Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Antibodies and Reagents
2.3. BKPyV Production
2.4. Cell Viability Assay
2.5. Immunofluorescence Staining
2.6. Western-Blot
2.7. Real-Time PCR
2.8. Kynurenine Assay
2.9. Statistical Analysis
3. Results
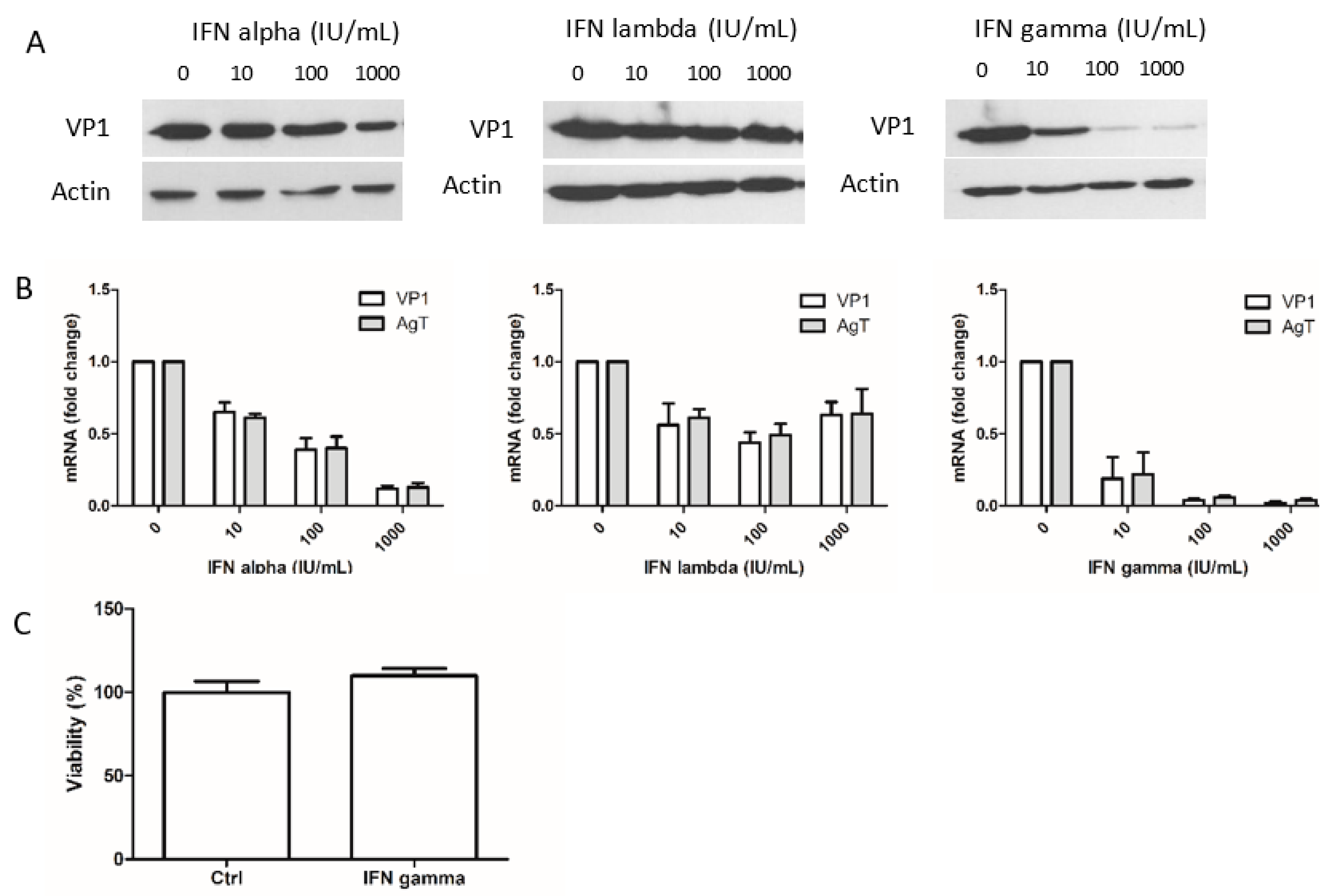
3.1. Interferon-Gamma Inhibits BKPyV Multiplication more Potently than IFN-Alpha and IFN-Lambda 1
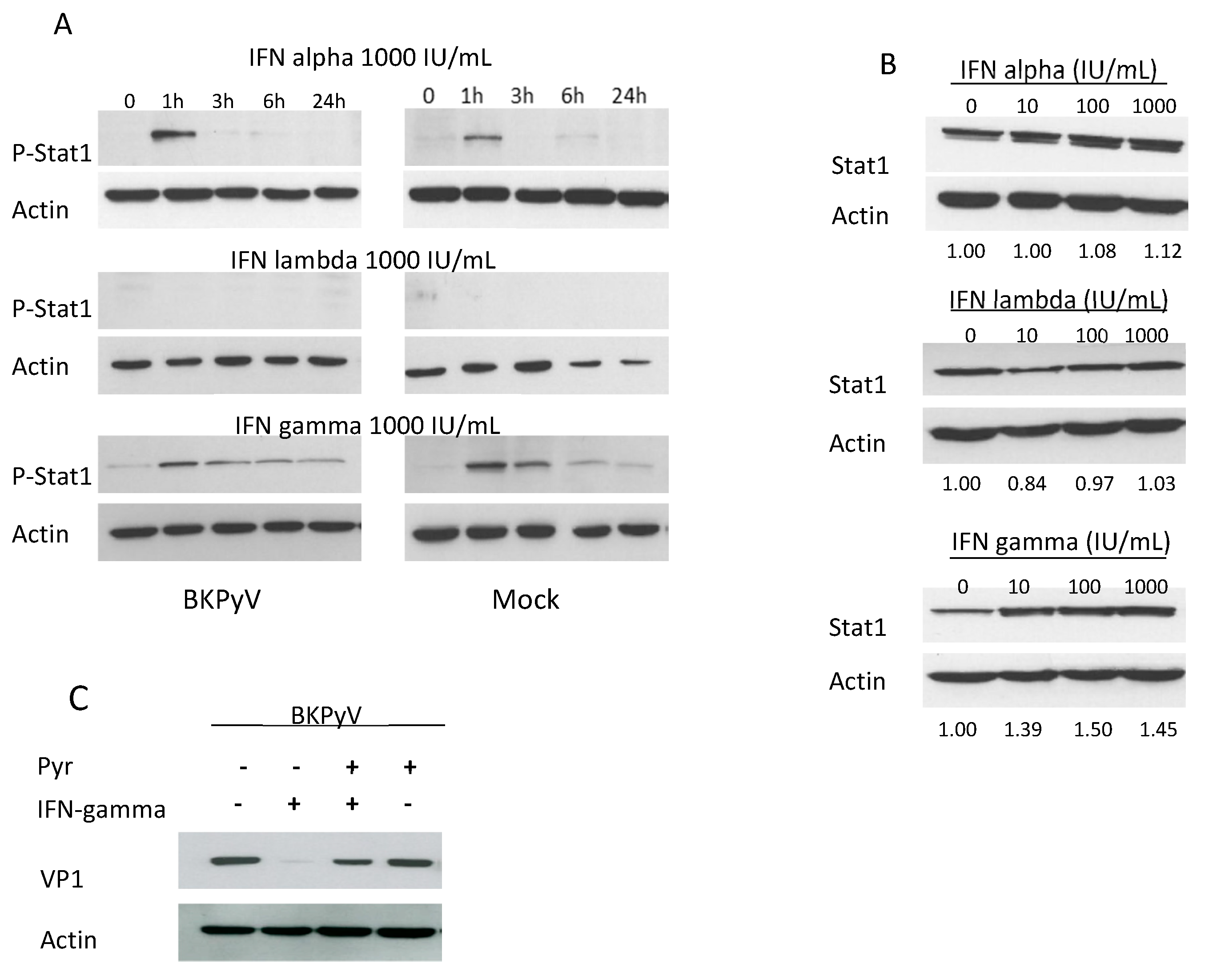
3.2. The Jak-Stat Pathway Is Involved in the Antiviral Effect of IFNs on BKPyV Infection
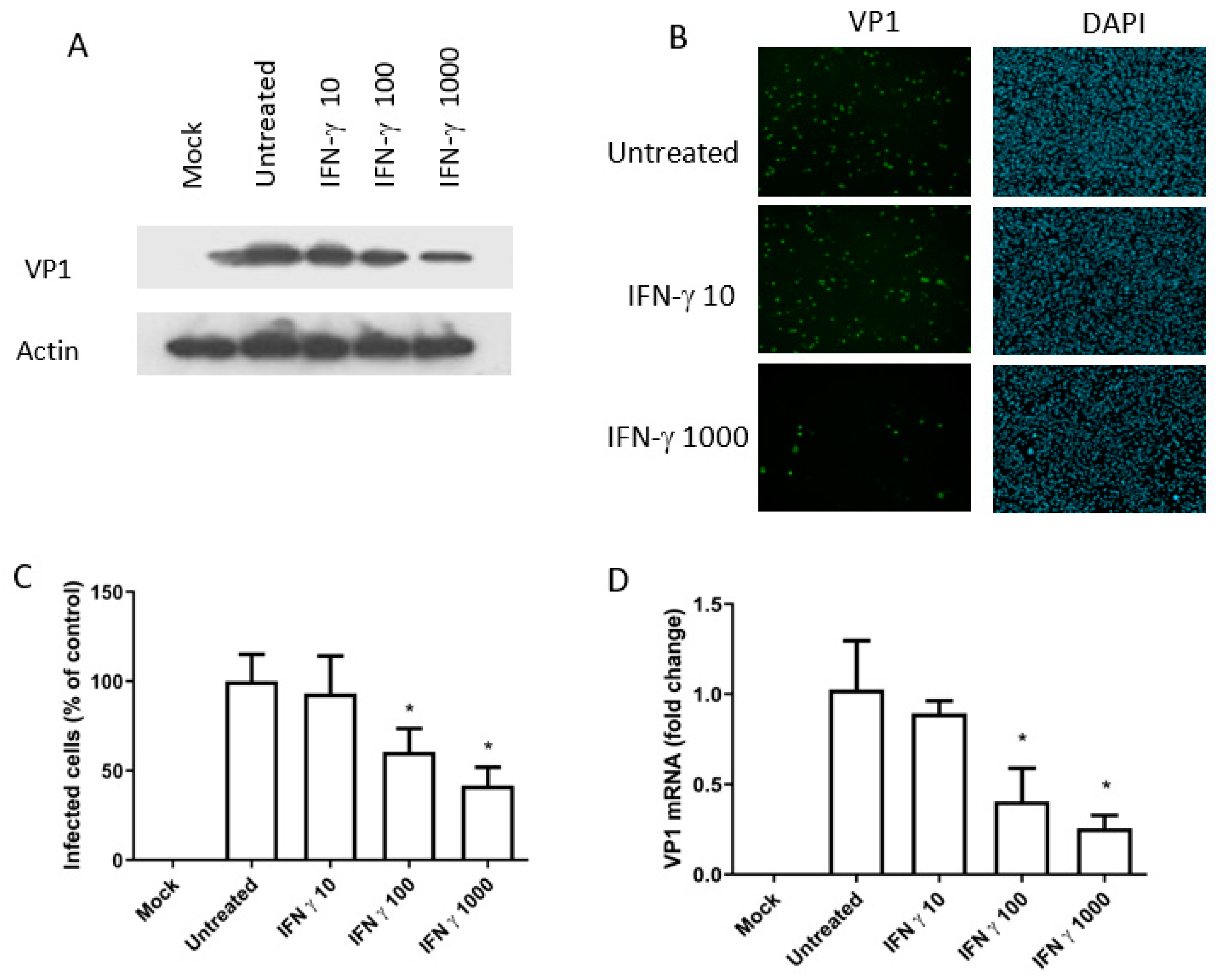
3.3. The Antiviral Activity of IFN-Gamma (BKPyV Infection of Caki-1 Cells)
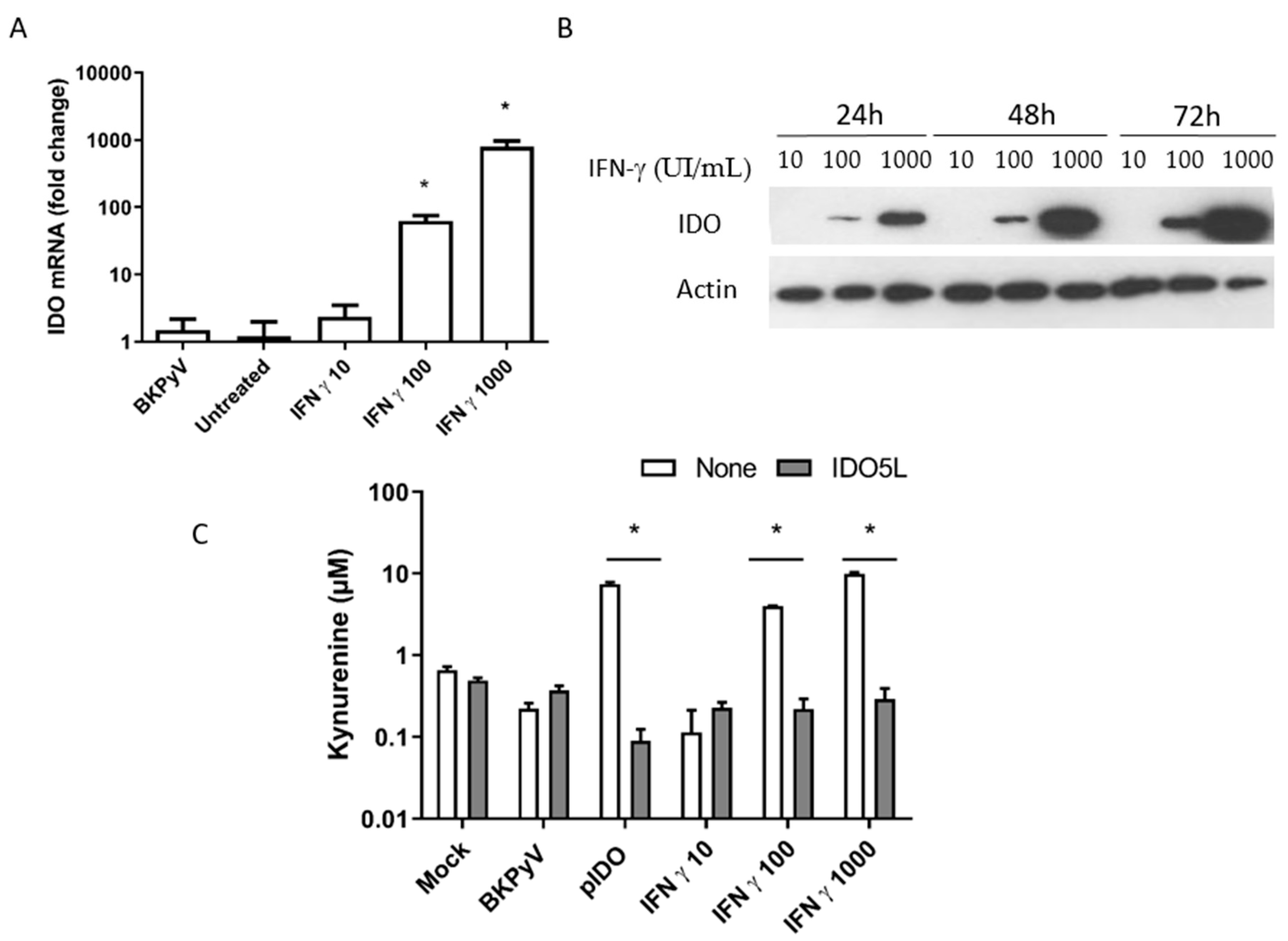
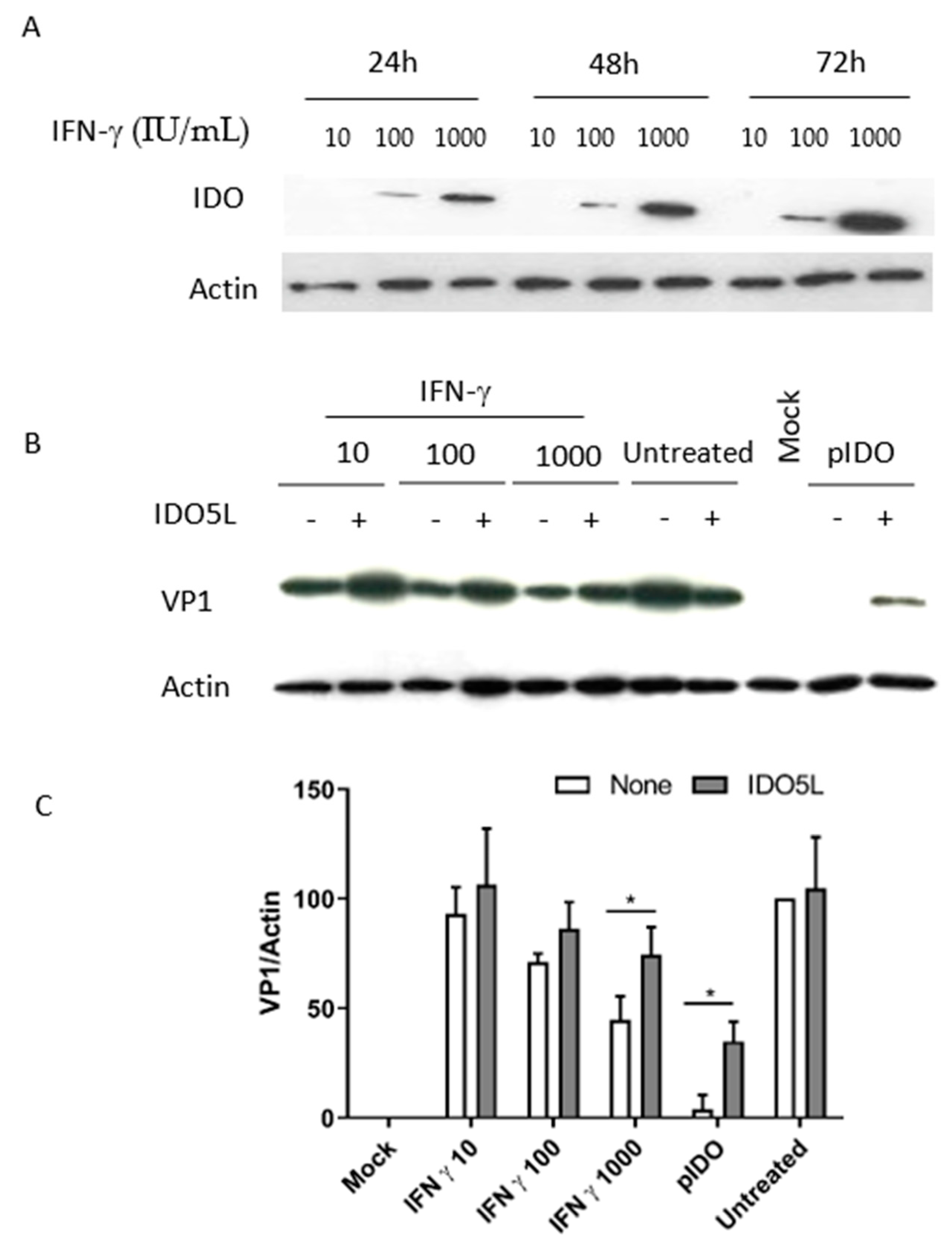
3.4. Expression of IDO in Caki-1 Cells
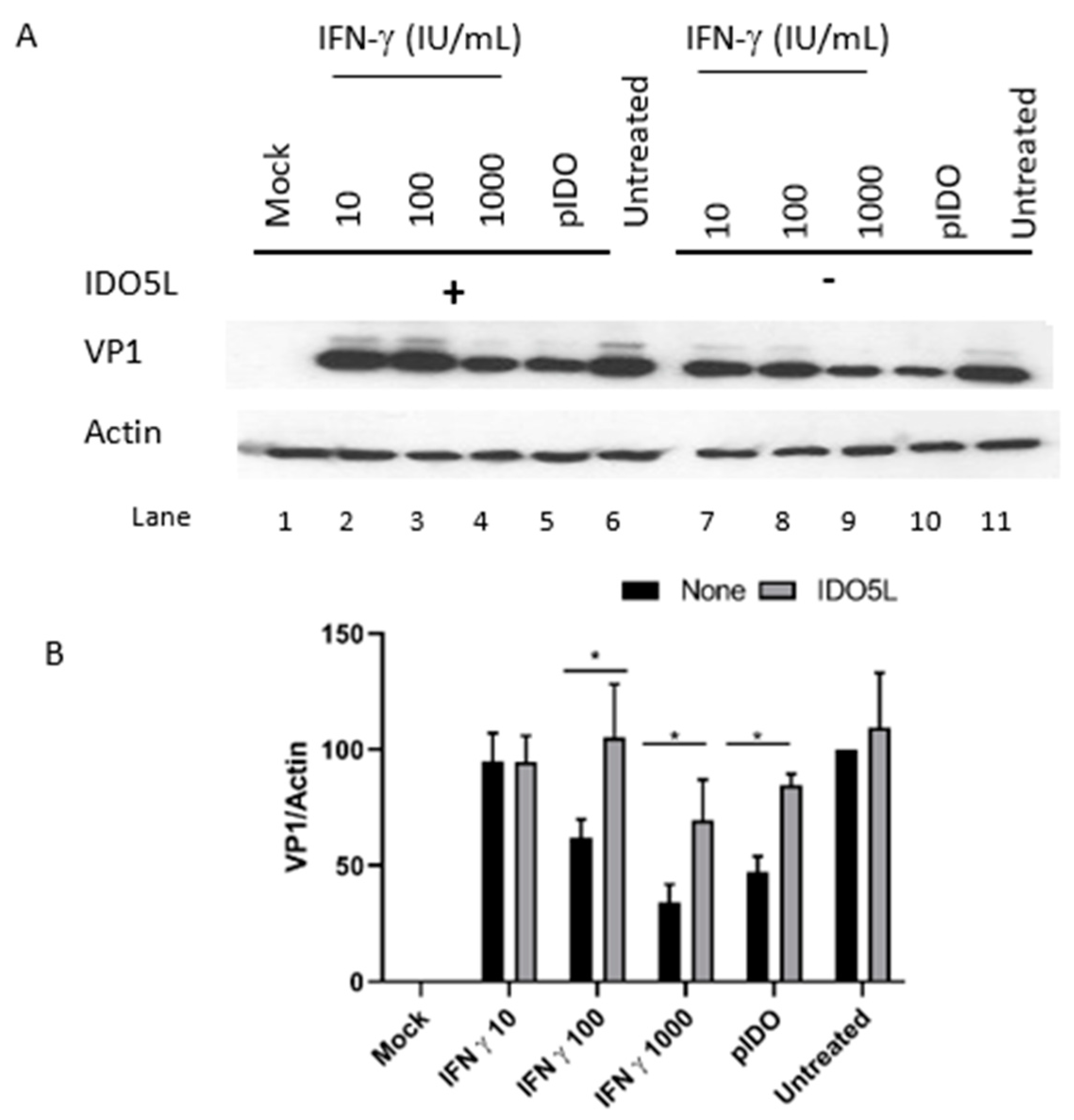
3.5. The Antiviral Effect of IDO (BKPyV Infection of Caki-1 Cells)
3.6. Antiviral Effect of IFN-Gamma and IDO in RPTE/TERT1 Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purighalla, R.; Shapiro, R.; McCauley, J.; Randhawa, P. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am. J. Kidney Dis. 1995, 26, 671–673. [Google Scholar] [CrossRef]
- Binet, I.; Nickeleit, V.; Hirsch, H.H.; Prince, O.; Dalquen, P.; Gudat, F.; Mihatsch, M.J.; Thiel, G. Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation 1999, 67, 918–922. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Randhawa, P.S. AST infectious diseases community of practice BK polyomavirus in solid organ transplantation—Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.S.; Finkelstein, S.; Scantlebury, V.; Shapiro, R.; Vivas, C.; Jordan, M.; Picken, M.M.; Demetris, A.J. Human Polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 1999, 67, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. J. Interferon Res. 1987, 7, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Abend, J.R.; Low, J.A.; Imperiale, M.J. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J. Virol. 2007, 81, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. Ido expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Bodaghi, B.; Goureau, O.; Zipeto, D.; Laurent, L.; Virelizier, J.L.; Michelson, S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J. Immunol. Baltim. Md 1950 1999, 162, 957–964. [Google Scholar]
- Adams, O.; Besken, K.; Oberdörfer, C.; MacKenzie, C.R.; Takikawa, O.; Däubener, W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 2004, 78, 2632–2636. [Google Scholar] [CrossRef]
- Obojes, K.; Andres, O.; Kim, K.S.; Däubener, W.; Schneider-Schaulies, J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J. Virol. 2005, 79, 7768–7776. [Google Scholar] [CrossRef]
- Terajima, M.; Leporati, A.M. Role of indoleamine 2,3-dioxygenase in antiviral activity of interferon-gamma against vaccinia virus. Viral Immunol. 2005, 18, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Dugan, A.S.; Gasparovic, M.L.; Tsomaia, N.; Mierke, D.F.; O’Hara, B.A.; Manley, K.; Atwood, W.J. Identification of amino acid residues in BK virus VP1 that are critical for viability and growth. J. Virol. 2007, 81, 11798–11808. [Google Scholar] [CrossRef]
- Handala, L.; Blanchard, E.; Raynal, P.-I.; Roingeard, P.; Morel, V.; Descamps, V.; Castelain, S.; Francois, C.; Duverlie, G.; Brochot, E.; et al. BK polyomavirus hijacks extracellular vesicles for En Bloc transmission. J. Virol. 2020, 94, e01834-19. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Helle, F.; Louandre, C.; Martin, E.; Brochot, E.; Izquierdo, L.; Fournier, C.; Hoffmann, T.W.; Castelain, S.; Duverlie, G.; et al. The kinase-inhibitor sorafenib inhibits multiple steps of the hepatitis C virus infectious cycle in vitro. Antivir. Res. 2015, 118, 93–102. [Google Scholar] [CrossRef]
- Handala, L.; Fiore, T.; Rouill, Y. QuantIF: An Imagej macro to automatically determine the percentage of infected cells after immunofluorescence. Viruses 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; François, C.; Descamps, V.; Fournier, C.; Wychowski, C.; Dubuisson, J.; Castelain, S.; Duverlie, G. Enhanced anti-HCV activity of interferon alpha 17 subtype. Virol. J. 2009, 6, 70. [Google Scholar] [CrossRef]
- Lepiller, Q.; Soulier, E.; Li, Q.; Lambotin, M.; Barths, J.; Fuchs, D.; Stoll-Keller, F.; Liang, T.J.; Barth, H. Antiviral and immunoregulatory effects of indoleamine-2,3-dioxygenase in hepatitis C virus infection. J. Innate Immun. 2015, 7, 530–544. [Google Scholar] [CrossRef]
- Yeung, A.W.S.; Wu, W.; Freewan, M.; Stocker, R.; King, N.J.C.; Thomas, S.R. Flavivirus infection induces indoleamine 2,3-dioxygenase in human monocyte-derived macrophages via tumor necrosis factor and NF-κB. J. Leukoc. Biol. 2012, 91, 657–666. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, J.; Jiang, D.; Cai, D.; Levy, J.M.; Cuconati, A.; Block, T.M.; Guo, J.-T.; Guo, H. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. J. Virol. 2011, 85, 1048–1057. [Google Scholar] [CrossRef]
- Adams, O.; Besken, K.; Oberdörfer, C.; MacKenzie, C.R.; Rüssing, D.; Däubener, W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004, 6, 806–812. [Google Scholar] [CrossRef]
- Interferon Gamma Prevents Infectious Entry of Human Papillomavirus 16 via an L2-Dependent Mechanism. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5411602/ (accessed on 12 May 2020).
- Assetta, B.; De Cecco, M.; O’Hara, B.; Atwood, W.J. JC polyomavirus infection of primary human renal epithelial cells is controlled by a type I IFN-induced response. mBio 2016, 7, e00903-16. [Google Scholar] [CrossRef] [PubMed]
- Jaitin, D.A.; Roisman, L.C.; Jaks, E.; Gavutis, M.; Piehler, J. Inquiring into the differential action of interferons (IFNs): An IFN-α2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-β. Mol. Cell. Biol. 2006, 26, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, T.; Müller, K.; Stein, M.; Diezemann, C.; Sefrin, A.; Babel, N.; Reinke, P. BK virus-specific immunity kinetics: A predictor of recovery from polyomavirus BK-associated nephropathy. Am. J. Transplant. 2011, 11, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, T.; Stein, M.; Babel, N.; Reinke, P. The loss of BKV-specific immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am. J. Transplant. 2015, 15, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Na, D.H.; Chang, J.-Y.; Park, K.H.; Min, J.W.; Ko, E.J.; Lee, H.; Yang, C.W.; Chung, B.H.; Oh, E.-J. Usefulness of BK virus-specific interferon-γ enzyme-linked immunospot assay for predicting the outcome of BK virus infection in kidney transplant recipients. Korean J. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.T.; Cao, J.S.; Zhao, J.; Yu, Y.; Qi, F.; Dai, X.C. IDO expressing dendritic cells suppress allograft rejection of small bowel transplantation in mice by expansion of Foxp3+ regulatory T cells. Transpl. Immunol. 2015, 33, 69–77. [Google Scholar] [CrossRef]
- Benavente, F.M.; Soto, J.A.; Pizarro-Ortega, M.S.; Bohmwald, K.; González, P.A.; Bueno, S.M.; Kalergis, A.M. Contribution of IDO to human respiratory syncytial virus infection. J. Leukoc. Biol. 2019, 106, 933–942. [Google Scholar] [CrossRef]
- Divanovic, S.; Sawtell, N.M.; Trompette, A.; Warning, J.I.; Dias, A.; Cooper, A.M.; Yap, G.S.; Arditi, M.; Shimada, K.; Duhadaway, J.B.; et al. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J. Infect. Dis. 2012, 205, 152–161. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, T.; Martin, E.; Descamps, V.; Brochot, E.; Morel, V.; Handala, L.; Dakroub, F.; Castelain, S.; Duverlie, G.; Helle, F.; et al. Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells. Viruses 2020, 12, 865. https://doi.org/10.3390/v12080865
Fiore T, Martin E, Descamps V, Brochot E, Morel V, Handala L, Dakroub F, Castelain S, Duverlie G, Helle F, et al. Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells. Viruses. 2020; 12(8):865. https://doi.org/10.3390/v12080865
Chicago/Turabian StyleFiore, Tony, Elodie Martin, Véronique Descamps, Etienne Brochot, Virginie Morel, Lynda Handala, Fatima Dakroub, Sandrine Castelain, Gilles Duverlie, François Helle, and et al. 2020. "Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells" Viruses 12, no. 8: 865. https://doi.org/10.3390/v12080865
APA StyleFiore, T., Martin, E., Descamps, V., Brochot, E., Morel, V., Handala, L., Dakroub, F., Castelain, S., Duverlie, G., Helle, F., & François, C. (2020). Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells. Viruses, 12(8), 865. https://doi.org/10.3390/v12080865





