [CrossRef]
- Kocsis, E.; Greenstone, H.L.; Locke, E.G.; Kessel, M.; Steven, A.C. Multiple conformational states of the bacteriophage T4 capsid surface lattice induced when expansion occurs without prior cleavage. J. Struct. Biol. 1997, 118, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.T.; Mohd Redzuan, N.H.; Barton, M.K.; Md Amin, N.A.; Desmond, M.I.; Adams, L.E.; Ali, B.; Pardo, S.; Molleur, D.; Wu, W.; et al. Global Proteomic Profiling of Salmonella Infection by a Giant Phage. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Chaikeeratisak, V.; Nguyen, K.; Egan, M.E.; Erb, M.L.; Vavilina, A.; Pogliano, J. The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages. Cell Rep. 2017, 20, 1563–1571. [Google Scholar] [CrossRef]
- Bayfield, O.W.; Klimuk, E.; Winkler, D.C.; Hesketh, E.L.; Chechik, M.; Cheng, N.; Dykeman, E.C.; Minakhin, L.; Ranson, N.A.; Severinov, K.; et al. Cryo-EM structure and in vitro DNA packaging of a thermophilic virus with supersized T = 7 capsids. Proc. Natl. Acad. Sci. USA 2019, 116, 3556–3561. [Google Scholar] [CrossRef]
- Ignatiou, A.; Brasiles, S.; El Sadek Fadel, M.; Burger, J.; Mielke, T.; Topf, M.; Tavares, P.; Orlova, E.V. Structural transitions during the scaffolding-driven assembly of a viral capsid. Nat. Commun. 2019, 10, 4840. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Z.; Fang, P.A.; Zhang, Q.; Wright, E.T.; Wu, W.; Zhang, C.; Vago, F.; Ren, Y.; Jakana, J.; et al. Capsid expansion mechanism of bacteriophage T7 revealed by multistate atomic models derived from cryo-EM reconstructions. Proc. Natl. Acad. Sci. USA 2014, 111, E4606–E4614. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; Heymann, J.B.; Cheng, N.; Trus, B.L.; Steven, A.C. Procapsid assembly, maturation, nuclear exit: Dynamic steps in the production of infectious herpesvirions. Adv. Exp. Med. Biol. 2012, 726, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; Moyer, A.L.; Cheng, N.; Thompson, C.D.; Dvoretzky, I.; Lowy, D.R.; Schiller, J.T.; Steven, A.C.; Buck, C.B.; Trus, B.L. Maturation of the human papillomavirus 16 capsid. mBio 2014, 5, e01104–e01114. [Google Scholar] [CrossRef] [PubMed]
- Church, G.A.; Wilson, D.W. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 1997, 71, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.F.; Duda, R.L.; Cheng, N.; Hendrix, R.W.; Steven, A.C. Proteolytic and conformational control of virus capsid maturation: The bacteriophage HK97 system. J. Mol. Biol. 1995, 253, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, W.W.; Homa, F.L.; Thomsen, D.R.; Trus, B.L.; Cheng, N.; Steven, A.; Booy, F.; Brown, J.C. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J. Virol. 1999, 73, 4239–4250. [Google Scholar] [CrossRef]
- Aksyuk, A.A.; Newcomb, W.W.; Cheng, N.; Winkler, D.C.; Fontana, J.; Heymann, J.B.; Steven, A.C. Subassemblies and asymmetry in assembly of herpes simplex virus procapsid. mBio 2015, 6, e01525-15. [Google Scholar] [CrossRef]
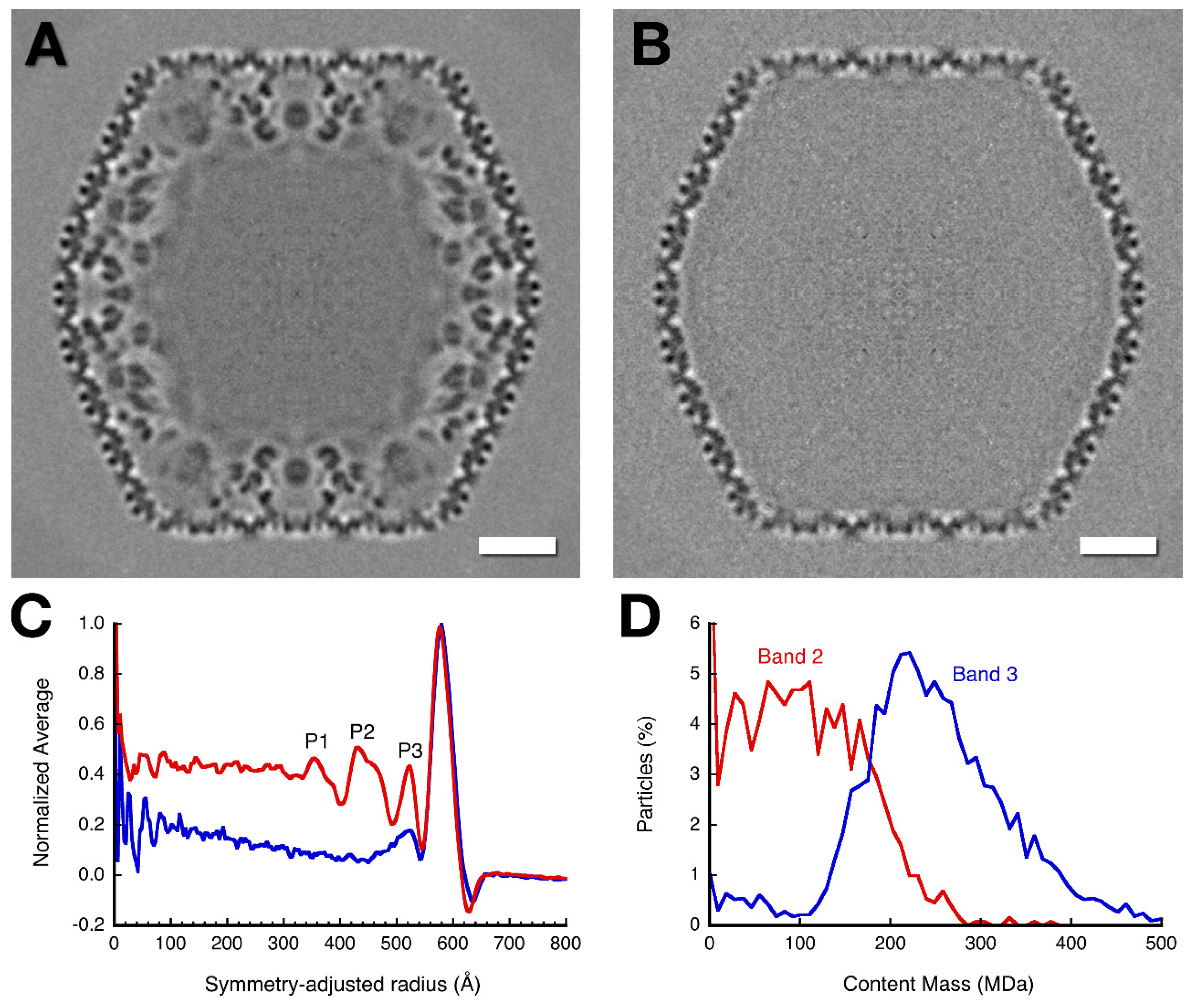
| Peak Label | Empty Capsid | Mottled Capsid | ||
|---|---|---|---|---|
| Peak Position | Mass (MDa) | Peak Position | Mass (MDa) | |
| Amorphous mass | <450 | 30 | <334 | 60 |
| P1 | 355 | 40 | ||
| P2 | 432 | 81 | ||
| P3 | 523 | 29 | 523 | 47 |
| Outer shell | 579 | 136 † | 576 | 136 † |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heymann, J.B.; Wang, B.; Newcomb, W.W.; Wu, W.; Winkler, D.C.; Cheng, N.; Reilly, E.R.; Hsia, R.-C.; Thomas, J.A.; Steven, A.C. The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell. Viruses 2020, 12, 910. https://doi.org/10.3390/v12090910
Heymann JB, Wang B, Newcomb WW, Wu W, Winkler DC, Cheng N, Reilly ER, Hsia R-C, Thomas JA, Steven AC. The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell. Viruses. 2020; 12(9):910. https://doi.org/10.3390/v12090910
Chicago/Turabian StyleHeymann, J. Bernard, Bing Wang, William W. Newcomb, Weimin Wu, Dennis C. Winkler, Naiqian Cheng, Erin R. Reilly, Ru-Ching Hsia, Julie A. Thomas, and Alasdair C. Steven. 2020. "The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell" Viruses 12, no. 9: 910. https://doi.org/10.3390/v12090910
APA StyleHeymann, J. B., Wang, B., Newcomb, W. W., Wu, W., Winkler, D. C., Cheng, N., Reilly, E. R., Hsia, R.-C., Thomas, J. A., & Steven, A. C. (2020). The Mottled Capsid of the Salmonella Giant Phage SPN3US, a Likely Maturation Intermediate with a Novel Internal Shell. Viruses, 12(9), 910. https://doi.org/10.3390/v12090910