Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Cells
2.3. Fluoroquinolone Compounds
2.4. Viral Replication Kinetics in Cell Culture
2.5. Determination of Half-Maximal Effective Concentration (EC50) against Select Flaviviruses
2.6. Determination of Half-Maximal Cytotoxic Concentration (CC50) of Fluoroquinolones
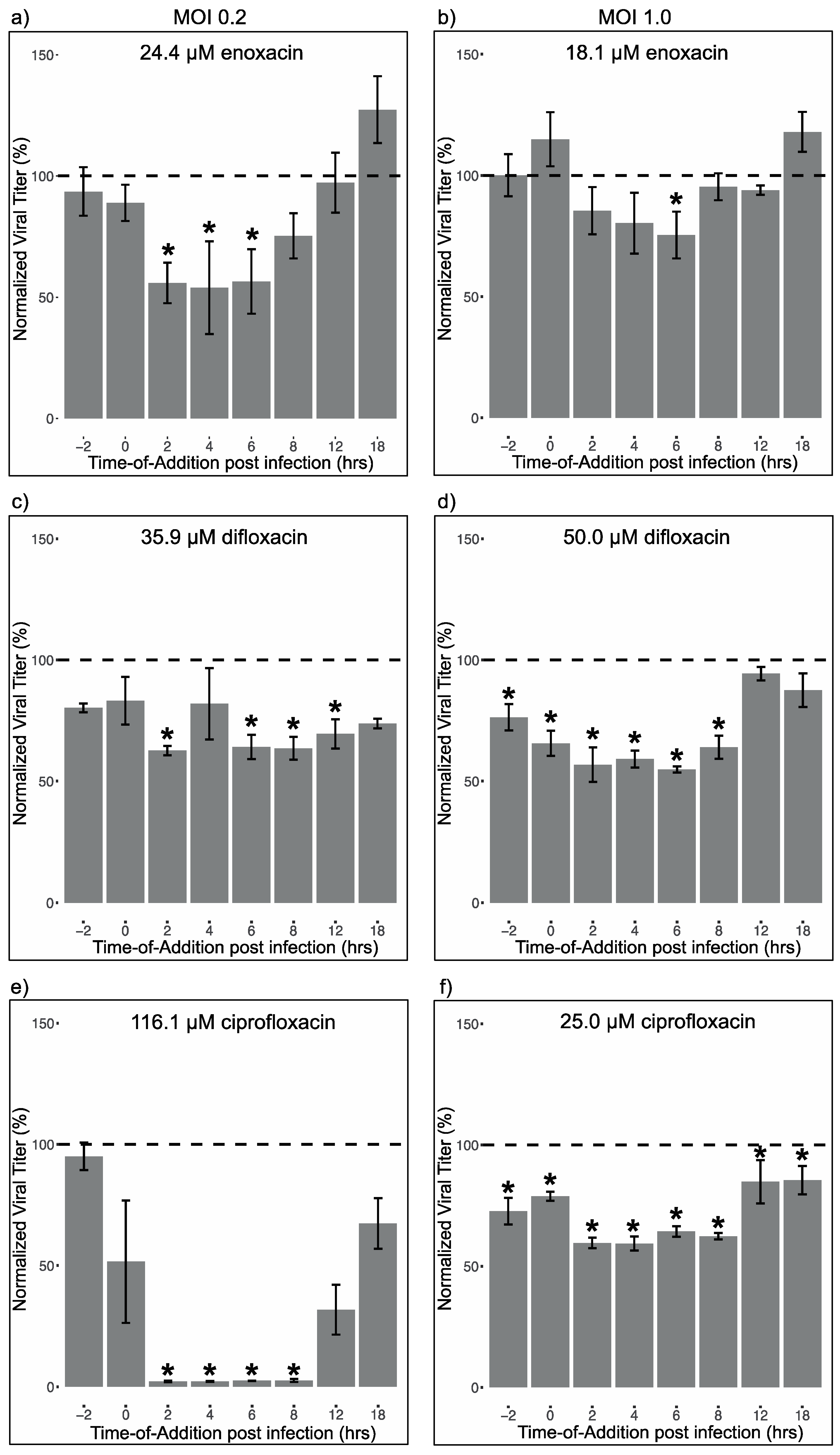
2.7. Time-Of-Addition Assays
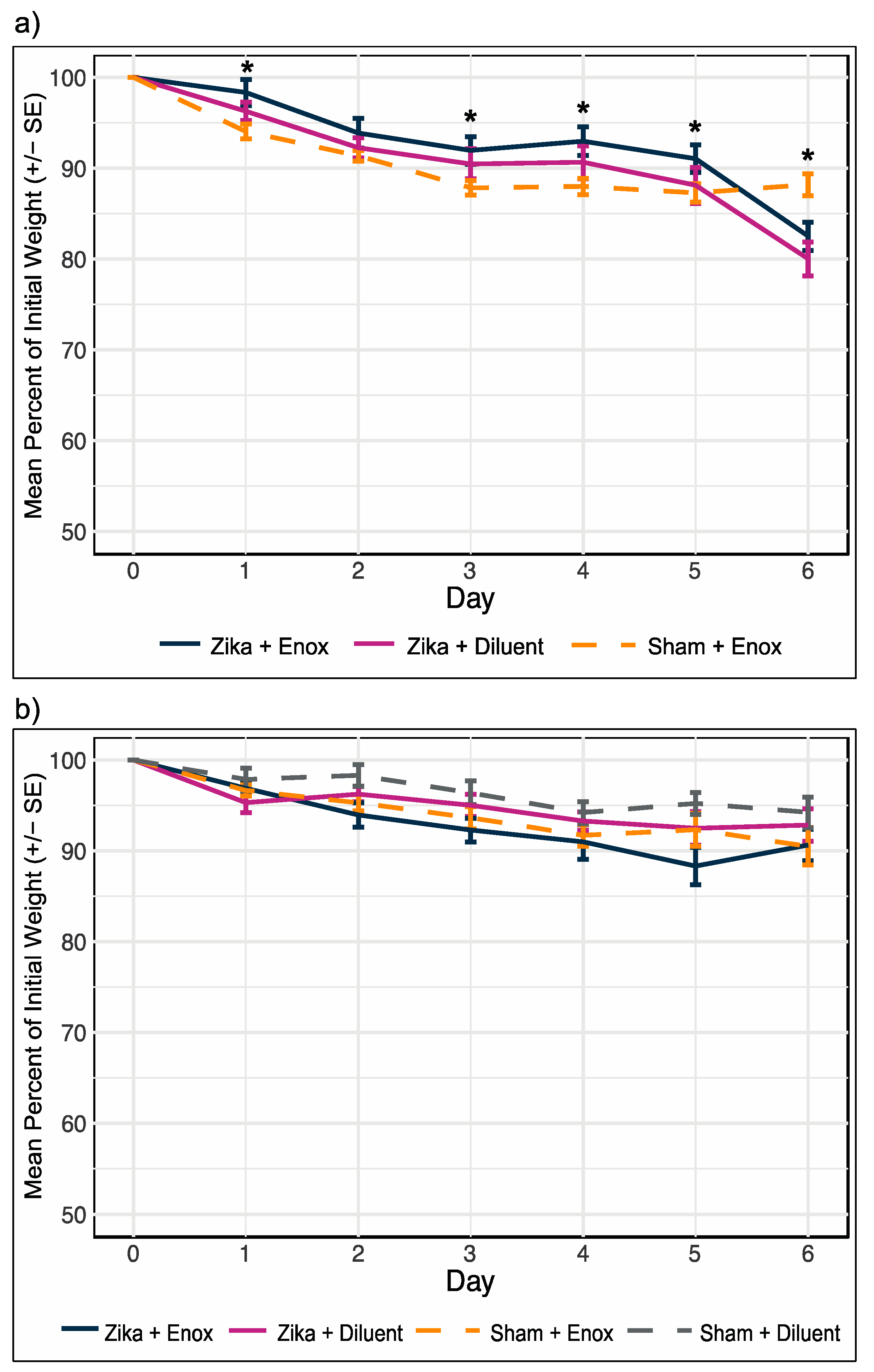
2.8. Determination of In Vivo Efficacy of Enoxacin
2.9. Statistical Analysis
3. Results
3.1. Flavivirus Replication Curves in Cultured Human Cells
3.2. Fluoroquinolones Suppress Flavivirus Replication in Cultured Human Cells
3.3. Fluoroquinolone Suppression of Different Life Cycle Stages of ZIKV
3.4. Enoxacin Treatment of ZIKV-Infected Mice Did Not Alleviate or Exacerbate Weight Loss
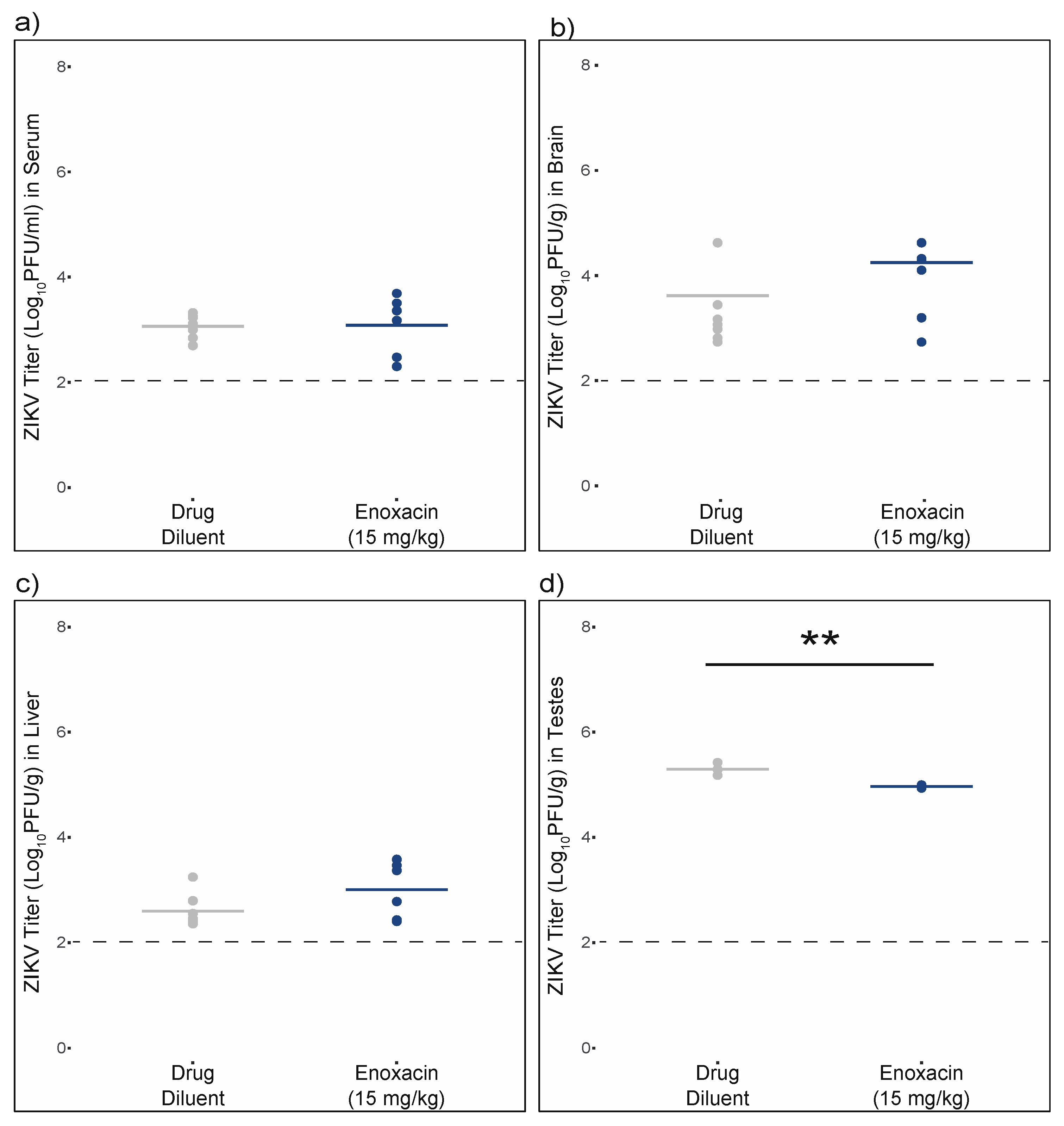
3.5. Enoxacin Suppressed ZIKV Replication in Mouse Testes, but Not Serum, Brain, or Liver
3.6. Enoxacin Does Not Inhibit ZIKV Replication in Mouse Sertoli Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Dawson, A.L.; Petersen, E.E.; Jones, A.M.; Lee, E.H.; Yazdy, M.M.; Ahmad, N.; Macdonald, J.; Evert, N.; Bingham, A.; et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 2016, 30333, 59–68. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Růžek, D.; Dobler, G.; Mantke, O.D. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef]
- Kemenesi, G.; Bányai, K. Tick-borne flaviviruses, with a focus on Powassan virus. Clin. Microbiol. Rev. 2018, 32, e00106-17. [Google Scholar] [CrossRef]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.-P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamanaka, A.; Konishi, E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 2014, 32, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.-C. Therapeutic Advances Against ZIKV: A Quick Response, a Long Way to Go. Pharmaceuticals 2019, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shi, P.-Y. West Nile virus drug discovery. Viruses 2013, 5, 2977–3006. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Wang, Q.-Y.; Noble, C.G.; Chen, Y.-L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D. Ten years of dengue drug discovery: Progress and prospects. Antivir. Res. 2013, 100, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, S.J.F.; Neyts, J. Towards antiviral therapies for treating dengue virus infections. Curr. Opin. Pharmacol. 2016, 30, 1–7. [Google Scholar] [CrossRef]
- Zou, J.; Shi, P.-Y. Strategies for Zika drug discovery. Curr. Opin. Virol. 2019, 35, 19–26. [Google Scholar] [CrossRef]
- Wolfson, J.S.; Hooper, D.C. The Fluoroquinolones: Structures, Mechanisms of Action and Resistance, and Spectra of Activity In Vitro. Antimicrob. Agents Chemother. 1985, 28, 581–586. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–62. [Google Scholar]
- Nagy, P.D.; Pogany, J. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 2012, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.; Villanueva, A.; Moutinho, C.; Davalos, V.; Spizzo, R.; Ivan, C.; Rossi, S.; Setien, F.; Casanovas, O.; Simo-Riudalbas, L. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc. Natl. Acad. Sci. USA 2011, 108, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Li, Y.; Zhang, J.; Li, W.; Szulwach, K.E.; Duan, R.; Faghihi, M.A.; Khalil, A.M.; Lu, L.; Paroo, Z. A small molecule enhances RNA interference and promotes microRNA processing. Nat. Biotechnol. 2008, 26, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Xi, Z. Enhancement of RNAi by a small molecule antibiotic enoxacin. Cell Res. 2008, 18, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.N.; Li, R.; Bernhoff, E.; Gutteberg, T.J.; Rinaldo, C.H. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antivir. Res. 2011, 92, 115–123. [Google Scholar] [CrossRef]
- Simon, N.; Bochman, M.L.; Seguin, S.; Brodsky, J.L.; Seibel, W.L.; Schwacha, A. Ciprofloxacin is an inhibitor of the Mcm2-7 replicative helicase. Biosci. Rep. 2013, 33, e00072. [Google Scholar] [CrossRef]
- Dalhoff, A. Immunomodulatory activities of fluoroquinolones. Infection 2005, 33, 55–70. [Google Scholar] [CrossRef]
- Tazi, K.A.; Moreau, R.; Hervé, P.; Dauvergne, A.; Cazals-Hatem, D.; Bert, F.; Poirel, O.; Rabiller, A.; Lebrec, D. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: Role of Akt signaling. Gastroenterology 2005, 129, 303–314. [Google Scholar] [CrossRef]
- Akamatsu, H.; Niwa, Y.; Sasaki, H.; Matoba, Y.; Asada, Y.; Horio, T. Effect of pyridone carboxylic acid anti-microbials on the generation of reactive oxygen species in vitro. J. Int. Med. Res. 1996, 24, 345–351. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Chiu, Y.-H.; Armstrong, A.J.; Kinchen, J.M.; Juncadella, I.J.; Bayliss, D.A.; Ravichandran, K.S. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 2014, 507, 329. [Google Scholar] [CrossRef]
- Fukumoto, R.; Cary, L.H.; Gorbunov, N.V.; Lombardini, E.D.; Elliott, T.B.; Kiang, J.G. Ciprofloxacin modulates cytokine/chemokine profile in serum, improves bone marrow repopulation, and limits apoptosis and autophagy in ileum after whole body ionizing irradiation combined with skin-wound trauma. PLoS ONE 2013, 8, e58389. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Siddiqui, S.; Rehmani, S.; Kazmi, S.U.; Ali, S.H. Fluoroquinolones inhibit HCV by targeting its helicase. Antivir. Ther. 2012, 17, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kaita, K.D.E.; Hawkins, K.; Uhanova, J.; Minuk, G.Y. Use of fluoroquinolones in patients with chronic hepatitis C virus-induced liver failure. Antimicrob. Agents Chemother. 2002, 46, 3280–3282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaya, M.; Nishimura, H.; Hatachi, Y.; Yasuda, H.; Deng, X.; Sasaki, T.; Mizuta, K.; Kubo, H.; Nagatomi, R. Levofloxacin inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Antimicrob. Agents Chemother. 2012, 56, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.R.; et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Karron, R.A.; Sun, W.; Vaughn, D.W.; Reynolds, M.J.; Perreault, J.R.; Thumar, B.; Men, R.; Lai, C.-J.; Elkins, W.R. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 2001, 65, 405–413. [Google Scholar] [CrossRef]
- Hanley, K.A.; Nelson, J.T.; Schirtzinger, E.E.; Whitehead, S.S.; Hanson, C.T. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BCM Ecol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, A.G. Infectious cDNA clone of attenuated Langat tick-borne flavivirus (strain E5) and a 3′ deletion mutant constructed from it exhibit decreased neuroinvasiveness in immunodeficient mice. Virology 2001, 282, 288–300. [Google Scholar] [CrossRef]
- Smith, D.R.; Sprague, T.R.; Hollidge, B.S.; Valdez, S.M.; Padilla, S.L.; Bellanca, S.A.; Golden, J.W.; Coyne, S.R.; Kulesh, D.A.; Miller, L.J. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. Am. J. Trop. Med. Hyg. 2018, 98, 432–444. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, Y.-L.; Schul, W.; Wang, Q.-Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef]
- Srivarangkul, P.; Yuttithamnon, W.; Suroengrit, A.; Pankaew, S.; Hengphasatporn, K.; Rungrotmongkol, T.; Phuwapraisirisan, P.; Ruxrungtham, K.; Boonyasuppayakorn, S. A novel flavanone derivative inhibits dengue virus fusion and infectivity. Antivir. Res. 2018, 151, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, J.; Melnick, J.L.; Rawls, W.E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 1968, 2, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Emeny, J.M.; Morgan, M.J. Regulation of the interferon system: Evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting flavivirus RNA dependent RNA polymerase through a pyridobenzothiazole inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Chuang, F.-K.; Liao, C.-L.; Hu, M.-K.; Chiu, Y.-L.; Lee, A.-R.; Huang, S.-M.; Chiu, Y.-L.; Tsai, P.-L.; Su, B.-C.; Chang, T.-H. Antiviral Activity of Compound L3 against Dengue and Zika Viruses In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 4050. [Google Scholar] [CrossRef] [PubMed]
- Qing, M.; Zou, G.; Wang, Q.Y.; Xu, H.Y.; Dong, H.; Yuan, Z.; Shi, P.Y. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob. Agents Chemother. 2010, 54, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Q.-Y.; Xu, H.Y.; Qing, M.; Kramer, L.; Yuan, Z.; Shi, P.-Y. Inhibition of dengue virus by targeting viral NS4B protein. J. Virol. 2011, 85, 11183–11195. [Google Scholar] [CrossRef]
- Byrd, C.M.; Grosenbach, D.W.; Berhanu, A.; Dai, D.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Yang, G.; Tyavanagimatt, S.; Harver, C.; et al. Novel benzoxazole inhibitor of dengue virus replication that targets the NS3 helicase. Antimicrob. Agents Chemother. 2013, 57, 1902–1912. [Google Scholar] [CrossRef]
- Byrd, C.M.; Dai, D.; Grosenbach, D.W.; Berhanu, A.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Wineinger, K.A.; Page, J.M.; Harver, C.; et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [Google Scholar] [CrossRef]
- Kato, F.; Ishida, Y.; Oishi, S.; Fujii, N.; Watanabe, S.; Vasudevan, S.G.; Tajima, S.; Takasaki, T.; Suzuki, Y.; Ichiyama, K.; et al. Novel antiviral activity of bromocriptine against dengue virus replication. Antivir. Res. 2016, 131, 141–147. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M. Flaviviruses. In Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bedos, J.-P.; Azoulay-Dupuis, E.; Moine, P.; Muffat-Joly, M.; Veber, B.; Pocidalo, J.-J.; Vallee, E. Pharmacodynamic activities of ciprofloxacin and sparfloxacin in a murine pneumococcal pneumonia model: Relevance for drug efficacy. J. Pharmacol. Exp. Ther. 1998, 286, 29–35. [Google Scholar]
- Chartrand, S.A.; Scribner, R.K.; Marks, M.I.; Dice, J. Enoxacin pharmacokinetics and efficacy in CF-1 mice. J. Antimicrob. Chemother. 1987, 19, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aty, A.M.; Goudah, A.; Ismail, M.; Shimoda, M. Disposition kinetics of difloxacin in rabbit after intravenous and intramuscular injection of Dicural. Vet. Res. Commun. 2005, 29, 297–304. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a novel murine model to study zika virus. Am. J. Trop. Med. Hyg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Black, A.; Dunky, A.; Wolf, R.; Sedman, A.; Latts, J.; Welling, P.G. Pharmacokinetics of intravenous and oral enoxacin in healthy volunteers. J. Antimicrob. Chemother. 1988, 21, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Bartosik-Wich, B.; Sörgel, F.; Gutzler, F. In vitro activity, pharmacokinetics, clinical safety and therapeutic efficacy of enoxacin in the treatment of patients with complicated urinary tract infections. Infection 1985, 13, 219–224. [Google Scholar] [CrossRef]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kumar, A.; Jovel, J.; Lopez-Orozco, J.; Limonta, D.; Airo, A.M.; Hou, S.; Stryapunina, I.; Fibke, C.; Moore, R.B.; Hobman, T.C. Human sertoli cells support high levels of zika virus replication and persistence. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Siemann, D.N.; Strange, D.P.; Maharaj, P.N.; Shi, P.-Y.; Verma, S. Zika virus infects human Sertoli cells and modulates the integrity of the in vitro blood-testis barrier model. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Mlera, L.; Bloom, M.E. Differential Zika Virus Infection of Testicular Cell Lines. Viruses 2019, 11, 42. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; ISBN 9241547871. [Google Scholar]
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive flavivirus infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Racsa, L.D.; Kraft, C.S.; Olinger, G.G.; Hensley, L.E. Viral hemorrhagic fever diagnostics. Clin. Infect. Dis. 2015, 62, 214–219. [Google Scholar] [PubMed]
- Debing, Y.; Neyts, J.; Delang, L. The future of antivirals: Broad-spectrum inhibitors. Curr. Opin. Infect. Dis. 2015, 28, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Santose, M.C.; Lee, N. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015, 13, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef]
- De Bernardi Schneider, A.; Machado, D.J.; Guirales, S.; Janies, D.A. FLAVi: An enhanced annotator for viral genomes of Flaviviridae. Viruses 2020, 12, 892. [Google Scholar] [CrossRef]
- Emmerson, A.M.; Jones, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef]
- Helenius, A.; Marsh, M.; White, J. Inhibition of Semliki Forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 1982, 58, 47–61. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Moore, A.C.; Kolokoltsov, A.A.; Davey, R.A. Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins Rab5 and Rab7. Virology 2007, 369, 78–91. [Google Scholar] [CrossRef]
- Zeichardt, H.; Wetz, K.; Willingmann, P.; Habermehl, K.O. Entry of poliovirus type 1 and Mouse Elberfeld (ME) virus into HEp-2 cells: Receptor-mediated endocytosis and endosomal or lysosomal uncoating. J. Gen. Virol. 1985, 66, 483–492. [Google Scholar] [CrossRef]
- Kronenberger, P.; Vrijsen, R.; Boeye, A. Chloroquine induces empty capsid formation during poliovirus eclipse. J.Virol. 1991, 65, 7008–7011. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Roa, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against chikungunya virus in Vero cells. J. Med. Virol. 2010, 88, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Nuckols, J.T.; McAuley, A.J.; Huang, Y.J.S.; Horne, K.M.; Higgs, S.; Davey, R.A.; Vanlandingham, D.L. pH-Dependent entry of chikungunya virus fusion into mosquito cells. Virol. J. 2014, 11, 4–7. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; De Barsy, T.; Poole, B.; Tulkens, P. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Delvecchio, R.; Higa, L.M.; Pezzuto, P.; Valadão, A.L.; Garcez, P.P.; Monteiro, F.L.; Loiola, E.C.; Dias, A.A.; Silva, F.J.M.; Aliota, M.T.; et al. Chloroquine, an endocytosis blocking agent, inhibits zika virus infection in different cell models. Viruses 2016, 8, 322. [Google Scholar] [CrossRef]
- Browning, D.J. Pharmacology of chloroquine and hydroxychloroquine. In Hydroxychloroquine and Chloroquine Retinopathy; Springer: New York, NY, USA, 2014; pp. 35–63. [Google Scholar]
- Shiryaev, S.A.; Mesci, P.; Pinto, A.; Fernandes, I.; Sheets, N.; Shresta, S.; Farhy, C.; Huang, C.-T.; Strongin, A.Y.; Muotri, A.R.; et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; Ji, X.; Quanquin, N.; Deng, Y.Q.; Tian, M.; Aliyari, R.; Zuo, X.; Yuan, L.; Afridi, S.K.; et al. Chloroquine, a FDA-approved Drug, Prevents Zika Virus Infection and its Associated Congenital Microcephaly in Mice. EBioMedicine 2017, 24, 189–194. [Google Scholar] [CrossRef]
- Farias, K.J.S.; Machado, P.R.L.; da Fonseca, B.A.L. Chloroquine inhibits dengue virus type 2 replication in Vero cells but not in C6/36 cells. Sci. World J. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Farias, K.J.S.; Machado, P.R.L.; de Almeida Junior, R.F.; de Aquino, A.A.; da Fonseca, B.A.L. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol. Immunol. 2014, 58, 318–326. [Google Scholar] [CrossRef]
- Cao, B.; Parnell, L.A.; Diamond, M.S.; Mysorekar, I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017, 214, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Farias, K.J.S.; Machado, P.R.L.; Muniz, J.A.P.C.; Imbeloni, A.A.; da Fonseca, B.A.L. Antiviral Activity of Chloroquine Against Dengue Virus Type 2 Replication in Aotus Monkeys. Viral Immunol. 2015, 28, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Minh, N.N.; Van, T.P.; Lee, S.J.; Farrar, J.; Wills, B.; Tran, H.T.; Simmons, C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010, 4, e785. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Castro, L.A.; Fonseca, B.A.L. Chloroquine use improves dengue-related symptoms. Mem. Inst. Oswaldo Cruz 2013, 108, 596–599. [Google Scholar] [CrossRef]
- Lau, B.D.; Pinto, B.L.; Thiemann, D.R.; Lehmann, C.U. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin. Ther. 2011, 33, 1792–1796. [Google Scholar] [CrossRef]
- Scheld, W.M. Quinolone therapy for infections of the central nervous system. Rev. Infect. Dis. 1989, 11, S1194–S1202. [Google Scholar] [CrossRef]
- Outman, W.R. Metabolism and the fluoroquinolones. Am. J. Med. 1989, 87, 37S–42S. [Google Scholar]
- Robson, R.A. Quinolone pharmacokinetics. Int. J. Antimicrob. Agents 1992, 2, 3–10. [Google Scholar] [CrossRef]
- McDonald, E.M.; Duggal, N.K.; Brault, A.C. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl. Trop. Dis. 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V. Zika virus infection damages the testes in mice. Nature 2016, 540, 438. [Google Scholar] [CrossRef]
- Kawiecki, A.B.; Mayton, E.H.; Dutuze, M.F.; Goupil, B.A.; Langohr, I.M.; Del Piero, F.; Christofferson, R.C. Tissue tropisms, infection kinetics, histologic lesions, and antibody response of the MR766 strain of Zika virus in a murine model. Virol. J. 2017, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peregrine, J.; Gurung, S.; Lindgren, M.C.; Husain, S.; Zavy, M.T.; Myers, D.A.; Papin, J.F. Zika virus infection, reproductive organ targeting, and semen transmission in the male olive baboon. J. Virol. 2019, 94, e01434-19. [Google Scholar] [CrossRef] [PubMed]
- Debeb, B.G.; Zhang, X.; Krishnamurthy, S.; Gao, H.; Cohen, E.; Li, L.; Rodriguez, A.A.; Landis, M.D.; Lucci, A.; Ueno, N.T. Characterizing cancer cells with cancer stem cell-like features in 293T human embryonic kidney cells. Mol. Cancer 2010, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Simanjuntak, Y.; Liang, J.J.; Chen, S.Y.; Li, J.K.; Lee, Y.L.; Wu, H.C.; Lin, Y.L. Ebselen alleviates testicular pathology in mice with Zika virus infection and prevents its sexual transmission. PLoS Pathog. 2018, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X. Zika virus causes testis damage and leads to male infertility in mice. Cell 2016, 167, 1511–1524. [Google Scholar] [CrossRef]
- Clancy, C.S.; Van Wettere, A.J.; Morrey, J.D.; Julander, J.G. Zika Virus Associated pathology and Antigen presence in the testicle in the Absence of sexual transmission During subacute to Chronic Infection in a Mouse Model. Sci. Rep. 2019, 9, 8325. [Google Scholar] [CrossRef]
- Demir, A.; Türker, P.; Önol, F.F.; Sirvanci, S.; Findik, A.; Tarcan, T. Effect of experimentally induced Escherichia coli epididymo-orchitis and ciprofloxacin treatment on rat spermatogenesis. Int. J. Urol. 2007, 14, 268–272. [Google Scholar] [CrossRef]
- Deng, Y.-Q.; Zhang, N.-N.; Li, C.-F.; Tian, M.; Hao, J.-N.; Xie, X.-P.; Shi, P.-Y.; Qin, C.-F. Adenosine analog NITD008 is a potent inhibitor of Zika virus. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 3, p. ofw175. [Google Scholar]
- Li, C.; Deng, Y.Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.Y.; Zhang, N.N.; Watanabe, M.; Dong, H.L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Hollidge, B.; Daye, S.; Zeng, X.; Blancett, C.; Kuszpit, K.; Bocan, T.; Koehler, J.W.; Coyne, S.; Minogue, T.; et al. Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type I Interferon. PLoS Negl. Trop. Dis. 2017, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.; Vasconcelos, P.F.C.; et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.F.; Yang, Y.; Zou, P.; Chen, J.; He, Y.; Shui, S.L.; Cui, Y.R.; Bai, R.; Liang, Y.J.; et al. AXL promotes Zika virus infection in astrocytes by antagonizing type i interferon signalling. Nat. Microbiol. 2018, 3, 302–309. [Google Scholar] [CrossRef]
- McDonald, E.M.; Duggal, N.K.; Delorey, M.J.; Oksanish, J.; Ritter, J.M.; Brault, A.C. Duration of seminal Zika viral RNA shedding in immunocompetent mice inoculated with Asian and African genotype viruses. Virology 2019, 535, 1–10. [Google Scholar] [CrossRef]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H.; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685. [Google Scholar] [CrossRef]
- Salazar, V.; Jagger, B.W.; Mongkolsapaya, J.; Burgomaster, K.E.; Dejnirattisai, W.; Winkler, E.S.; Fernandez, E.; Nelson, C.A.; Fremont, D.H.; Pierson, T.C.; et al. Dengue and Zika virus cross-reactive human monoclonal antibodies protect against Spondweni virus infection and pathogenesis in mice. Cell Rep. 2019, 26, 1585–1597.e4. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Dutertre, M.; Mengelle, C.; Fourcade, C.; Marchou, B.; Delobel, P.; Izopet, J.; Martin-Blondel, G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 2016, 16, 405. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y. Evidence of sexual transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596. [Google Scholar] [CrossRef]
- Wu, K.Y.; Zuo, G.L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Cao, W.C.; Qin, C.F.; Luo, Z.G. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016, 26, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.-J.J.; Bowen, R.A.; Brault, A.C. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef] [PubMed]



| Virus | Strain | Obtained from | Passage History |
|---|---|---|---|
| Zika virus (ZIKV) | MEX 1–7 | World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) | C6/36 (×3) |
| Zika virus (ZIKV) | FSS13025 | C6/36 (×1), Vero (×1) | |
| Dengue virus-1 (DENV-1) | Thailand 160087-1A | Laboratory of Dr. Stephen Whitehead, National Institutes of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH) | Vero (×5) |
| Dengue virus-2 (DENV-2) | NGC proto | C6/36 (×3), Vero (×2) | |
| Dengue virus-4 (rDENV-4) | Dominica p4-3b [36] | Vero (×4) | |
| Langat virus (LGTV) | E5 [38] | Laboratory of Dr. Alexander Pletnev, NIAID, NIH | Vero (×4) |
| Modoc virus (MODV) | 7/26/61 | WRCEVA | IC suckling mice (×9), Vero (×4) |
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| 1 × 105 pfu ZIKV | PBS Control | 1 × 102 pfu ZIKV | PBS Control | |
| Drug diluent | 7 (4 female, 3 male) | NA | 7 (3 female, 4 male) | 6 (3 female, 3 male) |
| Enoxacin (10 mg/kg) | 7 (3 female, 4 male) | 6 (2 female, 4 male) | Not tested | Not tested |
| Enoxacin (15 mg/kg) | 6 (0 female, 6 male) | 5 (3 female, 2 male) | 6 (3 female, 3 male) | 7 (5 female, 2 male) |
| Drug | CC50 (95% CI) | Virus | EC50 (95% CI) | Selectivity Index a |
|---|---|---|---|---|
Enoxacin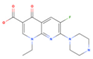 | 537.8 (430.1–700.0) | ZIKV (MOI: 0.2) | 24.4 (17.3–34.1) | 22.0 |
| ZIKV (MOI: 1.0) | 18.1 (14.6–22.4) | 29.7 | ||
| DENV-1 | 6.6 (6.0–7.3) | 81.5 | ||
| DENV-2 | 4.7 (3.5–6.2) | 114.4 | ||
| DENV-4 | 7.6 (7.1–8.2) | 70.8 | ||
| LGTV | <4.7 | n.d. | ||
| MODV | 14.6 (7.4–29.0) | 36.8 | ||
Difloxacin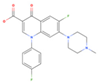 | >1000 | ZIKV (MOI: 0.2) | 35.9 (19.0–67.5) | n.d. |
| ZIKV (MOI: 1.0) | 25.4 (20.8–30.9) | n.d. | ||
| DENV-1 | 10.9 (9.2–12.9) | n.d. | ||
| DENV-2 | 5.7 (4.8–6.9) | n.d. | ||
| DENV-4 | 10.1 (9.1–11.3) | n.d. | ||
| LGTV | 8.2 (6.3–10.6) | n.d. | ||
| MODV | >150 | n.d. | ||
Ciprofloxacin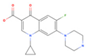 | 759.6 (649.3–912.9) | ZIKV (MOI: 0.2) | 116.1(68.9–179.0) | 6.5 |
| ZIKV (MOI: 1.0) | 56.8 (39.6–81.5) | 13.4 | ||
| DENV-1 | 27.8 (22.1–34.9) | 27.3 | ||
| DENV-2 | 8.0 (5.0–12.9) | 95.0 | ||
| DENV-4 | 19.6 (16.5–23.2) | 38.8 | ||
| LGTV | 7.4 (3.9–14.0) | 102.6 | ||
| MODV | 11.2 (3.8–32.6) | 67.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scroggs, S.L.P.; Andrade, C.C.; Chinnasamy, R.; Azar, S.R.; Schirtzinger, E.E.; Garcia, E.I.; Arterburn, J.B.; Hanley, K.A.; Rossi, S.L. Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses. Viruses 2020, 12, 1022. https://doi.org/10.3390/v12091022
Scroggs SLP, Andrade CC, Chinnasamy R, Azar SR, Schirtzinger EE, Garcia EI, Arterburn JB, Hanley KA, Rossi SL. Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses. Viruses. 2020; 12(9):1022. https://doi.org/10.3390/v12091022
Chicago/Turabian StyleScroggs, Stacey L. P., Christy C. Andrade, Ramesh Chinnasamy, Sasha R. Azar, Erin E. Schirtzinger, Erin I. Garcia, Jeffrey B. Arterburn, Kathryn A. Hanley, and Shannan L. Rossi. 2020. "Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses" Viruses 12, no. 9: 1022. https://doi.org/10.3390/v12091022
APA StyleScroggs, S. L. P., Andrade, C. C., Chinnasamy, R., Azar, S. R., Schirtzinger, E. E., Garcia, E. I., Arterburn, J. B., Hanley, K. A., & Rossi, S. L. (2020). Old Drugs with New Tricks: Efficacy of Fluoroquinolones to Suppress Replication of Flaviviruses. Viruses, 12(9), 1022. https://doi.org/10.3390/v12091022






