Investigation of Novel RNA Elements in the 3′UTR of Tobacco Necrosis Virus-D
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Construction
2.2. Preparation of Viral RNAs
2.3. RNA Secondary Structure Prediction
2.4. In Vitro Translation Assay
2.5. Protoplast Infections
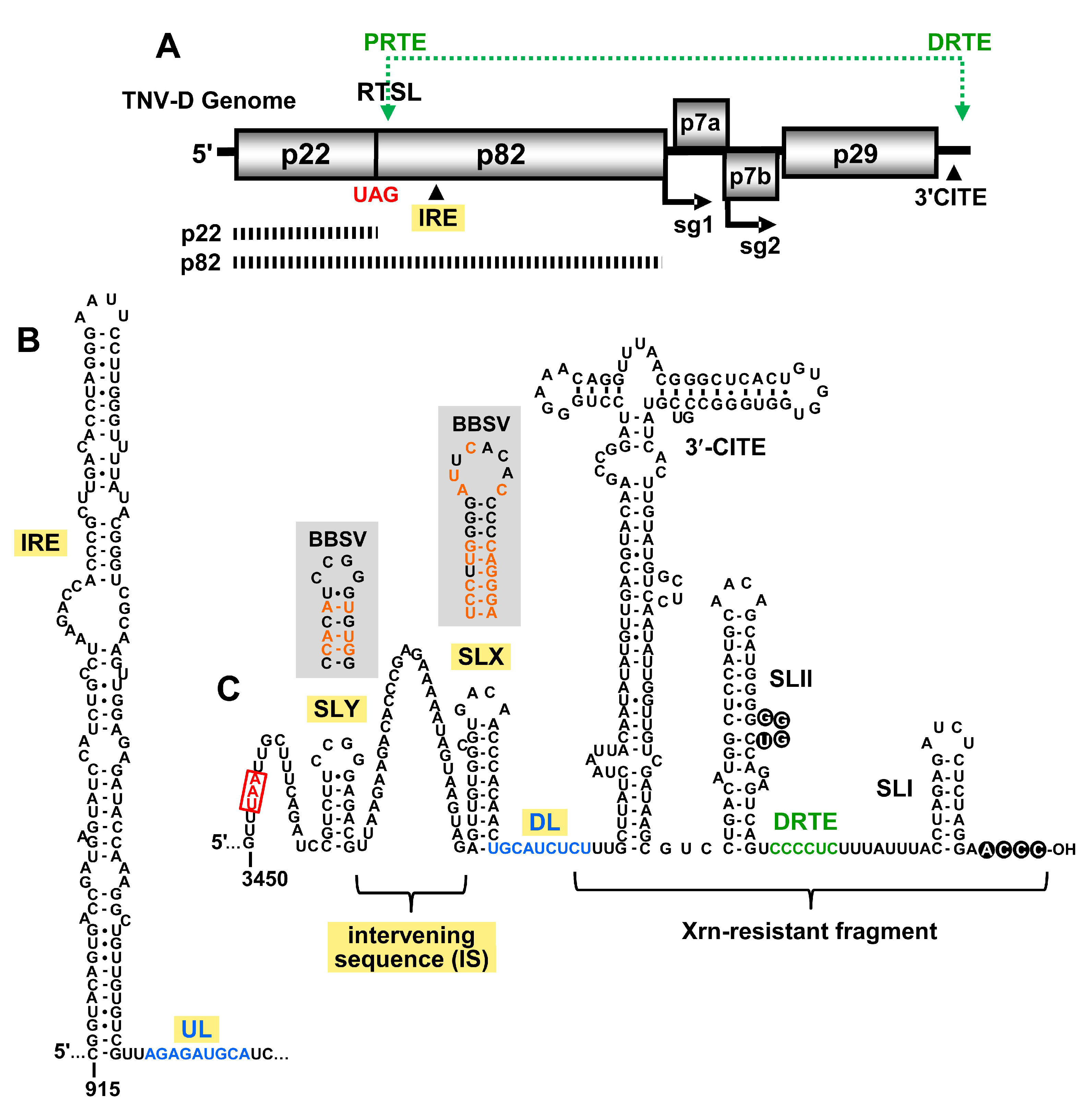
3. Results
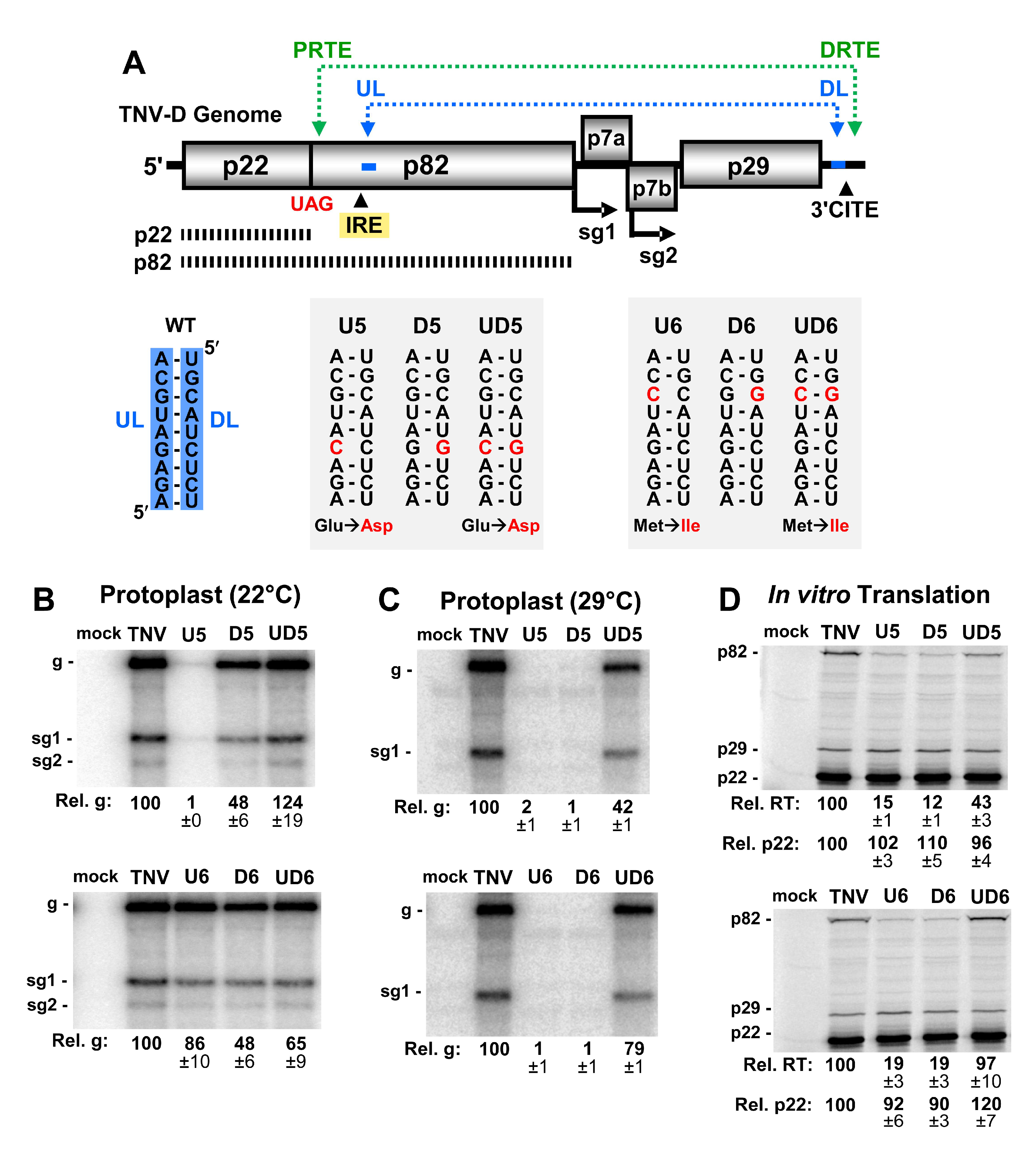
3.1. A Long-Range RNA–RNA Interaction Promotes Genome Accumulation and Translational Readthrough
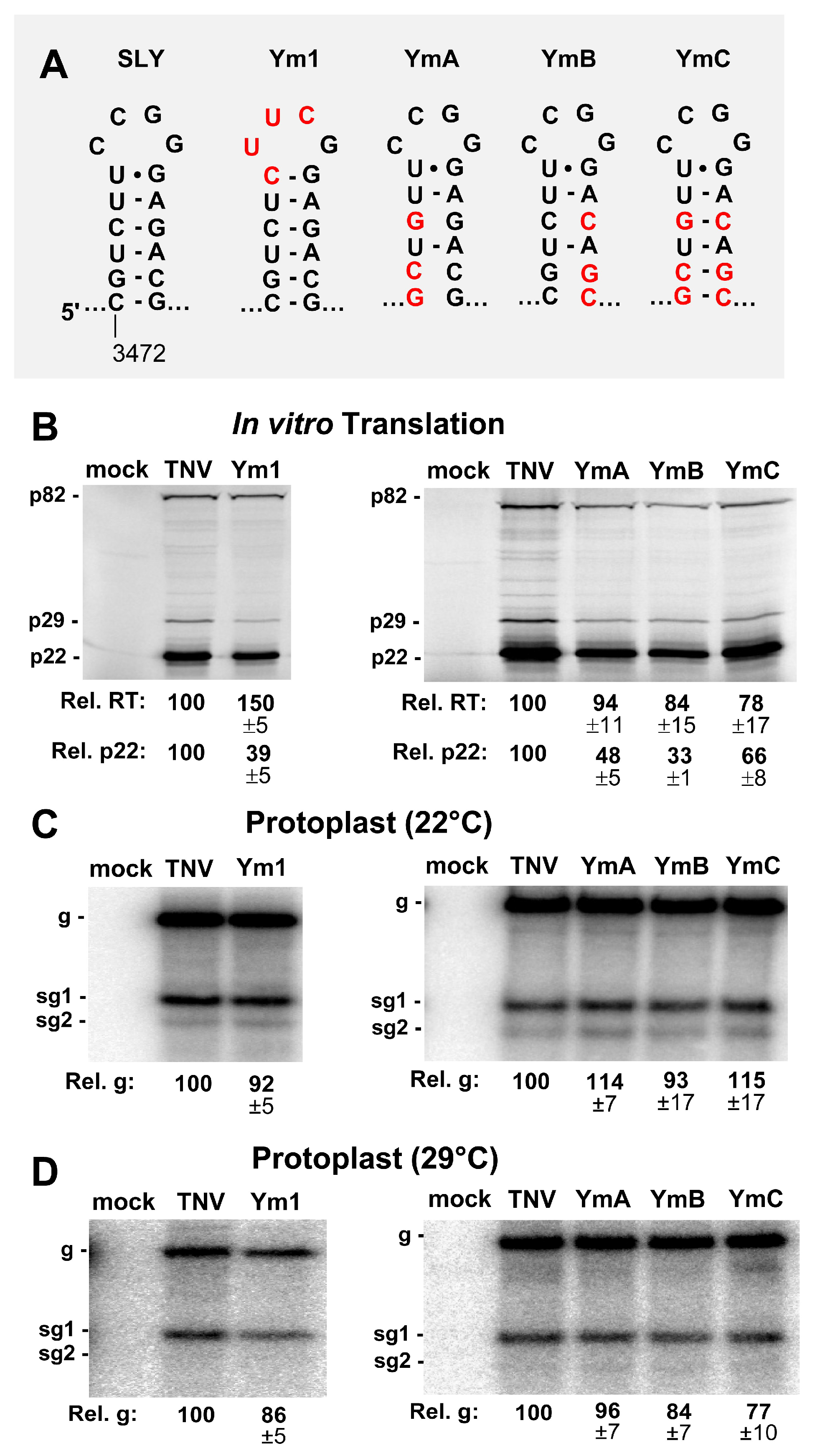
3.2. SLY Affects Translation In Vitro but not Viral RNA Accumulation in Protoplasts
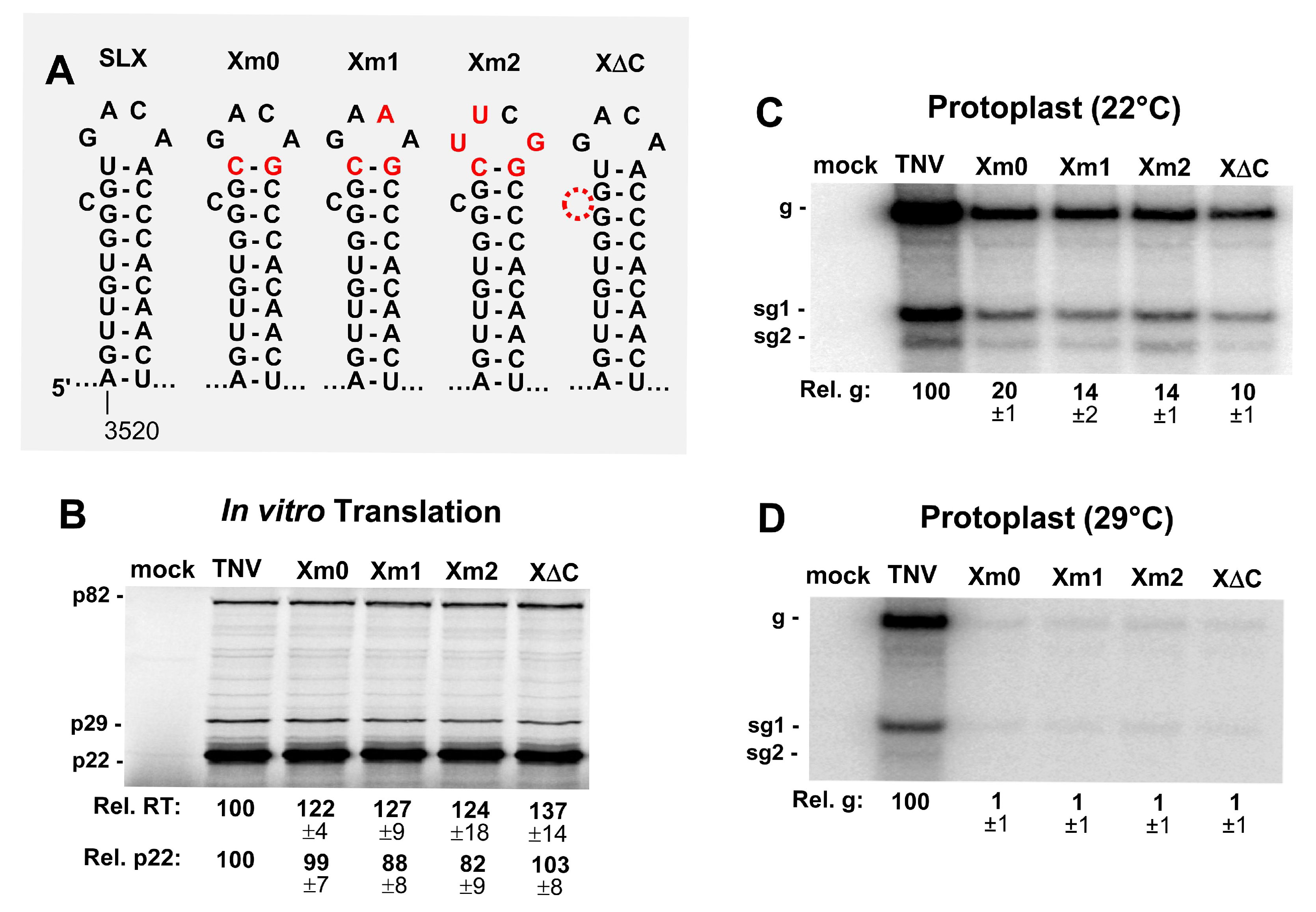
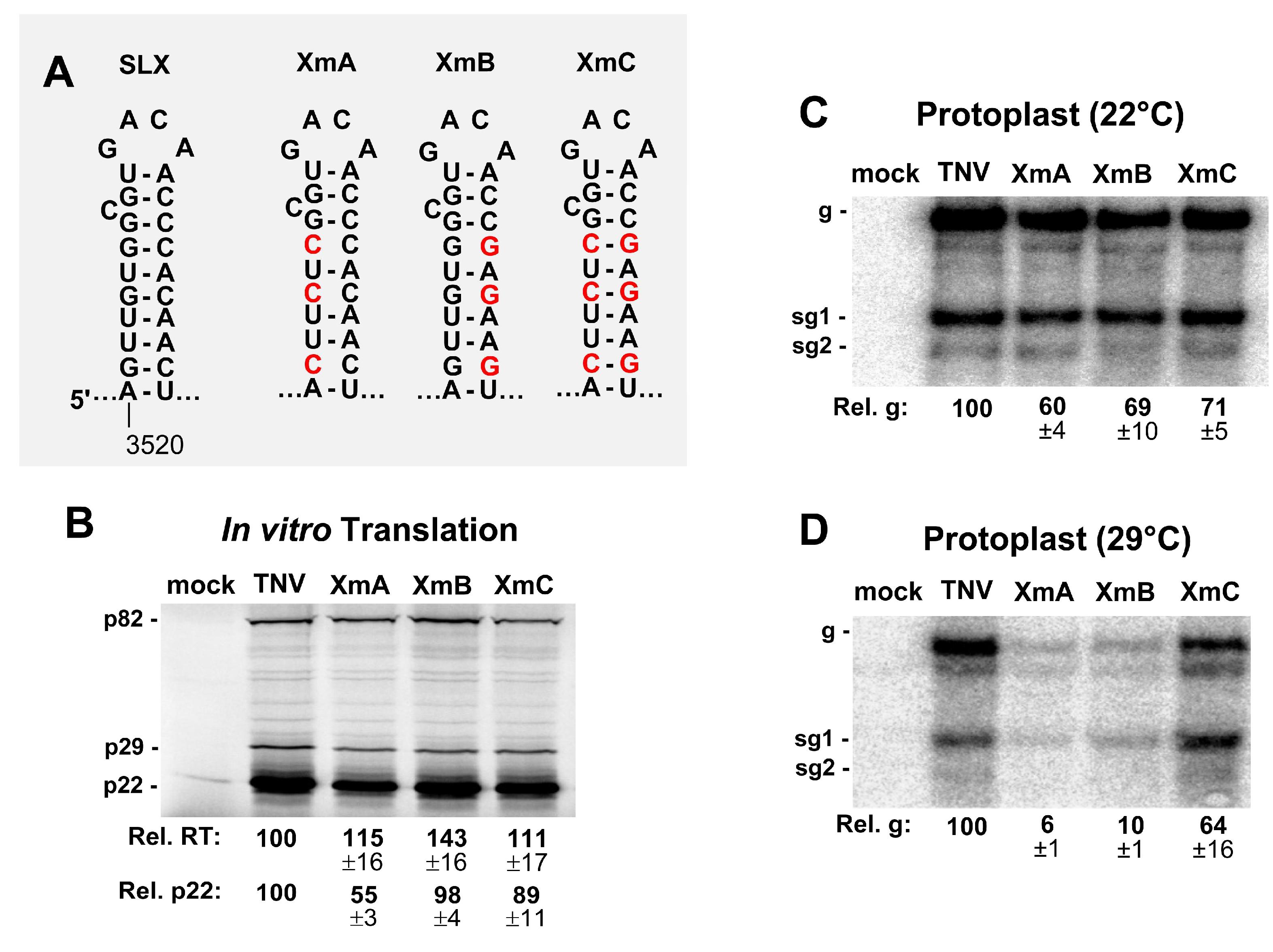
3.3. SLX Is Important for Viral RNA Accumulation in Protoplast Infections
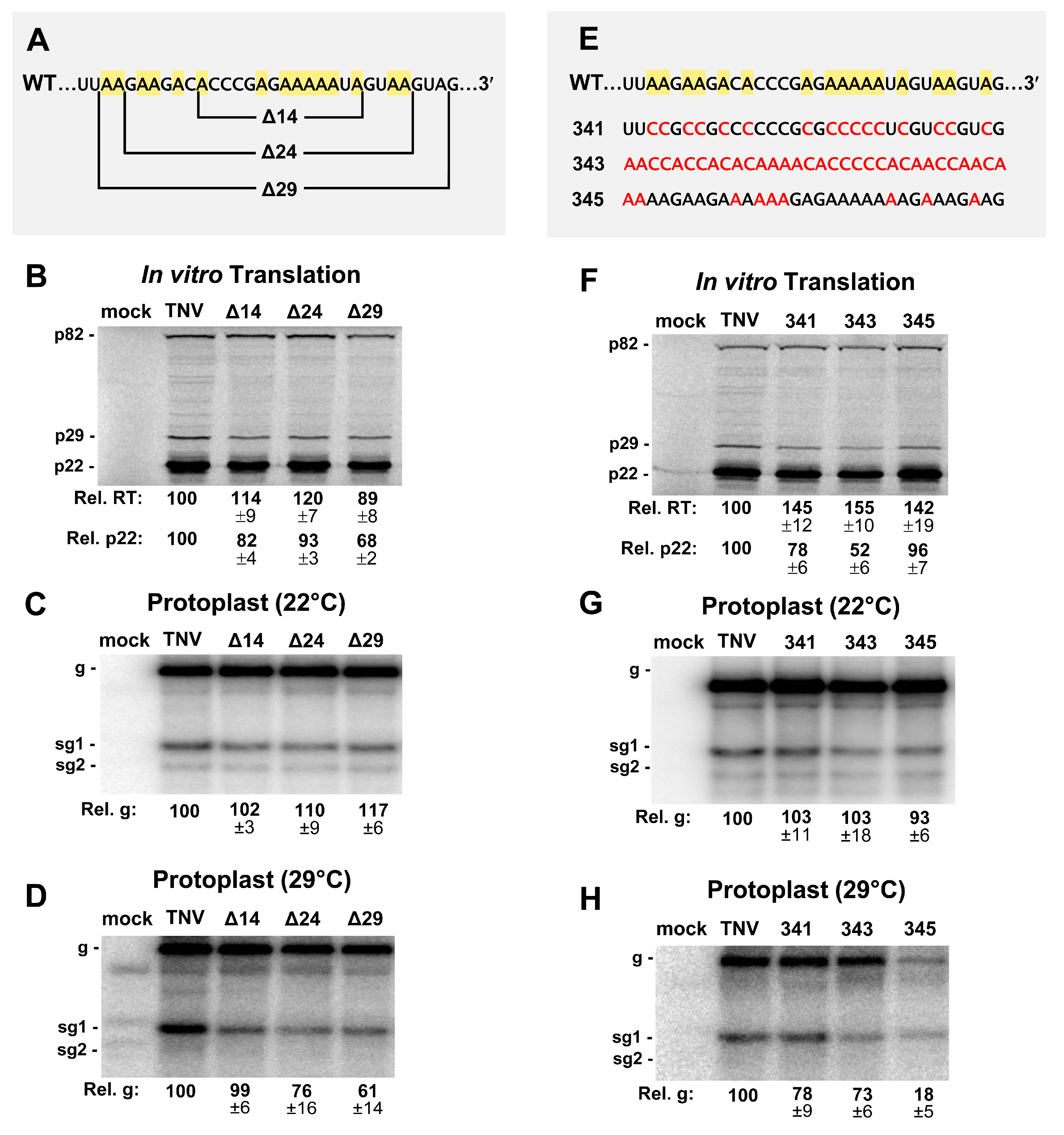
3.4. The Intervening Sequence (IS) between SLX and SLY Is not Vital for Viral Translation or Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newburn, L.R.; Nicholson, B.L.; Yosefi, M.; Cimino, P.A.; White, K.A. Translational readthrough in tobacco necrosis virus-D. Virology 2014, 450, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Newburn, L.R.; White, K.A. Atypical RNA elements modulate translational readthrough in tobacco necrosis virus D. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Chkuaseli, T.; Newburn, L.R.; Bakhshinyan, D.; White, K.A. Protein expression strategies in Tobacco necrosis virus-D. Virology 2015, 486, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.L.; Lee, P.K.; White, K.A. Internal RNA replication elements are prevalent in Tombusviridae. Front. Microbiol. 2012, 3, 279. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.J.; Treder, K.; Peterson, M.S.; Miller, W.A. Cation-dependent folding of 3’ cap-independent translation elements facilitates interaction of a 17-nucleotide conserved sequence with eIF4G. Nucleic Acids Res. 2013, 41, 3398–3413. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Miller, W.A. The 3’ untranslated region of tobacco necrosis virus RNA contains a barley yellow dwarf virus-like cap-independent translation element. J. Virol. 2004, 78, 4655–4664. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Miller, W.A. Structures required for poly(A) tail-independent translation overlap with, but are distinct from, cap-independent translation and RNA replication signals at the 3’ end of Tobacco necrosis virus RNA. Virology 2007, 358, 448–458. [Google Scholar] [CrossRef]
- Newburn, L.R.; White, K.A. Cis-acting RNA elements in positive-strand RNA plant virus genomes. Virology 2015, 479, 434–443. [Google Scholar] [CrossRef]
- Sit, T.L.; Lommel, S.A. Tombusviridae. In Encyclopedia of Life Sciences; John Wiley & Sons: Chichester, UK, 2010; pp. 1–9. [Google Scholar]
- Coutts, R.H.A.; Rigden, J.E.; Slabas, A.R.; Lomonossoff, G.P.; Wise, P.J. The complete nucleotide sequence of tobacco necrosis virus strain D. J. Gen. Virol. 1991, 72, 1521–1529. [Google Scholar] [CrossRef]
- Molnár, A.; Havelda, Z.; Dalmay, T.; Szutorisz, H.; Burgyán, J. Complete nucleotide sequence of tobacco necrosis virus strain DH and genes required for RNA replication and virus movement. J. Gen. Virol. 1997, 78, 1235–1239. [Google Scholar] [CrossRef]
- Fang, L.; Coutts, R.H. Investigations on the tobacco necrosis virus D p60 replicase protein. PLoS ONE 2013, 8, e80912. [Google Scholar] [CrossRef]
- Offei, S.K.; Coutts, R.H.A. Location of the 5′ termini of tobacco necrosis virus strain D subgenomic mRNAs. J. Phytopathol. 1996, 144, 13–17. [Google Scholar] [CrossRef]
- Gunawardene, C.D.; Newburn, L.R.; White, K.A. A 212-nt long RNA structure in the tobacco necrosis virus-D RNA genome is resistant to Xrn degradation. Nucleic Acids Res. 2019, 47, 9329–9342. [Google Scholar] [CrossRef]
- Newburn, L.R.; White, K.A. A trans-activator-like structure in RCNMV RNA1 evokes the origin of the trans-activator in RNA. PLoS Pathog. 2020, 16, e1008271. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H.; Sabina, J.; Zuker, M.; Turner, D.H. Expanded sequence dependence of thermodynamic parameters provides robust prediction of RNA secondary structure. J. Mol. Biol. 1999, 288, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Morris, T.J. Nonhomologous RNA recombination in tombusviruses: Generation and evolution of defective interfering RNAs by stepwise deletions. J. Virol. 1994, 68, 14–24. [Google Scholar] [CrossRef]
- Monkewich, S.; Lin, H.X.; Fabian, M.R.; Xu, W.; Na, H.; Debashish, R.; Chernysheva, O.A.; Nagy, P.D.; White, K.A. The p92 polymerase coding region contains an internal RNA element required at an early step in tombusvirus genome replication. J. Virol. 2005, 79, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Pogany, J.; White, K.A.; Nagy, P.D. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA Is essential for replication. J. Virol. 2005, 79, 4859–4869. [Google Scholar] [CrossRef]
- Pathak, K.B.; Pogany, J.; Xu, K.; White, K.A.; Nagy, P.D. Defining the roles of cis-acting RNA elements in tombusvirus replicase assembly in vitro. J. Virol. 2012, 86, 156–171. [Google Scholar] [CrossRef]
- Wu, B.; Pogany, J.; Na, H.; Nicholson, B.L.; Nagy, P.D.; White, K.A. A discontinuous RNA platform mediates RNA virus replication: Building an integrated model for RNA-based regulation of viral processes. PLoS Pathog. 2009, 5, e1000323. [Google Scholar] [CrossRef] [PubMed]
- Cimino, P.A.; Nicholson, B.L.; Wu, B.; Xu, W.; White, K.A. Multifaceted regulation of translational readthrough by RNA replication elements in a tombusvirus. PLoS Pathog. 2011, 7, e1002423. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, C.D.; Jaluba, K.; White, K.A. Conserved motifs in a tombusvirus polymerase modulate genome replication, subgenomic transcription, and amplification of defective interfering RNAs. J. Virol. 2015, 89, 3236–3246. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Tajima, Y.; Taniguchi, T.; Kaido, M.; Mise, K.; Tomari, Y.; Taniguchi, H.; Okuno, T. Poly(A)-binding protein facilitates translation of an uncapped/nonpolyadenylated viral RNA by binding to the 3’ untranslated region. J. Virol. 2012, 86, 7836–7849. [Google Scholar] [CrossRef]
- Chkuaseli, T.; White, K.A. Intragenomic long-distance RNA-RNA interactions in plus-strand RNA plant viruses. Front. Microbiol. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.K.; White, K.A. Construction and characterization of an aureusvirus defective RNA. Virology 2014, 452, 67–74. [Google Scholar] [CrossRef][Green Version]
- Rubino, L.; Russo, M.; Martelli, G.P. Sequence analysis of pothos latent virus genomic RNA. J. Gen. Virol. 1995, 76, 2835–2839. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newburn, L.R.; Wu, B.; White, K.A. Investigation of Novel RNA Elements in the 3′UTR of Tobacco Necrosis Virus-D. Viruses 2020, 12, 856. https://doi.org/10.3390/v12080856
Newburn LR, Wu B, White KA. Investigation of Novel RNA Elements in the 3′UTR of Tobacco Necrosis Virus-D. Viruses. 2020; 12(8):856. https://doi.org/10.3390/v12080856
Chicago/Turabian StyleNewburn, Laura R., Baodong Wu, and K. Andrew White. 2020. "Investigation of Novel RNA Elements in the 3′UTR of Tobacco Necrosis Virus-D" Viruses 12, no. 8: 856. https://doi.org/10.3390/v12080856
APA StyleNewburn, L. R., Wu, B., & White, K. A. (2020). Investigation of Novel RNA Elements in the 3′UTR of Tobacco Necrosis Virus-D. Viruses, 12(8), 856. https://doi.org/10.3390/v12080856




