Analysis of the Function of the Lymphocytic Choriomeningitis Virus S Segment Untranslated Region on Growth Capacity In Vitro and on Virulence In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Immunofocus Assay and Immunofluorescence Assay (IFA)
2.3. Plasmids
2.4. Transfection, Minigenome, and Reverse Genetics System
2.5. Rescue of LCMV RNA Analogs into LCMV-Like Particles
2.6. RNA Secondary Structure Prediction
2.7. Luciferase Assay
2.8. Viral Growth Kinetics
2.9. Viral Genetic Stability Test
2.10. Animal Experiments
2.11. Neutralization Assay
2.12. Western Blotting
3. Results
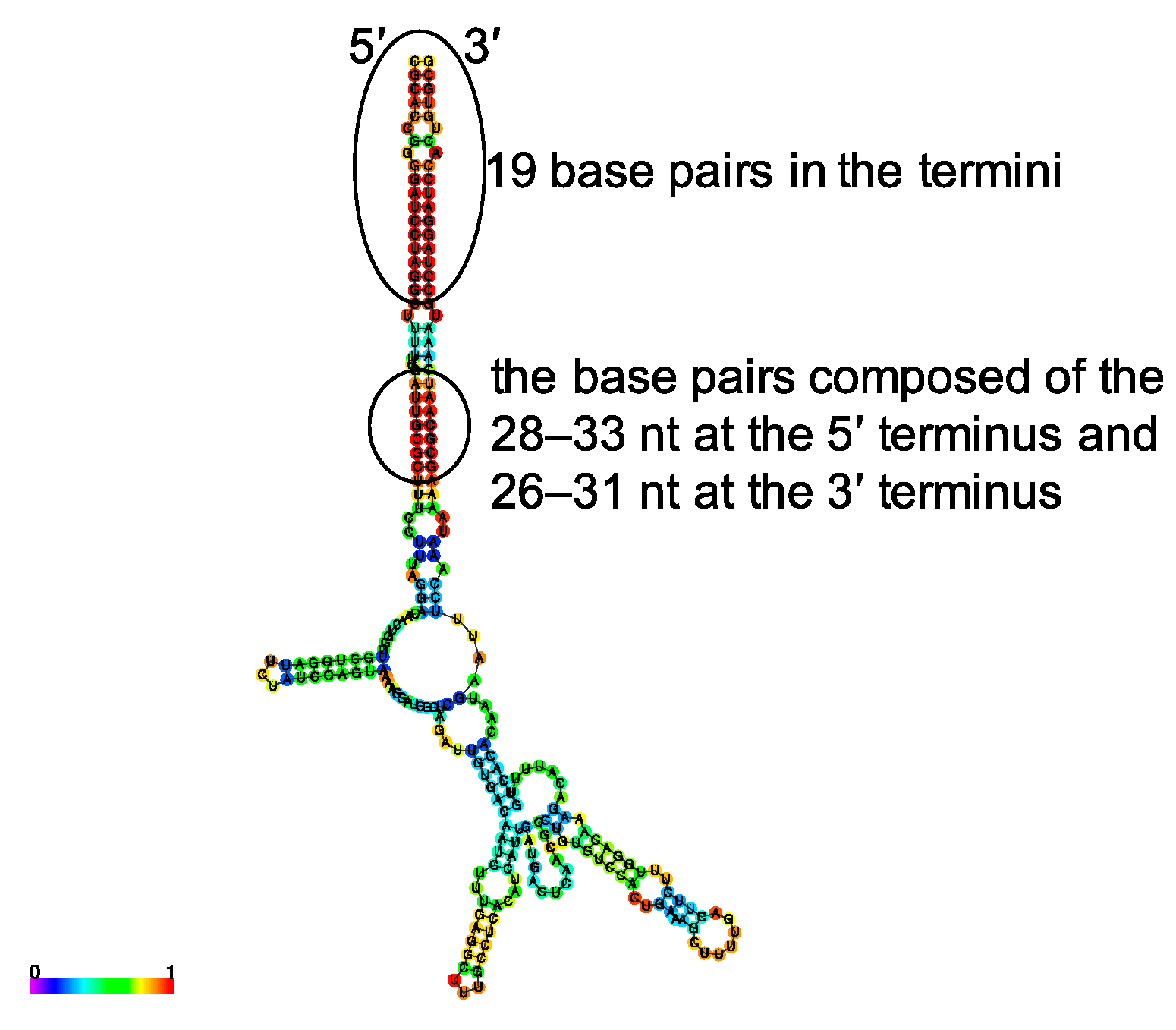
3.1. Design of Plasmids for Recombinant LCMV with Mutations in the S Segment UTRs and Prediction of Their RNA Secondary Structure
3.2. Rescue and Characterization of Recombinant LCMVs
3.3. Genetic Stability of rLCMVs
3.4. Pathogenicity of rLCMVs in Mice
3.5. Acquired Immunity against LCMV in Mice Induced by Infection With rLCMVs
3.6. Minigenome Assay and VLP Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knipe, D.M.; Howley, P.M. Fields Virology, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Chapter 50; pp. 1792–1827. [Google Scholar]
- Jahrling, P.B.; Peters, C.J. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch. Pathol. Lab. Med. 1992, 116, 486–488. [Google Scholar] [PubMed]
- Barton, L.L.; Mets, M.B.; Beauchamp, C.L. Lymphocytic choriomeningitis virus: Emerging fetal teratogen. Am. J. Obstet. Gynecol. 2002, 187, 1715–1716. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.A.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J. Lymphocytic choriomeningitis virus--an old enemy up to new tricks. N. Engl. J. Med. 2006, 354, 2208–2211. [Google Scholar] [CrossRef]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef]
- Armstrong, C.; Lillie, R.D. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Public Health Rep. 1934, 49, 1019–1027. [Google Scholar] [CrossRef]
- Rivers, T.M.; McNair Scott, T.F. Meningitis in Man Caused by a Filterable Virus. Science 1935, 81, 439–440. [Google Scholar] [CrossRef]
- Zinkernagel, R.M. Lymphocytic choriomeningitis virus and immunology. Curr. Top Microbiol. Immunol. 2002, 263, 1–5. [Google Scholar]
- Lee, K.J.; Novella, I.S.; Teng, M.N.; Oldstone, M.B.; de La Torre, J.C. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 2000, 74, 3470–3477. [Google Scholar] [CrossRef]
- Lee, K.J.; Perez, M.; Pinschewer, D.D.; de la Torre, J.C. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 2002, 76, 6393–6397. [Google Scholar] [CrossRef]
- Sanchez, A.B.; de la Torre, J.C. Genetic and biochemical evidence for an oligomeric structure of the functional L polymerase of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2005, 79, 7262–7268. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Bergthaler, A.; de la Torre, J.C.; Pinschewer, D.D. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4663–4668. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.B.; de la Torre, J.C. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology 2006, 350, 370–380. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Reverse genetics approaches to combat pathogenic arenaviruses. Antiviral. Res. 2008, 80, 239–250. [Google Scholar] [CrossRef]
- Martinez-Sobrido, L.; Emonet, S.; Giannakas, P.; Cubitt, B.; Garcia-Sastre, A.; de la Torre, J.C. Identification of amino acid residues critical for the anti-interferon activity of the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2009, 83, 11330–11340. [Google Scholar] [CrossRef]
- Emonet, S.E.; Urata, S.; de la Torre, J.C. Arenavirus reverse genetics: New approaches for the investigation of arenavirus biology and development of antiviral strategies. Virology 2011, 411, 416–425. [Google Scholar] [CrossRef]
- Buchmeier, M.J.; Welsh, R.M.; Dutko, F.J.; Oldstone, M.B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 1980, 30, 275–331. [Google Scholar]
- Djavani, M.; Lukashevich, I.S.; Salvato, M.S. Sequence comparison of the large genomic RNA segments of two strains of lymphocytic choriomeningitis virus differing in pathogenic potential for guinea pigs. Virus Genes 1998, 17, 151–155. [Google Scholar] [CrossRef]
- Oehen, S.; Hengartner, H.; Zinkernagel, R.M. Vaccination for disease. Science 1991, 251, 195–198. [Google Scholar] [CrossRef]
- Takagi, T.; Ohsawa, M.; Yamanaka, H.; Matsuda, N.; Sato, H.; Ohsawa, K. Difference of two new LCMV strains in lethality and viral genome load in tissues. Exp. Anim. 2017, 66, 199–208. [Google Scholar] [CrossRef]
- Lukashevich, I.S.; Rodas, J.D.; Tikhonov, I.I.; Zapata, J.C.; Yang, Y.; Djavani, M.; Salvato, M.S. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch. Virol. 2004, 149, 2319–2336. [Google Scholar] [CrossRef] [PubMed]
- Danes, L.; Benda, R.; Fuchsova, M. Experimental Inhalation Infection of Monkeys of the Macacus Cynomolgus and Macacus Rhesus Species with the Virus of Lymphocytic Choriomeningitis (We). Bratisl. Lek. Listy 1963, 2, 71–79. [Google Scholar] [PubMed]
- Lukashevich, I.S.; Djavani, M.; Rodas, J.D.; Zapata, J.C.; Usborne, A.; Emerson, C.; Mitchen, J.; Jahrling, P.B.; Salvato, M.S. Hemorrhagic fever occurs after intravenous, but not after intragastric, inoculation of rhesus macaques with lymphocytic choriomeningitis virus. J. Med. Virol. 2002, 67, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Lukashevich, I.S.; Tikhonov, I.; Rodas, J.D.; Zapata, J.C.; Yang, Y.; Djavani, M.; Salvato, M.S. Arenavirus-mediated liver pathology: Acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J. Virol. 2003, 77, 1727–1737. [Google Scholar] [CrossRef]
- Zapata, J.C.; Pauza, C.D.; Djavani, M.M.; Rodas, J.D.; Moshkoff, D.; Bryant, J.; Ateh, E.; Garcia, C.; Lukashevich, I.S.; Salvato, M.S. Lymphocytic choriomeningitis virus (LCMV) infection of macaques: A model for Lassa fever. Antiviral. Res. 2011, 92, 125–138. [Google Scholar] [CrossRef]
- Salvato, M.S.; Lukashevich, I.S.; Yang, Y.; Medina-Moreno, S.; Djavani, M.; Bryant, J.; Rodas, J.D.; Zapata, J.C. A Primate Model for Viral Hemorrhagic Fever. Methods Mol. Biol. 2018, 1604, 279–290. [Google Scholar] [CrossRef]
- Iwasaki, M.; Ngo, N.; Cubitt, B.; Teijaro, J.R.; de la Torre, J.C. General Molecular Strategy for Development of Arenavirus Live-Attenuated Vaccines. J. Virol. 2015, 89, 12166–12177. [Google Scholar] [CrossRef]
- Perez, M.; de la Torre, J.C. Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2003, 77, 1184–1194. [Google Scholar] [CrossRef]
- Hass, M.; Westerkofsky, M.; Muller, S.; Becker-Ziaja, B.; Busch, C.; Gunther, S. Mutational analysis of the lassa virus promoter. J. Virol. 2006, 80, 12414–12419. [Google Scholar] [CrossRef]
- Saijo, M.; Georges-Courbot, M.C.; Marianneau, P.; Romanowski, V.; Fukushi, S.; Mizutani, T.; Georges, A.J.; Kurata, T.; Kurane, I.; Morikawa, S. Development of recombinant nucleoprotein-based diagnostic systems for Lassa fever. Clin. Vaccine Immunol. 2007, 14, 1182–1189. [Google Scholar] [CrossRef]
- Sato, K.; Hamada, M.; Asai, K.; Mituyama, T. CENTROIDFOLD: A web server for RNA secondary structure prediction. Nucleic Acids Res. 2009, 37, W277–W280. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Sakai, M.; Yoshii, K.; Sunden, Y.; Yokozawa, K.; Hirano, M.; Kariwa, H. Variable region of the 3’ UTR is a critical virulence factor in the Far-Eastern subtype of tick-borne encephalitis virus in a mouse model. J. Gen. Virol. 2014, 95, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Muto, M.; Hirano, M.; Kariwa, H.; Yoshii, K. Virulence of tick-borne encephalitis virus is associated with intact conformational viral RNA structures in the variable region of the 3′-UTR. Virus Res. 2015, 203, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kloc, A.; Rai, D.K.; Rieder, E. The Roles of Picornavirus Untranslated Regions in Infection and Innate Immunity. Front. Microbiol. 2018, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.I.; Hedrick, S.M.; Nielsen, E.A.; D’Eustachio, P.; Ruddle, F.; Steinberg, A.D.; Paul, W.E.; Davis, M.M. Isolation of a cDNA clone corresponding to an X-linked gene family (XLR) closely linked to the murine immunodeficiency disorder xid. Nature 1985, 314, 369–372. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Sendo, F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin. Exp. Immunol. 2000, 120, 125–133. [Google Scholar] [CrossRef]
- Chen, L.; Sendo, F. Cytokine and chemokine mRNA expression in neutrophils from CBA/NSlc mice infected with Plasmodium berghei ANKA that induces experimental cerebral malaria. Parasitol. Int. 2001, 50, 139–143. [Google Scholar] [CrossRef]
- Scher, I.; Steinberg, A.D.; Berning, A.K.; Paul, W.E. X-linked B-lymphocyte immune defect in CBA/N mice. II. Studies of the mechanisms underlying the immune defect. J. Exp. Med. 1975, 142, 637–650. [Google Scholar] [CrossRef]
- Rawlings, D.J.; Saffran, D.C.; Tsukada, S.; Largaespada, D.A.; Grimaldi, J.C.; Cohen, L.; Mohr, R.N.; Bazan, J.F.; Howard, M.; Copeland, N.G.; et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 1993, 261, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.S.; Dallman, M.J.; Dayan, A.D.; Martin, A.; Mosedale, B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 1980, 283, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Plows, D.; Kontogeorgos, G.; Kollias, G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J. Immunol. 1999, 162, 1018–1023. [Google Scholar] [PubMed]
- Park, J.K.; Byun, J.Y.; Park, J.A.; Kim, Y.Y.; Lee, Y.J.; Oh, J.I.; Jang, S.Y.; Kim, Y.H.; Song, Y.W.; Son, J.; et al. HM71224, a novel Bruton’s tyrosine kinase inhibitor, suppresses B cell and monocyte activation and ameliorates arthritis in a mouse model: A potential drug for rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Battegay, M.; Moskophidis, D.; Waldner, H.; Brundler, M.A.; Fung-Leung, W.P.; Mak, T.W.; Hengartner, H.; Zinkernagel, R.M. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 1993, 151, 5408–5415. [Google Scholar] [PubMed]
- Mazel-Sanchez, B.; Elliott, R.M. Attenuation of bunyamwera orthobunyavirus replication by targeted mutagenesis of genomic untranslated regions and creation of viable viruses with minimal genome segments. J. Virol. 2012, 86, 13672–13678. [Google Scholar] [CrossRef]
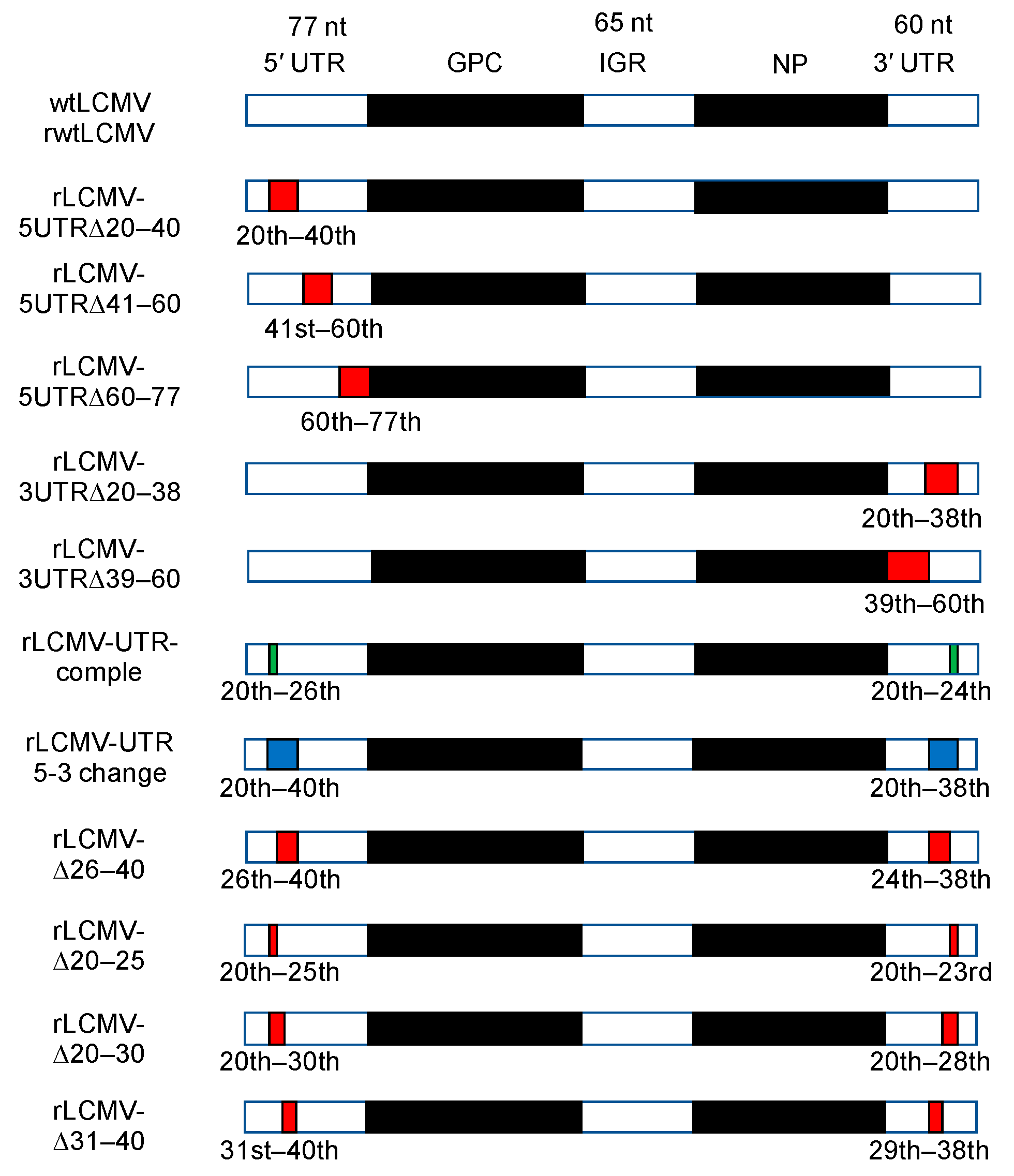
| Plasmid | 5′ UTR Nucleotide Sequence (from 5′ to 3′, vRNA Polarity) |
|---|---|
| pRF-WE-SRG or SMG | CGCACCGGGGATCCTAGGCTTTTTGGATTGCGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG (or SMG)-5UTRΔ20–40 | CGCACCGGGGATCCTAGGC---------------------TAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG (or SMG)-5UTRΔ41–60 | CGCACCGGGGATCCTAGGCTTTTTGGATTGCGCTTTCCTT--------------------TTCTATCCAGTAAAAGG |
| pRF-WE-SRG (or SMG) -5UTRΔ60–77 | CGCACCGGGGATCCTAGGCTTTTTGGATTGCGCTTTCCTTTAGGACAACTGGGTGCTGG------------------ |
| pRF-WE-SRG (or SMG)-3UTRΔ20–38 | CGCACCGGGGATCCTAGGCTTTTTGGATTGCGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG (or SMG)-3UTRΔ39–60 | CGCACCGGGGATCCTAGGCTTTTTGGATTGCGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG (or SMG)-UTR-comple | CGCACCGGGGATCCTAGGCCCAAAAAATTGCGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG-UTR 5-3 change | CGCACCGGGGATCCTAGGCTAAA--CTAACGCGAAAATAATAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG-Δ26–40 | CGCACCGGGGATCCTAGGCTTTTTG---------------TAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG-Δ20–25 | CGCACCGGGGATCCTAGGC------GATTGCGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG-Δ20–30 | CGCACCGGGGATCCTAGGC-----------CGCTTTCCTTTAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| pRF-WE-SRG-Δ31–40 | CGCACCGGGGATCCTAGGCTTTTTGGATTG----------TAGGACAACTGGGTGCTGGATTCTATCCAGTAAAAGG |
| Plasmid | 3′ UTR Nucleotide Sequence (from 3′ to 5′, cRNA Polarity) |
|---|---|
| pRF-WE-SRG or SMG | GCGTGTCACCTAGGATCCGTAAA--CTAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG (or SMG)-5UTRΔ20–40 | GCGTGTCACCTAGGATCCGTAAA--CTAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG (or SMG)-5UTRΔ41–60 | GCGTGTCACCTAGGATCCGTAAA--CTAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG (or SMG)-5UTRΔ60–77 | GCGTGTCACCTAGGATCCGTAAA--CTAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG (or SMG)-3UTRΔ20–38 | GCGTGTCACCTAGGATCCG---------------------ACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG (or SMG)-3UTRΔ39–60 | GCGTGTCACCTAGGATCCGTAAA--CTAACGCGAAAATAA---------------------- |
| pRF-WE-SRG (or SMG)-UTR-comple | GCGTGTCACCTAGGATCCG-GTTTA-TAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG-UTR 5-3 change | GCGTGTCACCTAGGATCCGTTTTTGGATTGCGCTTTCCTTACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG-Δ26–40 | GCGTGTCACCTAGGATCCGTAAA-----------------ACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG-Δ20–25 | GCGTGTCACCTAGGATCCG------CTAACGCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG-Δ20–30 | GCGTGTCACCTAGGATCCG-----------GCGAAAATAAACCTTTAAGTAACACACTGTTT |
| pRF-WE-SRG-Δ31–40 | GCGTGTCACCTAGGATCCGTAAA-CTAAC-----------ACCTTTAAGTAACACACTGTTT |
| Plasmid Name | Number of Nucleotides Forming a Panhandle Structure at the Termini | Base Pairs with High Base-Pairing Probability, Except for the 19 Base Pairs at the Termini | ||
|---|---|---|---|---|
| At the 5′ Terminus | At the 3′ Terminus | At the 5′ Terminus | At the 3′ Terminus | |
| pRF-WE-SRG | 45 nt | 42 nt | 28–33 | 26–31 |
| pRF-WE-SRG-5UTRΔ20–40 | 19 nt | 19 nt | none | none |
| pRF-WE-SRG-5UTRΔ41–60 | 36 nt | 34 nt | 28–33 | 26–31 |
| pRF-WE-SRG-5UTRΔ60–77 | 45 nt | 42 nt | 28–33 | 26–31 |
| pRF-WE-SRG-3UTRΔ20–38 | 27 nt | 23 nt | none | none |
| pRF-WE-SRG-3UTRΔ39–60 | 36 nt | 34 nt | 28–33 | 26–31 |
| pRF-WE-SRG-UTR-comple | 45 nt | 42 nt | 28–33 | 26–31 |
| pRF-WE-SRG-UTR 5-3 change | 34 nt | 36 nt | 26–31 | 28–33 |
| pRF-WE-SRG-Δ26–40 | 25 nt | 25 nt | none | none |
| pRF-WE-SRG-Δ20–25 | 39 nt | 38 nt | 20–27 | 20–27 |
| pRF-WE-SRG-Δ20–30 | 34 nt | 33 nt | 20–22 | 20–22 |
| pRF-WE-SRG-Δ31–40 | 35 nt | 32 nt | none | none |
| Plasmid Name | Virus Name | Viral Growth Efficiency In Vitro Compared to That of rtLCMV in Vero Cell | Viral Pathogenicity In Vivo (Mortality Rate) | ||
|---|---|---|---|---|---|
| CBA/NSlc Mice | DBA/1JJmsSlc Mice | Immunity Acquired | |||
| pRF-WE-SRG | rwtLCMV # | 80% | 60% | Acquired | |
| pRF-WE-SRG-5UTRΔ20–40 | rLCMV-5UTRΔ20–40 | None | Not done (ND) | ND | ND |
| pRF-WE-SRG-5UTRΔ41–60 | rLCMV-5UTRΔ41–60 | Equal | 40% | 0% | Acquired |
| pRF-WE-SRG-5UTRΔ60–77 | rLCMV-5UTRΔ60–77 | Equal | 80% | 0% | Acquired |
| pRF-WE-SRG-3UTRΔ20–38 | rLCMV-3UTRΔ20–38 | None | ND | ND | ND |
| pRF-WE-SRG-3UTRΔ39–60 | rLCMV-3UTRΔ39–60 ## | Less | 0% | 20% | Acquired |
| pRF-WE-SRG-UTR-comple | rLCMV-UTR-comple | Less | 0% | 0% | Acquired * |
| pRF-WE-SRG-UTR 5-3 change | rLCMV-UTR 5-3 change | Less | 0% | 0% | Acquired ** |
| pRF-WE-SRG-Δ26–40 | rLCMV-Δ26–40 | Less | 0% | 0% | Acquired *** |
| pRF-WE-SRG-Δ20–25 | rLCMV-Δ20–25 | None | ND | ND | ND |
| pRF-WE-SRG-Δ20–30 | rLCMV-Δ20–30 | None | ND | ND | ND |
| pRF-WE-SRG-Δ31–40 | rLCMV-Δ31–40 | None | ND | ND | ND |
| Virus Name | Segment | Nt Position (vRNA Sense) | P0 * | P10 ** | Gene Name | Amino Acid Change | Remarks |
|---|---|---|---|---|---|---|---|
| rLCMV-5UTRΔ60–77 | L | 929 | C | T and C *** | L | V2091I | |
| rLCMV-UTR-comple | S | 20 | C | C and T *** | 5’ UTR | The authentic nucleic acid sequence of wtLCMV at the 20th position was T. | |
| S | 21 | C | C and T *** | 5’ UTR | The authentic nucleic acid sequence of wtLCMV at the 21st position was T. | ||
| S | 3355 | T | T and A *** | 3’ UTR | The authentic nucleic acid sequence of wtLCMV at the 3355th position was A. | ||
| S | 3356 | G | G and A *** | 3’ UTR | The authentic nucleic acid sequence of wtLCMV at the 3356th position was A. | ||
| rLCMV-UTR 5-3 change | S | 32 | A | C and A *** | 5’ UTR | ||
| S | 969 | T | T and C *** | GPC | C298C | Synonymous change. | |
| S | 3334 | C | T and C *** | 3’ UTR | |||
| L | 2463 | A | G and A *** | L | C1579C | Synonymous change. | |
| L | 2524 | A | G and A *** | L | V1559A | ||
| L | 2614 | A | G and A *** | L | L1529P | ||
| L | 2689 | A | G and A *** | L | F1504S | ||
| L | 3757 | C | T and C *** | L | S1148N | ||
| L | 6698 | A | G and A *** | L | L168L | Synonymous change. | |
| L | 6699 | A | G and A *** | L | L167L | Synonymous change. | |
| rLCMV-Δ26–40 | L | 2708 | C | T | L | E1498K | |
| L | 5332 | T | A | L | K623M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, S.; Yoshikawa, T.; Shimojima, M.; Fukushi, S.; Kurosu, T.; Tani, H.; Fukuma, A.; Kato, F.; Nakayama, E.; Maeki, T.; et al. Analysis of the Function of the Lymphocytic Choriomeningitis Virus S Segment Untranslated Region on Growth Capacity In Vitro and on Virulence In Vivo. Viruses 2020, 12, 896. https://doi.org/10.3390/v12080896
Taniguchi S, Yoshikawa T, Shimojima M, Fukushi S, Kurosu T, Tani H, Fukuma A, Kato F, Nakayama E, Maeki T, et al. Analysis of the Function of the Lymphocytic Choriomeningitis Virus S Segment Untranslated Region on Growth Capacity In Vitro and on Virulence In Vivo. Viruses. 2020; 12(8):896. https://doi.org/10.3390/v12080896
Chicago/Turabian StyleTaniguchi, Satoshi, Tomoki Yoshikawa, Masayuki Shimojima, Shuetsu Fukushi, Takeshi Kurosu, Hideki Tani, Aiko Fukuma, Fumihiro Kato, Eri Nakayama, Takahiro Maeki, and et al. 2020. "Analysis of the Function of the Lymphocytic Choriomeningitis Virus S Segment Untranslated Region on Growth Capacity In Vitro and on Virulence In Vivo" Viruses 12, no. 8: 896. https://doi.org/10.3390/v12080896
APA StyleTaniguchi, S., Yoshikawa, T., Shimojima, M., Fukushi, S., Kurosu, T., Tani, H., Fukuma, A., Kato, F., Nakayama, E., Maeki, T., Tajima, S., Lim, C.-K., Ebihara, H., Kyuwa, S., Morikawa, S., & Saijo, M. (2020). Analysis of the Function of the Lymphocytic Choriomeningitis Virus S Segment Untranslated Region on Growth Capacity In Vitro and on Virulence In Vivo. Viruses, 12(8), 896. https://doi.org/10.3390/v12080896





