Generation of Combinatorial Lentiviral Vectors Expressing Multiple Anti-Hepatitis C Virus shRNAs and Their Validation on a Novel HCV Replicon Double Reporter Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
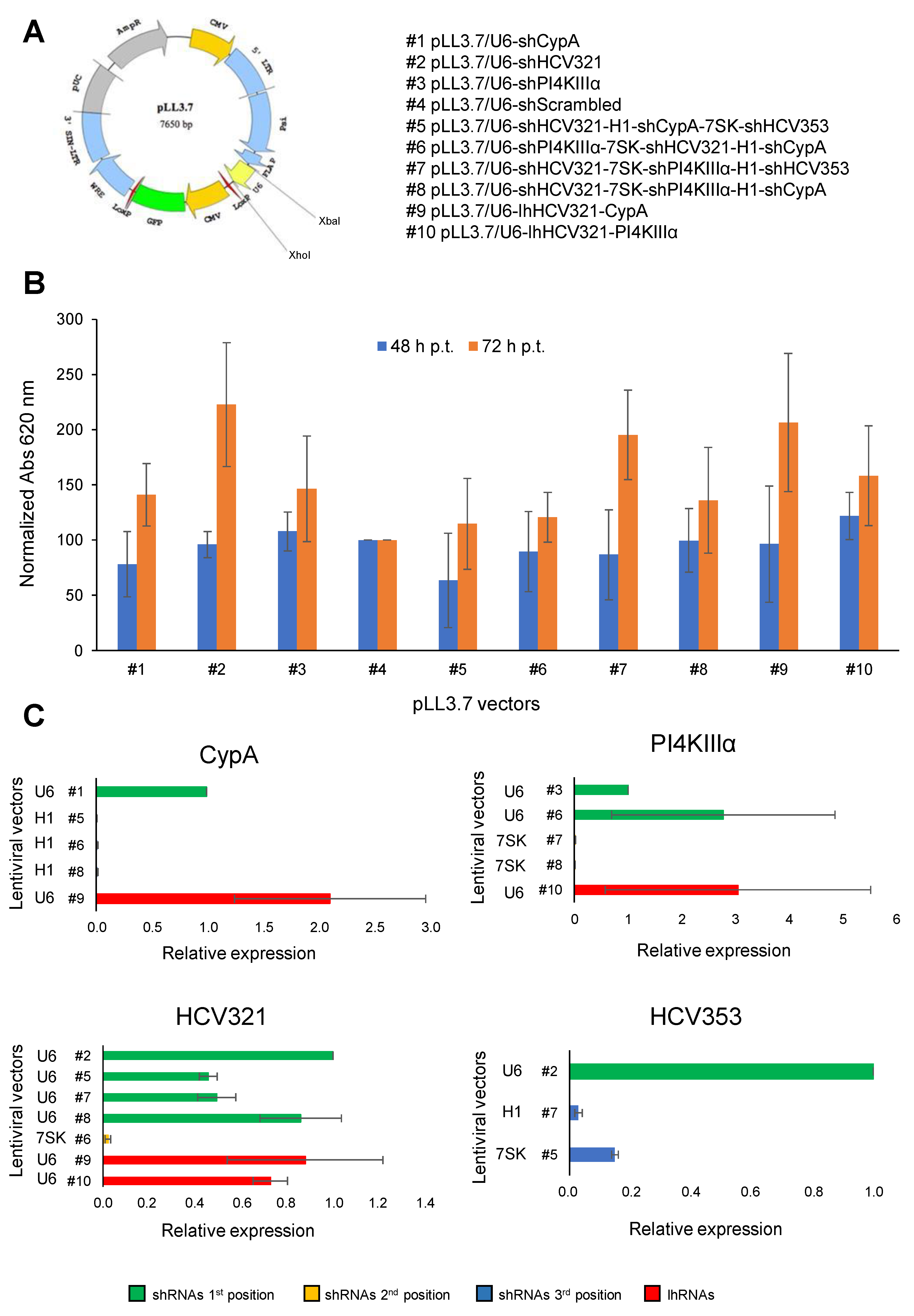
2.2. Plasmids
2.3. Cell Lines
2.4. In Vitro Transcription
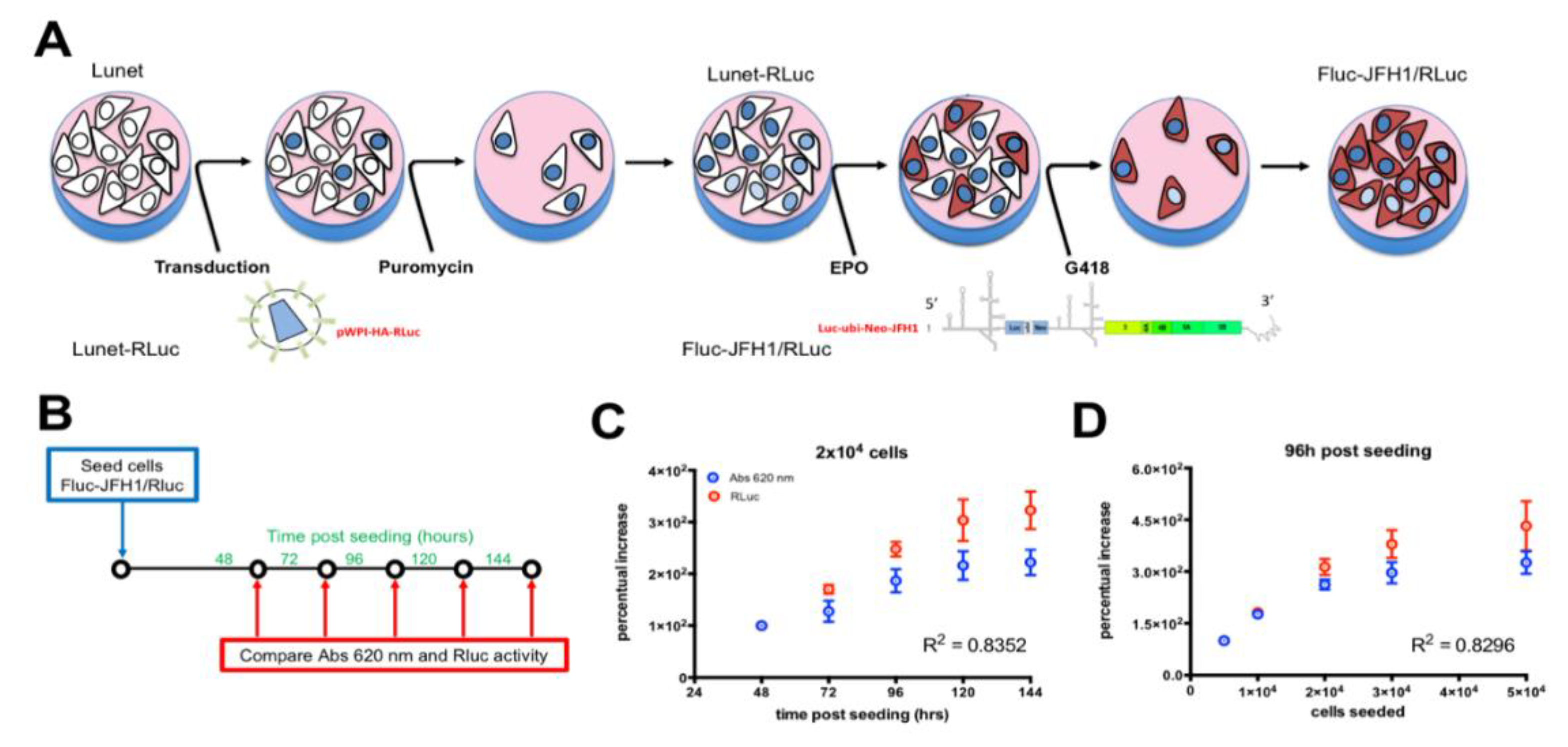
2.5. Generation of HCV Replicon Cell Lines
2.6. MTT Cell Viability Assay
2.7. Quantification of HCV Replication
2.8. Statistical Analysis
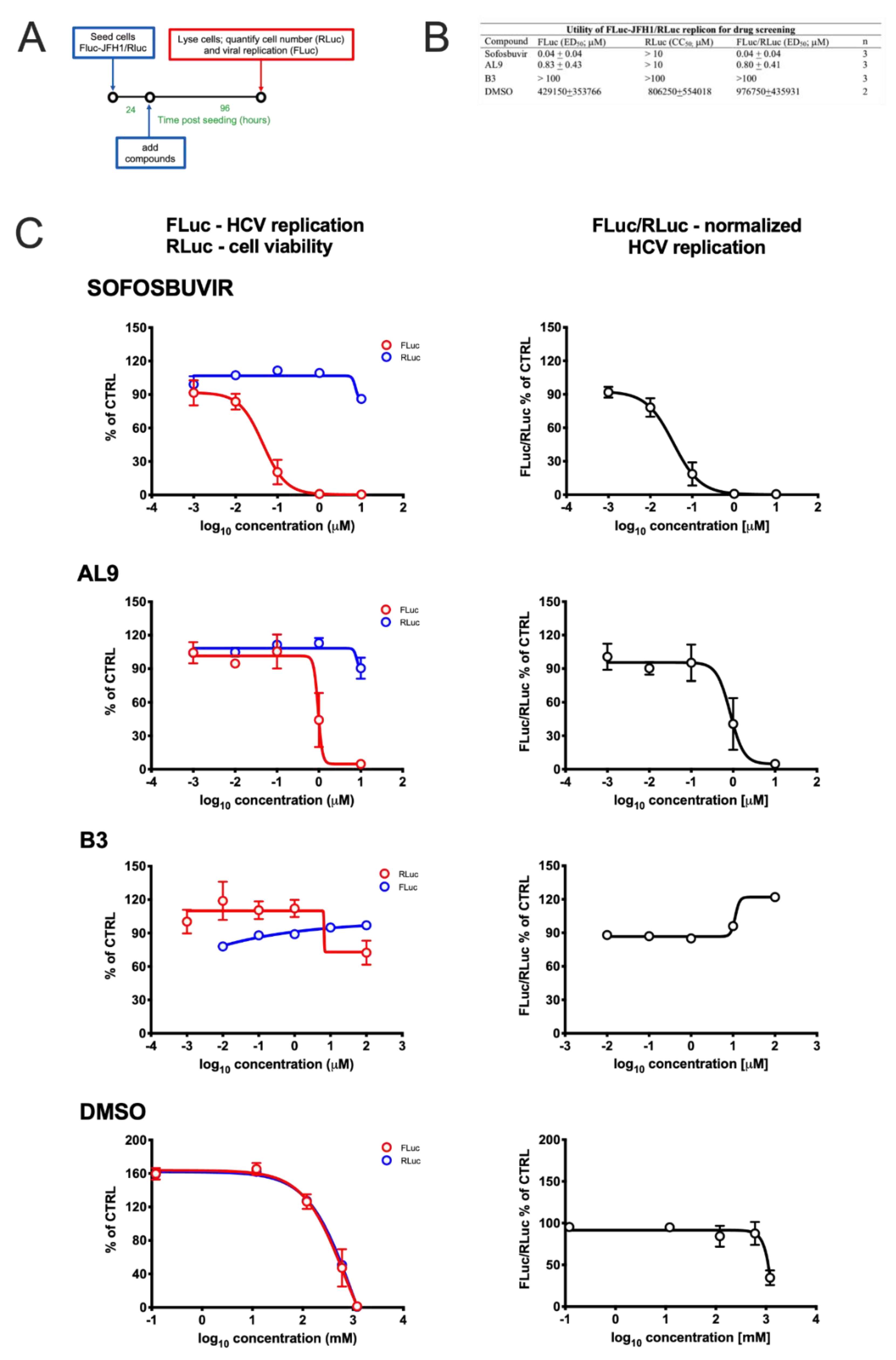
2.9. Cell Treatment with Antiviral Drugs
2.10. Production and Titration of Lentiviral Particles
2.11. Quantification of Anti-HCV shRNA/lhRNAs Expression by Real Time PCR
2.12. Western Blotting
3. Results
3.1. Generation of Lentiviral Vectors Expressing Different Arrays of Anti-HCV shRNAs
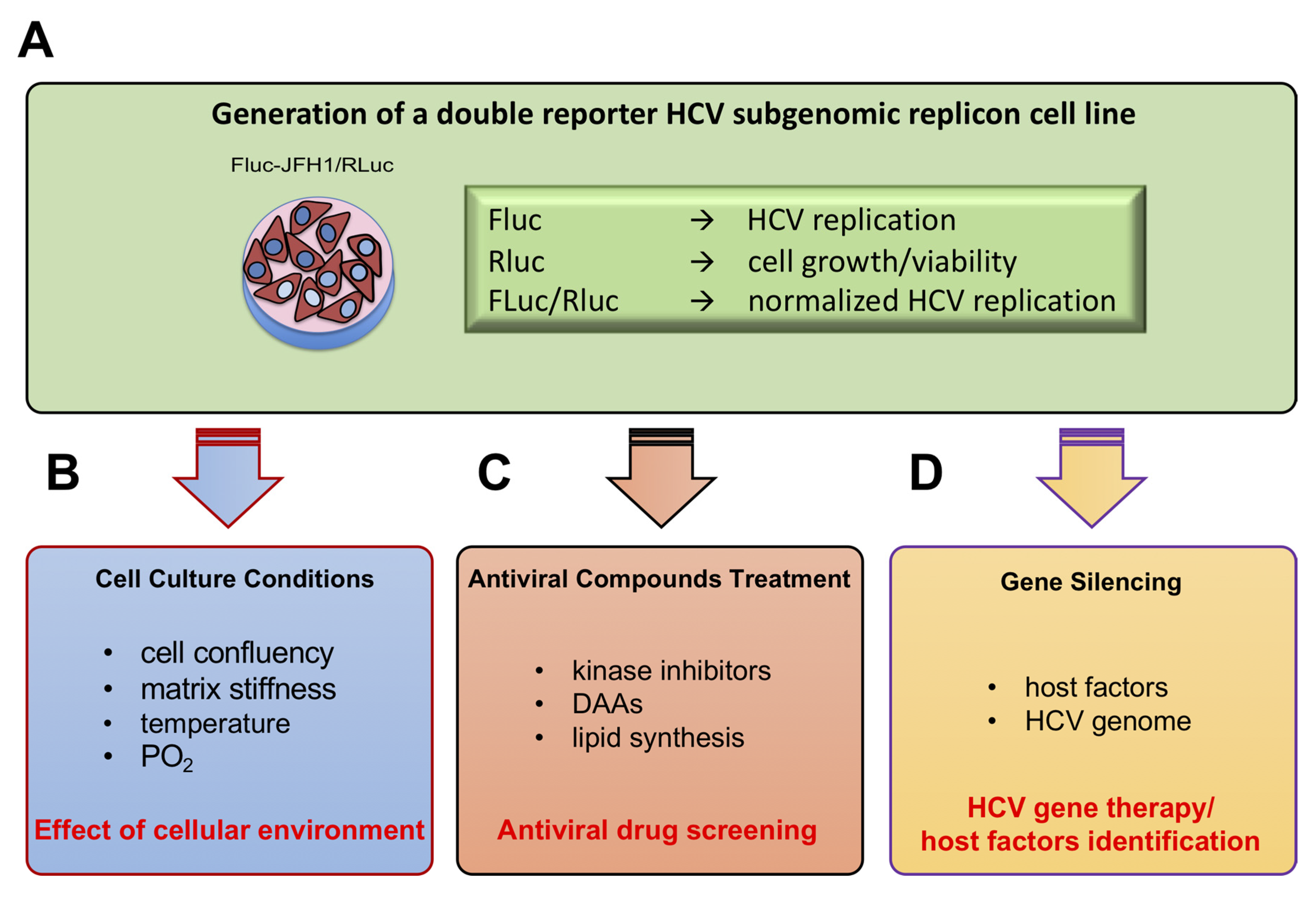
3.2. Establishment of a Novel HCV Replicon Double Reporter Cell Line
3.3. Monitoring HCV Replication Inhibition with the FLuc-JFH1/RLuc Cell Line
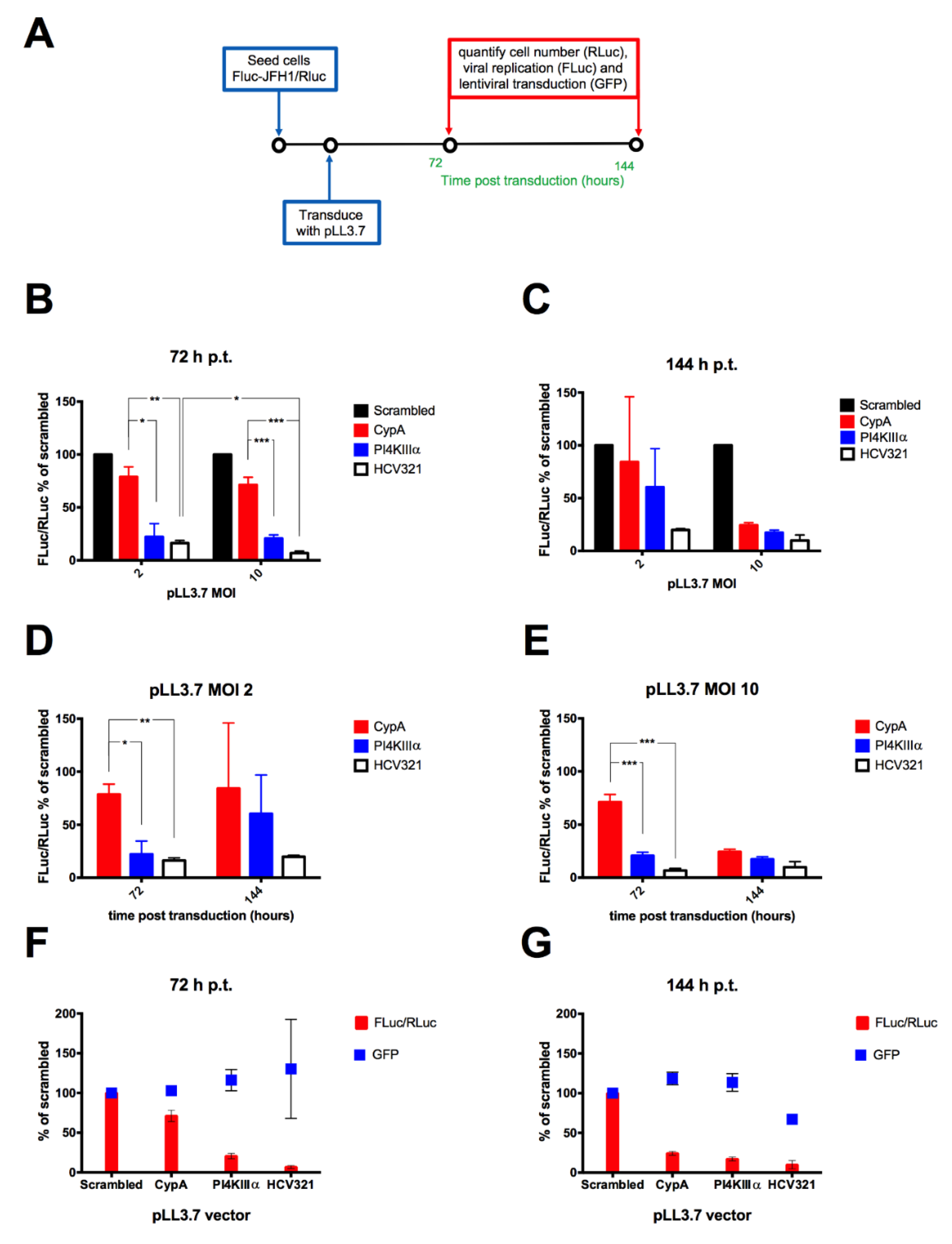
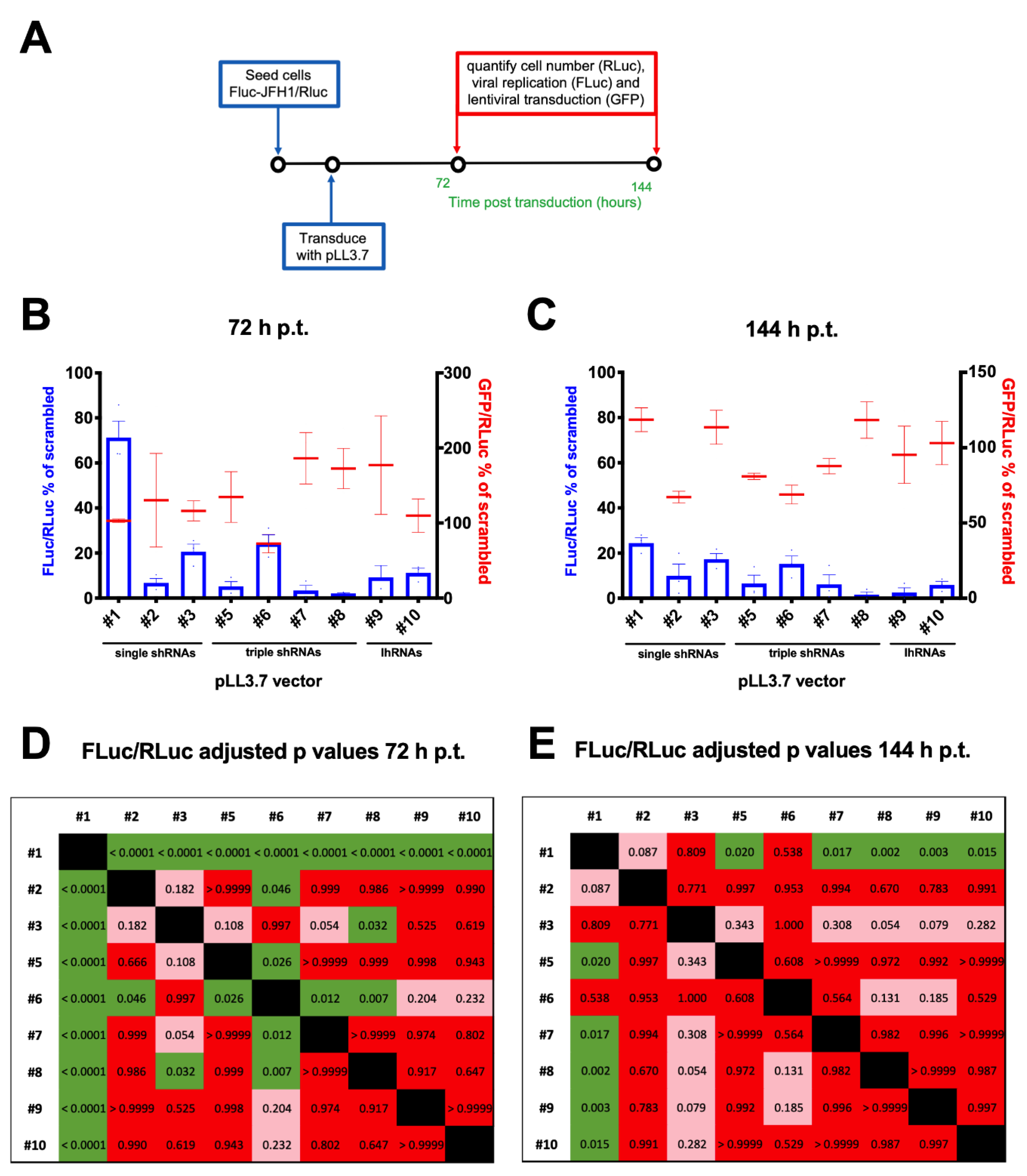
3.4. Lentiviral Transduction of the FLuc-JFH1/RLuc Replicon Cell Line Results in MOI-Dependent Inhibition of Viral Replication
3.5. A Combinatorial Platform to Efficiently Inhibit HCV Replication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Afdhal, N.; Reddy, K.R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef]
- Gritsenko, D.; Hughes, G. Ledipasvir/Sofosbuvir (harvoni): Improving options for hepatitis C virus infection. Pharm. Ther. 2015, 40, 256–276. [Google Scholar]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Samreen, B.; Khaliq, S.; Ijaz, B.; Khan, M.; Siddique, M.H.; Ahmad, W.; Hassan, S. HCV entry receptors as potential targets for siRNA-based inhibition of HCV. Genet. Vaccines 2011, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Small interfering RNA targeted to hepatitis C virus 5’ nontranslated region exerts potent antiviral effect. J. Virol. 2007, 81, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, S.; Jahan, S.; Ijaz, B.; Ahmad, W.; Asad, S.; Hassan, S. Inhibition of hepatitis C virus genotype 3a by siRNAs targeting envelope genes. Arch. Virol. 2011, 156, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.H.; Kim, E.J.; Cho, H.; Lee, W.; Kim, G.W.; Park, H.J.; Cho, S.W.; Lee, C.; Oh, J.W. Inhibition of hepatitis C virus in mice by a small interfering RNA targeting a highly conserved sequence in viral IRES pseudoknot. PLoS ONE 2016, 11, e0146710. [Google Scholar] [CrossRef][Green Version]
- Randall, G.; Panis, M.; Cooper, J.D.; Tellinghuisen, T.L.; Sukhodolets, K.E.; Pfeffer, S.; Landthaler, M.; Landgraf, P.; Kan, S.; Lindenbach, B.D.; et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. USA 2007, 104, 12884–12889. [Google Scholar] [CrossRef]
- Verstegen, M.M.; Pan, Q.; van der Laan, L.J. Gene therapies for hepatitis C virus. Adv. Exp. Med. Biol. 2015, 848, 1–29. [Google Scholar]
- Wilson, J.A.; Jayasena, S.; Khvorova, A.; Sabatinos, S.; Rodrigue-Gervais, I.G.; Arya, S.; Sarangi, F.; Harris-Brandts, M.; Beaulieu, S.; Richardson, C.D. RNA interference blocks gene expression and RNA synthesis from hepatitis C replicons propagated in human liver cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.S.; Elemeery, M.N.; Eldein, S.S.; Ghareeb, D.A. Silencing HCV replication in its reservoir. Open Access Maced. J. Med. Sci. 2018, 6, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.C.; Carneiro, B.M.; Batista, M.N.; Akinaga, M.M.; Rahal, P. Inhibition of hepatitis C virus using siRNA targeted to the virus and Hsp90. Cell Stress Chaperones 2017, 22, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Kundu, A.K.; Hazari, S.; Chandra, S.; Bao, L.; Ooms, T.; Morris, G.F.; Wu, T.; Mandal, T.K.; Dash, S. Inhibition of hepatitis C virus replication by intracellular delivery of multiple siRNAs by nanosomes. Mol. Ther. 2012, 20, 1724–1736. [Google Scholar] [CrossRef]
- Korf, M.; Jarczak, D.; Beger, C.; Manns, M.P.; Kruger, M. Inhibition of hepatitis C virus translation and subgenomic replication by siRNAs directed against highly conserved HCV sequence and cellular HCV cofactors. J. Hepatol. 2005, 43, 225–234. [Google Scholar] [CrossRef]
- Suhy, D.A.; Kao, S.C.; Mao, T.; Whiteley, L.; Denise, H.; Souberbielle, B.; Burdick, A.D.; Hayes, K.; Wright, J.F.; Lavender, H.; et al. Safe, long-term hepatic expression of anti-HCV shRNA in a nonhuman primate model. Mol. Ther. 2012, 20, 1737–1749. [Google Scholar] [CrossRef]
- Reiss, S.; Harak, C.; Romero-Brey, I.; Radujkovic, D.; Klein, R.; Ruggieri, A.; Rebhan, I.; Bartenschlager, R.; Lohmann, V. The lipid kinase phosphatidylinositol-4 kinase III alpha regulates the phosphorylation status of hepatitis C virus NS5A. PLoS Pathog. 2013, 9, e1003359. [Google Scholar] [CrossRef]
- Reiss, S.; Rebhan, I.; Backes, P.; Romero-Brey, I.; Erfle, H.; Matula, P.; Kaderali, L.; Poenisch, M.; Blankenburg, H.; Hiet, M.S.; et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 2011, 9, 32–45. [Google Scholar] [CrossRef]
- Ciesek, S.; Steinmann, E.; Wedemeyer, H.; Manns, M.P.; Neyts, J.; Tautz, N.; Madan, V.; Bartenschlager, R.; von Hahn, T.; Pietschmann, T. Cyclosporine A inhibits hepatitis C virus nonstructural protein 2 through cyclophilin A. Hepatology 2009, 50, 1638–1645. [Google Scholar] [CrossRef]
- Kaul, A.; Stauffer, S.; Berger, C.; Pertel, T.; Schmitt, J.; Kallis, S.; Zayas, M.; Lopez, M.Z.; Lohmann, V.; Luban, J.; et al. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog. 2009, 5, e1000546. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, F.; Robotham, J.M.; Tang, H. Critical role of cyclophilin A and its prolyl-peptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J. Virol. 2009, 83, 6554–6565. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Robotham, J.M.; Nelson, H.B.; Irsigler, A.; Kenworthy, R.; Tang, H. Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principal mediator of cyclosporine resistance in vitro. J. Virol. 2008, 82, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Henke, J.I.; Goergen, D.; Zheng, J.; Song, Y.; Schuttler, C.G.; Fehr, C.; Junemann, C.; Niepmann, M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008, 27, 3300–3310. [Google Scholar] [CrossRef]
- Jopling, C.L. Regulation of hepatitis C virus by microRNA-122. Biochem. Soc. Trans. 2008, 36, 1220–1223. [Google Scholar] [CrossRef]
- Shimakami, T.; Yamane, D.; Welsch, C.; Hensley, L.; Jangra, R.K.; Lemon, S.M. Base pairing between hepatitis C virus RNA and microRNA 122 3’ of its seed sequence is essential for genome stabilization and production of infectious virus. J. Virol. 2012, 86, 7372–7383. [Google Scholar] [CrossRef]
- van der Ree, M.H.; van der Meer, A.J.; de Bruijne, J.; Maan, R.; van Vliet, A.; Welzel, T.M.; Zeuzem, S.; Lawitz, E.J.; Rodriguez-Torres, M.; Kupcova, V.; et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antivir. Res. 2014, 111, 53–59. [Google Scholar] [CrossRef]
- Zeuzem, S.; Flisiak, R.; Vierling, J.M.; Mazur, W.; Mazzella, G.; Thongsawat, S.; Abdurakhmanov, D.; Van Kinh, N.; Calistru, P.; Heo, J.; et al. Randomised clinical trial: Alisporivir combined with peginterferon and ribavirin in treatment-naive patients with chronic HCV genotype 1 infection (ESSENTIAL II). Aliment. Pharm. 2015, 42, 829–844. [Google Scholar] [CrossRef]
- Bianco, A.; Reghellin, V.; Donnici, L.; Fenu, S.; Alvarez, R.; Baruffa, C.; Peri, F.; Pagani, M.; Abrignani, S.; Neddermann, P.; et al. Metabolism of phosphatidylinositol 4-kinase IIIalpha-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog. 2012, 8, e1002576. [Google Scholar] [CrossRef]
- Simmonds, P.; Bukh, J.; Combet, C.; Deleage, G.; Enomoto, N.; Feinstone, S.; Halfon, P.; Inchauspe, G.; Kuiken, C.; Maertens, G.; et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005, 42, 962–973. [Google Scholar] [CrossRef]
- Friebe, P.; Lohmann, V.; Krieger, N.; Bartenschlager, R. Sequences in the 5’ nontranslated region of hepatitis C virus required for RNA replication. J. Virol. 2001, 75, 12047–12057. [Google Scholar] [CrossRef]
- ElHefnawi, M.; Kim, T.; Kamar, M.A.; Min, S.; Hassan, N.M.; El-Ahwany, E.; Kim, H.; Zada, S.; Amer, M.; Windisch, M.P. In silico design and experimental validation of siRNAs targeting conserved regions of multiple hepatitis c virus genotypes. PLoS ONE 2016, 11, e0159211. [Google Scholar] [CrossRef]
- Spanevello, F.; Calistri, A.; Del Vecchio, C.; Mantelli, B.; Frasson, C.; Basso, G.; Palu, G.; Cavazzana, M.; Parolin, C. Development of lentiviral vectors simultaneously expressing multiple siRNAs against CCR5, vif and tat/rev genes for an HIV-1 gene therapy approach. Mol. Nucleic Acids 2016, 5, e312. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Jans, D.; Ripalti, A. Human cytomegalovirus (HCMV) DNA polymerase processivity factor ppUL44 dimerizes in the cytosol before translocation to the nucleus. Biochemistry 2006, 45, 6866–6872. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Paolini, L.; Contarini, A.; Zambarda, C.; Di Antonio, V.; Colosini, A.; Mercandelli, N.; Timmoneri, M.; Palu, G.; Caimi, L.; et al. Intersectin goes nuclear: Secret life of an endocytic protein. Biochem. J. 2018, 475, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Di Antonio, V.; Bellucci, L.; Thomas, D.R.; Caporuscio, F.; Ciccarese, F.; Ghassabian, H.; Wagstaff, K.M.; Forwood, J.K.; Jans, D.A.; et al. Contribution of the residue at position 4 within classical nuclear localization signals to modulating interaction with importins and nuclear targeting. Biochim. Biophys. Acta 2018, 1865, 1114–1129. [Google Scholar] [CrossRef]
- Jo, J.; Aichele, U.; Kersting, N.; Klein, R.; Aichele, P.; Bisse, E.; Sewell, A.K.; Blum, H.E.; Bartenschlager, R.; Lohmann, V.; et al. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology 2009, 136, 1391–1401. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Di Antonio, V.; Radeghieri, A.; Palu, G.; Ghildyal, R.; Alvisi, G. Molecular requirements for self-interaction of the respiratory syncytial virus matrix protein in living mammalian cells. Viruses 2018, 10, 109. [Google Scholar] [CrossRef]
- Friebe, P.; Boudet, J.; Simorre, J.-P.; Bartenschlager, R. Kissing-loop interaction in the 3’ end of the hepatitis C virus genome essential for RNA replication. J. Virol. 2005, 79, 380–392. [Google Scholar] [CrossRef]
- Eberle, C.A.; Zayas, M.; Stukalov, A.; Pichlmair, A.; Alvisi, G.; Muller, A.C.; Bennett, K.L.; Bartenschlager, R.; Superti-Furga, G. The lysine methyltransferase SMYD3 interacts with hepatitis C virus NS5A and is a negative regulator of viral particle production. Virology 2014, 462–463, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Dazert, E.; Metz, P.; Hofmann, S.; Bergeest, J.P.; Mazur, J.; Bankhead, P.; Hiet, M.S.; Kallis, S.; Alvisi, G.; et al. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe 2012, 12, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Kandasamy, K.; Alvisi, G.; Mulhern, O.; Sacco, R.; Habjan, M.; Binder, M.; Stefanovic, A.; Eberle, C.A.; Goncalves, A.; et al. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature 2012, 487, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, V.; Korner, F.; Koch, J.; Herian, U.; Theilmann, L.; Bartenschlager, R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 1999, 285, 110–113. [Google Scholar] [CrossRef]
- Van den Hoff, M.J.; Moorman, A.F.; Lamers, W.H. Electroporation in ‘intracellular’ buffer increases cell survival. Nucleic Acids Res. 1992, 20, 2902. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Alvisi, G.; Roth, D.M.; Camozzi, D.; Pari, G.S.; Loregian, A.; Ripalti, A.; Jans, D.A. The flexible loop of the human cytomegalovirus DNA polymerase processivity factor ppUL44 is required for efficient DNA binding and replication in cells. J. Virol. 2009, 83, 9567–9576. [Google Scholar] [CrossRef]
- Rubinson, D.A.; Dillon, C.P.; Kwiatkowski, A.V.; Sievers, C.; Yang, L.; Kopinja, J.; Rooney, D.L.; Zhang, M.; Ihrig, M.M.; McManus, M.T.; et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 2003, 33, 401–406. [Google Scholar] [CrossRef]
- Nelson, H.B.; Tang, H. Effect of cell growth on hepatitis C virus (HCV) replication and a mechanism of cell confluence-based inhibition of HCV RNA and protein expression. J. Virol. 2006, 80, 1181–1190. [Google Scholar] [CrossRef]
- Murakami, E.; Tolstykh, T.; Bao, H.; Niu, C.; Steuer, H.M.; Bao, D.; Chang, W.; Espiritu, C.; Bansal, S.; Lam, A.M.; et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J. Biol. Chem. 2010, 285, 34337–34347. [Google Scholar] [CrossRef]
- Younossi, Z.; Papatheodoridis, G.; Cacoub, P.; Negro, F.; Wedemeyer, H.; Henry, L.; Hatzakis, A. The comprehensive outcomes of hepatitis C virus infection: A multi-faceted chronic disease. J. Viral Hepat. 2018, 25, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Hartung, D.; Rahman, B.; Wasson, N.; Cottrell, E.B.; Fu, R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: A systematic review. Ann. Intern. Med. 2013, 158, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Yeo, Y.H.; Wei, M.T.; Ogawa, E.; Enomoto, M.; Lee, D.H.; Iio, E.; Lubel, J.; Wang, W.; Wei, B.; et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Manzoor, S.; Khattak, N.M.; Khalid, M.; Ahmed, Q.L.; Parvaiz, F.; Tariq, M.; Ashraf, J.; Ashraf, W.; Azam, S.; et al. Current and future therapies for hepatitis C virus infection: From viral proteins to host targets. Arch. Virol. 2014, 159, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, G.; Yu, L.; Feng, Y.; Li, X.; Zhang, Z.; Wang, Y.; Sun, D.; Pradhan, S. High-efficiency generation of induced pluripotent mesenchymal stem cells from human dermal fibroblasts using recombinant proteins. Stem Cell Res. 2016, 7, 99. [Google Scholar] [CrossRef][Green Version]
- Perales, C.; Chen, Q.; Soria, M.E.; Gregori, J.; Garcia-Cehic, D.; Nieto-Aponte, L.; Castells, L.; Imaz, A.; Llorens-Revull, M.; Domingo, E.; et al. Baseline hepatitis C virus resistance-associated substitutions present at frequencies lower than 15% may be clinically significant. Infect. Drug Resist. 2018, 11, 2207–2210. [Google Scholar] [CrossRef]
- Konishi, M.; Wu, C.H.; Kaito, M.; Hayashi, K.; Watanabe, S.; Adachi, Y.; Wu, G.Y. siRNA-resistance in treated HCV replicon cells is correlated with the development of specific HCV mutations. J. Viral Hepat. 2006, 13, 756–761. [Google Scholar] [CrossRef]
- Mandal, A.; Ganta, K.K.; Chaubey, B. Combinations of siRNAs against La Autoantigen with NS5B or hVAP-A have additive effect on inhibition of HCV replication. Hepat. Res. Treat. 2016, 2016, 9671031. [Google Scholar] [CrossRef]
- Bartenschlager, R.; Pietschmann, T. Efficient hepatitis C virus cell culture system: What a difference the host cell makes. Proc. Natl. Acad. Sci. USA 2005, 102, 9739–9740. [Google Scholar] [CrossRef]
- Targett-Adams, P.; McLauchlan, J. Development and characterization of a transient-replication assay for the genotype 2a hepatitis C virus subgenomic replicon. J. Gen. Virol. 2005, 86, 3075–3080. [Google Scholar] [CrossRef]
- Vitiello, A. Alvisi G. Material not intended for publication, 2019.
- ter Brake, O.; t Hooft, K.; Liu, Y.P.; Centlivre, M.; von Eije, K.J.; Berkhout, B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol. Ther. 2008, 16, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Amberkar, S.; Kiani, N.A.; Bartenschlager, R.; Alvisi, G.; Kaderali, L. High-throughput RNA interference screens integrative analysis: Towards a comprehensive understanding of the virus-host interplay. World J. Virol. 2013, 2, 18–31. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbadawy, H.M.; Mohammed Abdul, M.I.; Aljuhani, N.; Vitiello, A.; Ciccarese, F.; Shaker, M.A.; Eltahir, H.M.; Palù, G.; Di Antonio, V.; Ghassabian, H.; et al. Generation of Combinatorial Lentiviral Vectors Expressing Multiple Anti-Hepatitis C Virus shRNAs and Their Validation on a Novel HCV Replicon Double Reporter Cell Line. Viruses 2020, 12, 1044. https://doi.org/10.3390/v12091044
Elbadawy HM, Mohammed Abdul MI, Aljuhani N, Vitiello A, Ciccarese F, Shaker MA, Eltahir HM, Palù G, Di Antonio V, Ghassabian H, et al. Generation of Combinatorial Lentiviral Vectors Expressing Multiple Anti-Hepatitis C Virus shRNAs and Their Validation on a Novel HCV Replicon Double Reporter Cell Line. Viruses. 2020; 12(9):1044. https://doi.org/10.3390/v12091044
Chicago/Turabian StyleElbadawy, Hossein M., Mohi I. Mohammed Abdul, Naif Aljuhani, Adriana Vitiello, Francesco Ciccarese, Mohamed A. Shaker, Heba M. Eltahir, Giorgio Palù, Veronica Di Antonio, Hanieh Ghassabian, and et al. 2020. "Generation of Combinatorial Lentiviral Vectors Expressing Multiple Anti-Hepatitis C Virus shRNAs and Their Validation on a Novel HCV Replicon Double Reporter Cell Line" Viruses 12, no. 9: 1044. https://doi.org/10.3390/v12091044
APA StyleElbadawy, H. M., Mohammed Abdul, M. I., Aljuhani, N., Vitiello, A., Ciccarese, F., Shaker, M. A., Eltahir, H. M., Palù, G., Di Antonio, V., Ghassabian, H., Del Vecchio, C., Salata, C., Franchin, E., Ponterio, E., Bahashwan, S., Thabet, K., Abouzied, M. M., Shehata, A. M., Parolin, C., ... Alvisi, G. (2020). Generation of Combinatorial Lentiviral Vectors Expressing Multiple Anti-Hepatitis C Virus shRNAs and Their Validation on a Novel HCV Replicon Double Reporter Cell Line. Viruses, 12(9), 1044. https://doi.org/10.3390/v12091044









