Crocetin Improves Dengue Virus-Induced Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. DENV Infection and Crocetin Treatment in Mice
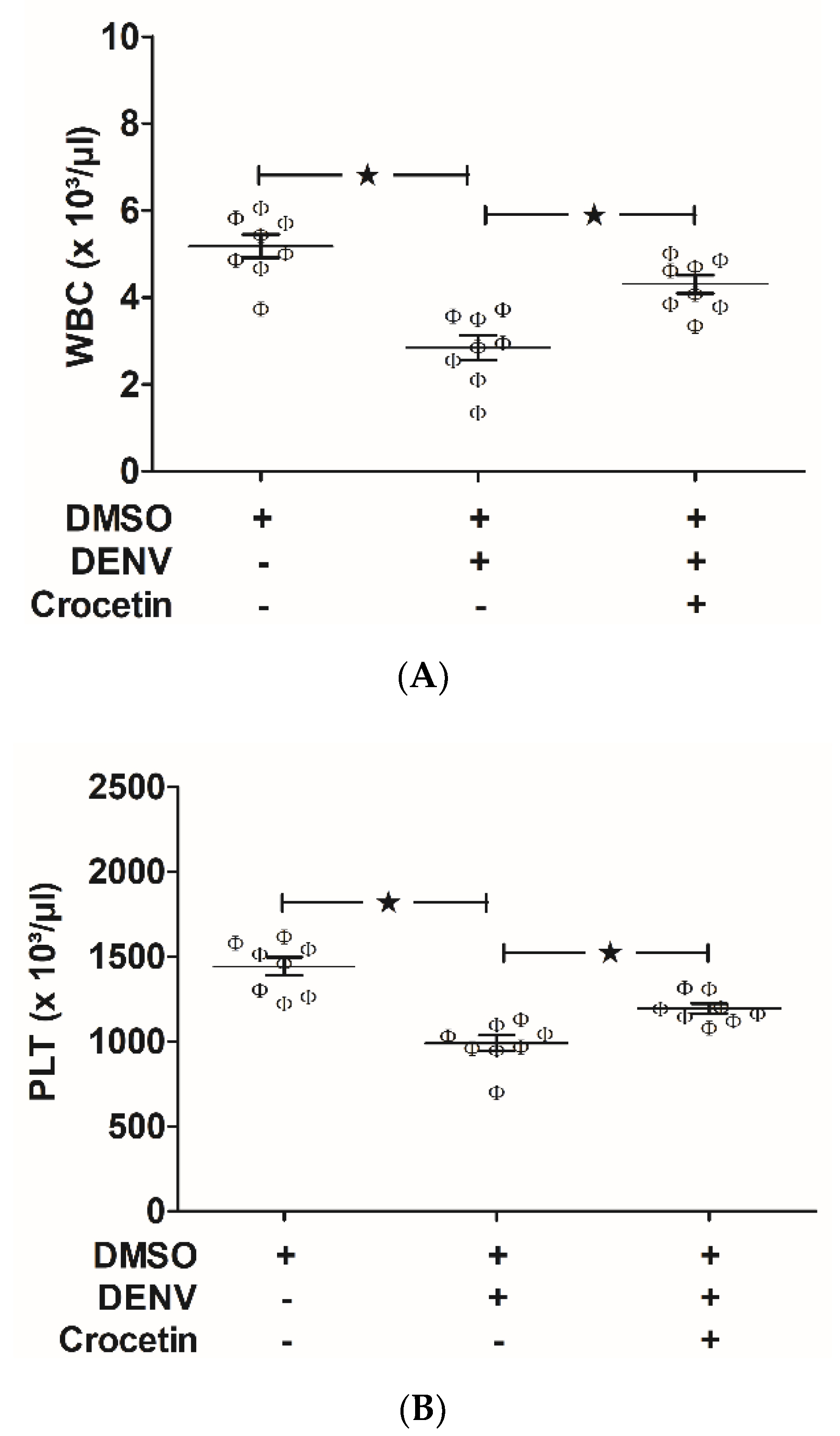
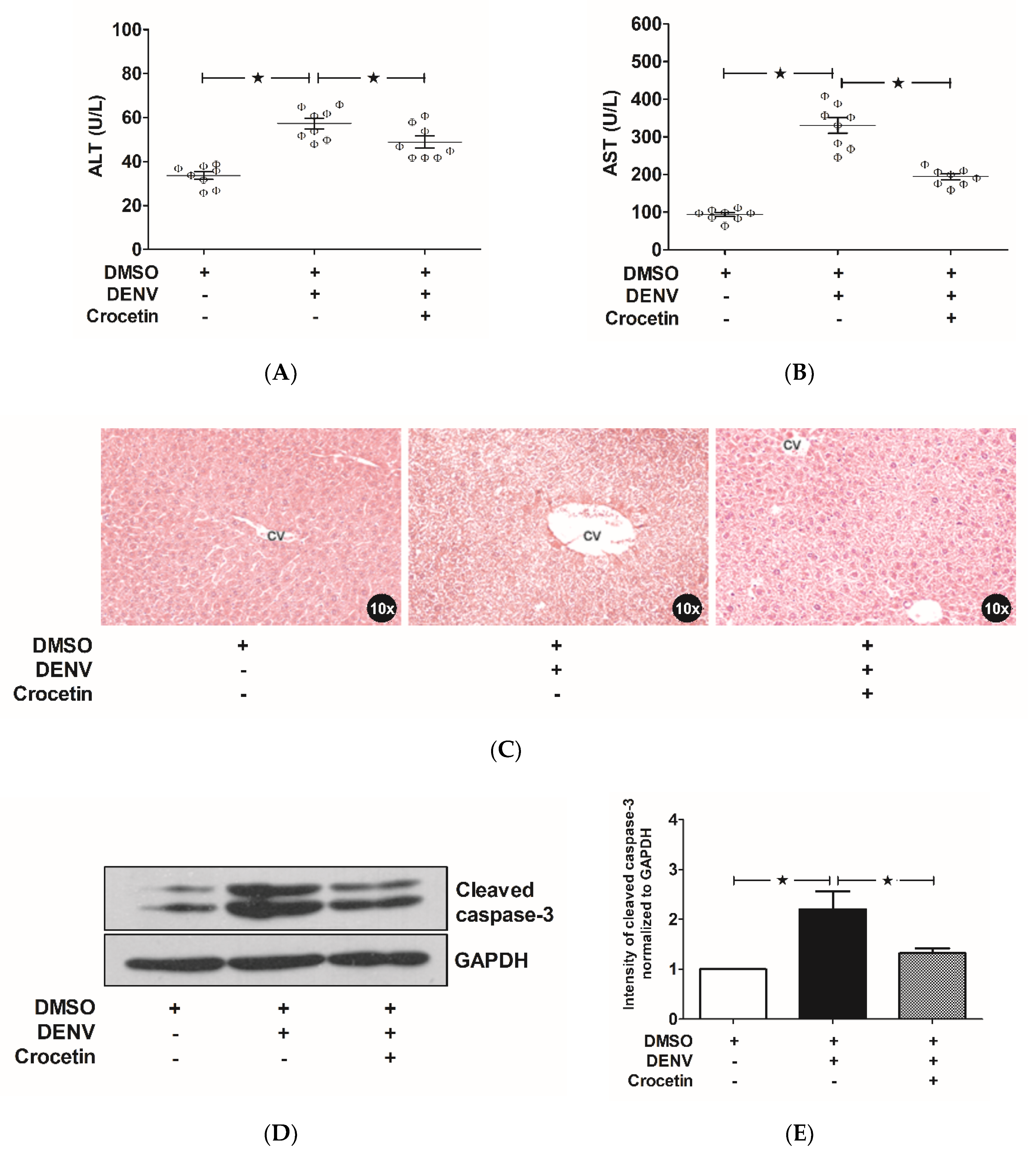
2.2. Hematology and Liver Transaminases
2.3. Histopathology Analysis
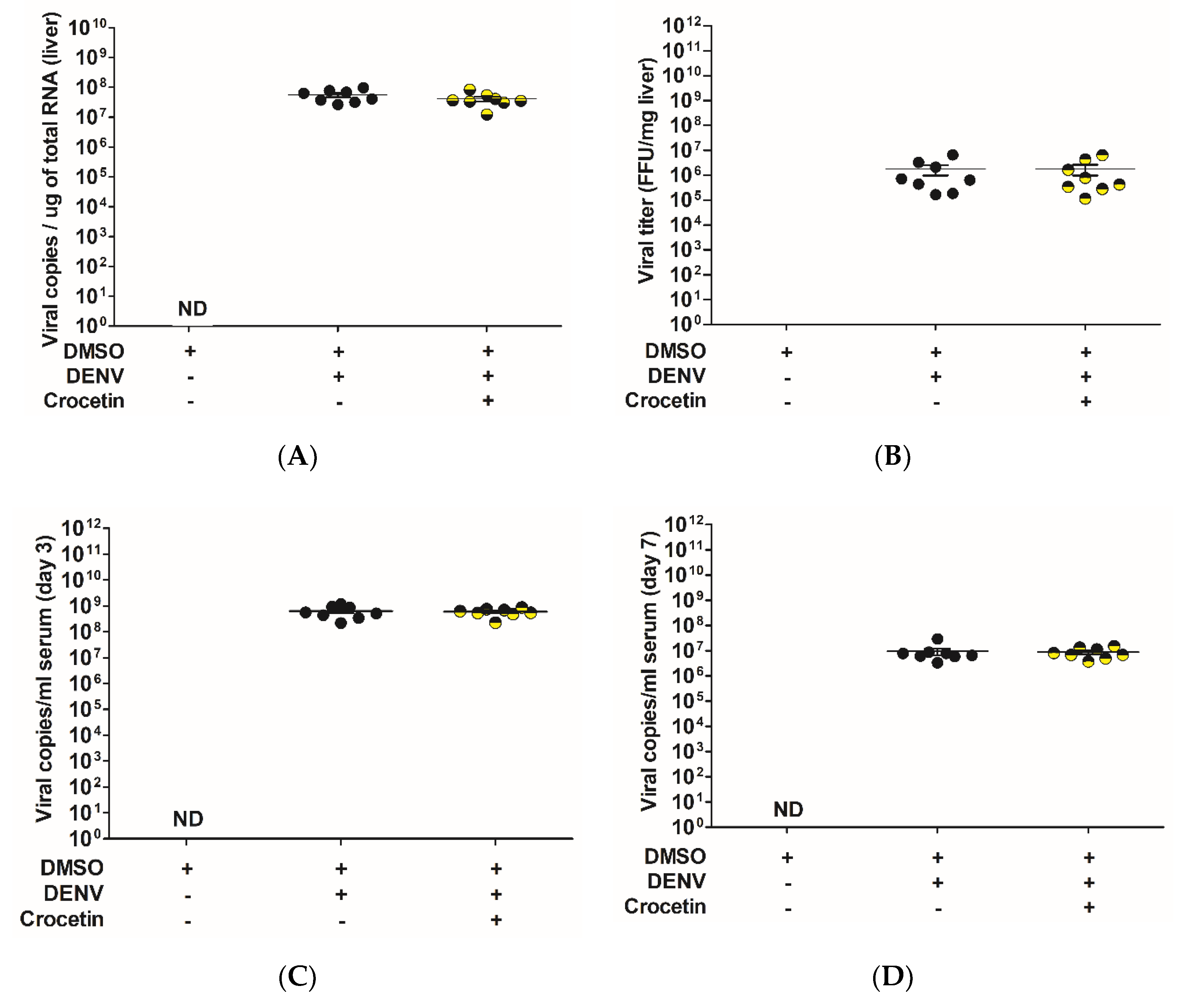
2.4. DENV-NS1 Viral RNA Quantification from the Liver
2.5. Focus-Forming Unit (FFU) Assay in Liver Homogenates
2.6. Superoxide Dismutase and Catalase Activity
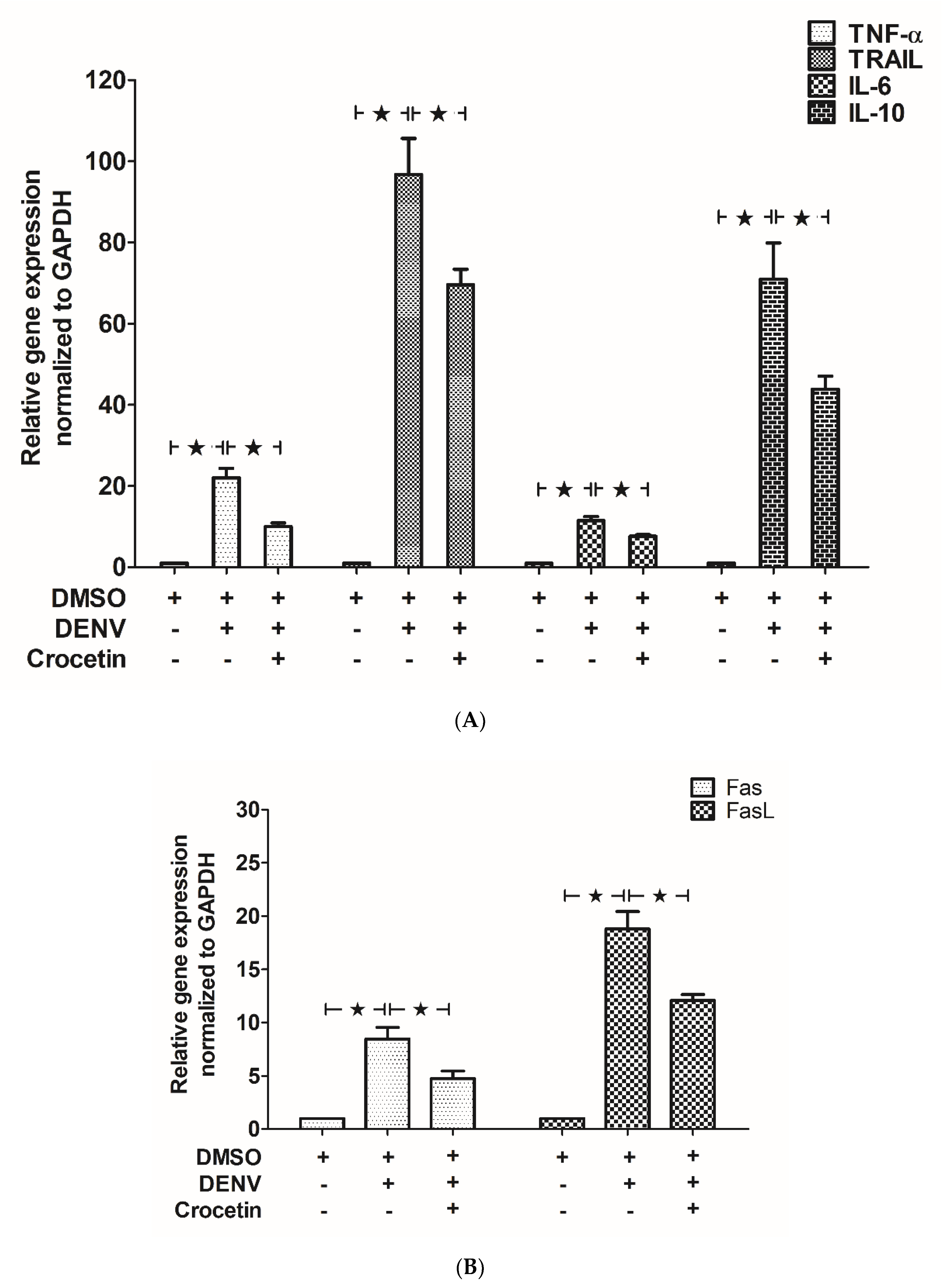
2.7. Gene Expression Profiler (RT-PCR Array)
2.8. RT-PCR Analysis
2.9. Cytosolic and Nuclear Fractionation of Proteins from Liver Tissues
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Crocetin Did not Reduce Dengue Virus Production in the Liver of DENV-Infected Mice
3.2. Crocetin Improved DENV-Associated Clinical Manifestations in Mice
3.3. Crocetin Improved Liver Injury in DENV-Infected Mice
3.4. Crocetin Reduced Apoptosis in the Livers of DENV-Infected Mice
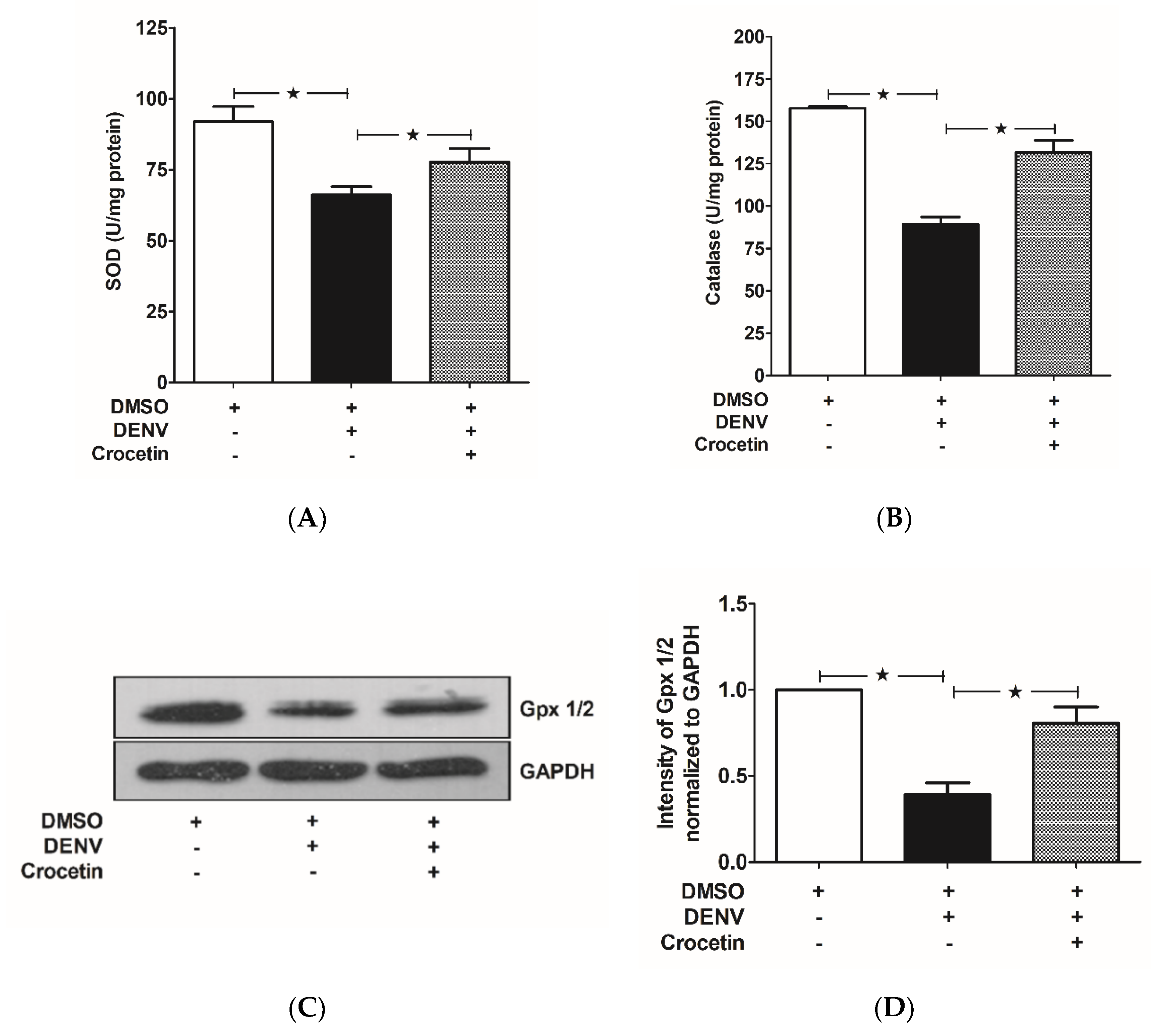
3.5. Crocetin Balanced Antioxidant Enzymes in the Livers of DENV-Infected Mice
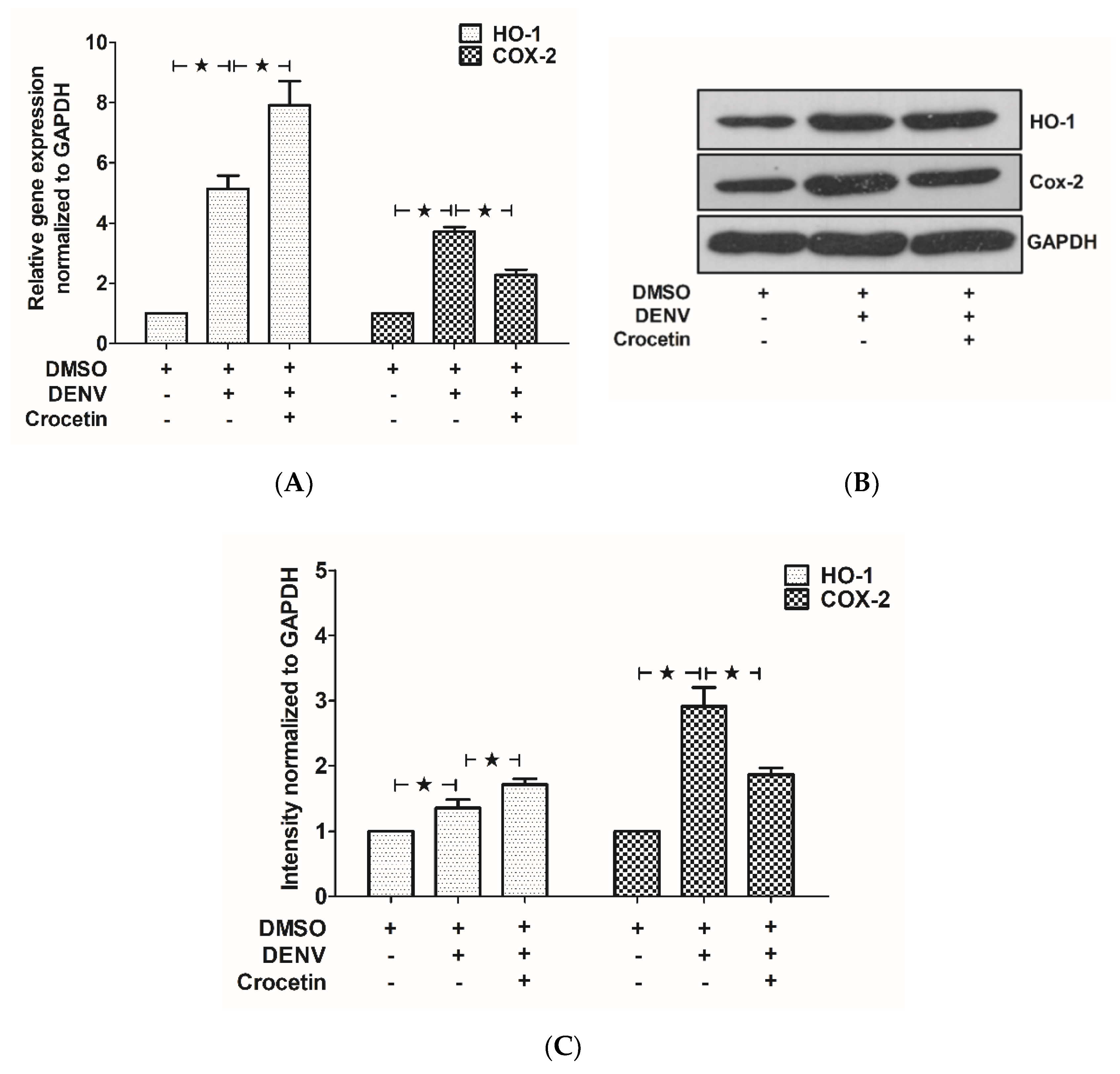
3.6. Crocetin Modulated HO-1 and COX-2 Expression in the Livers of DENV-Infected Mice
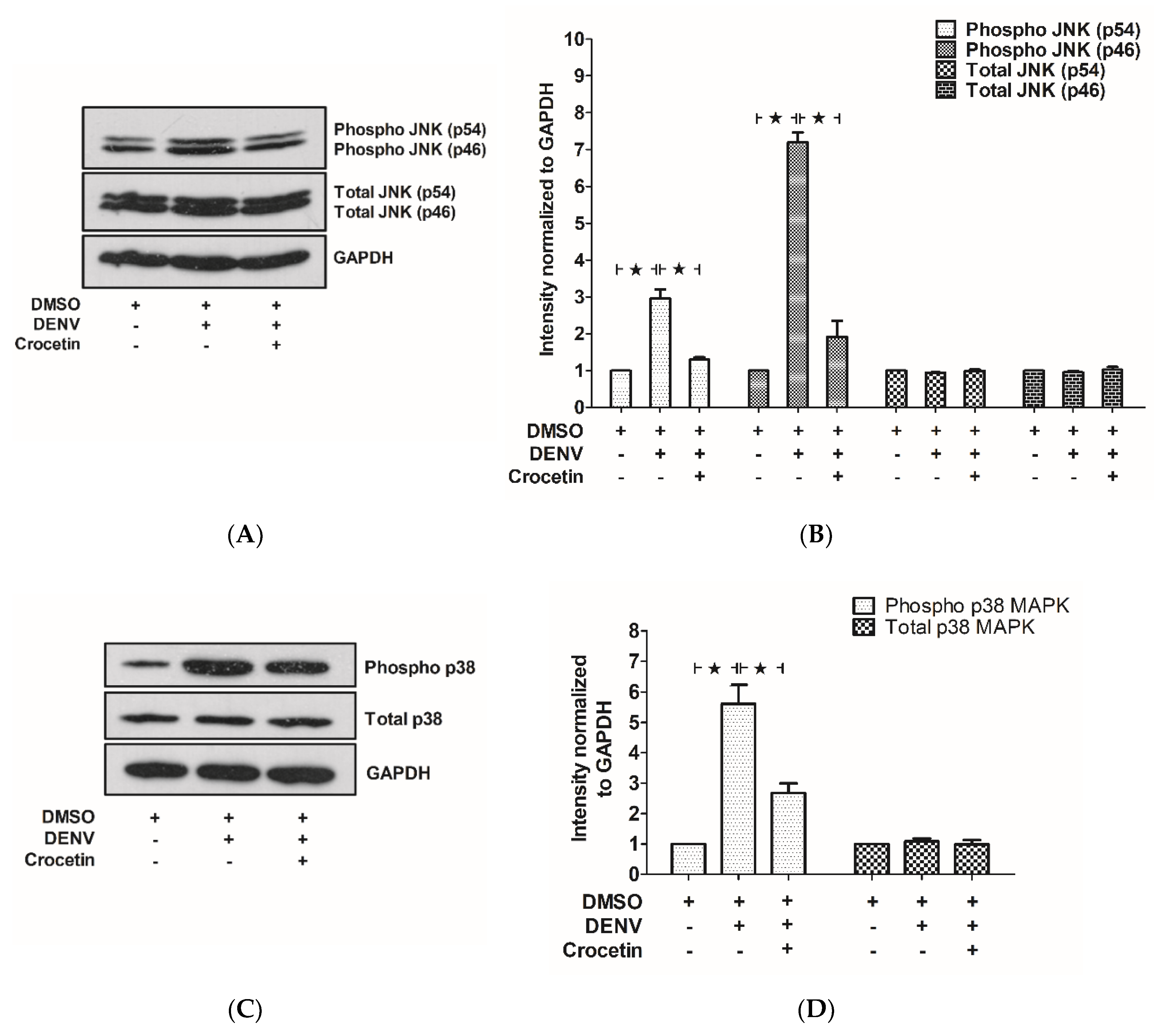
3.7. Crocetin Reduced Phosphorylated JNK and p38 in the Livers of DENV-Infected Mice
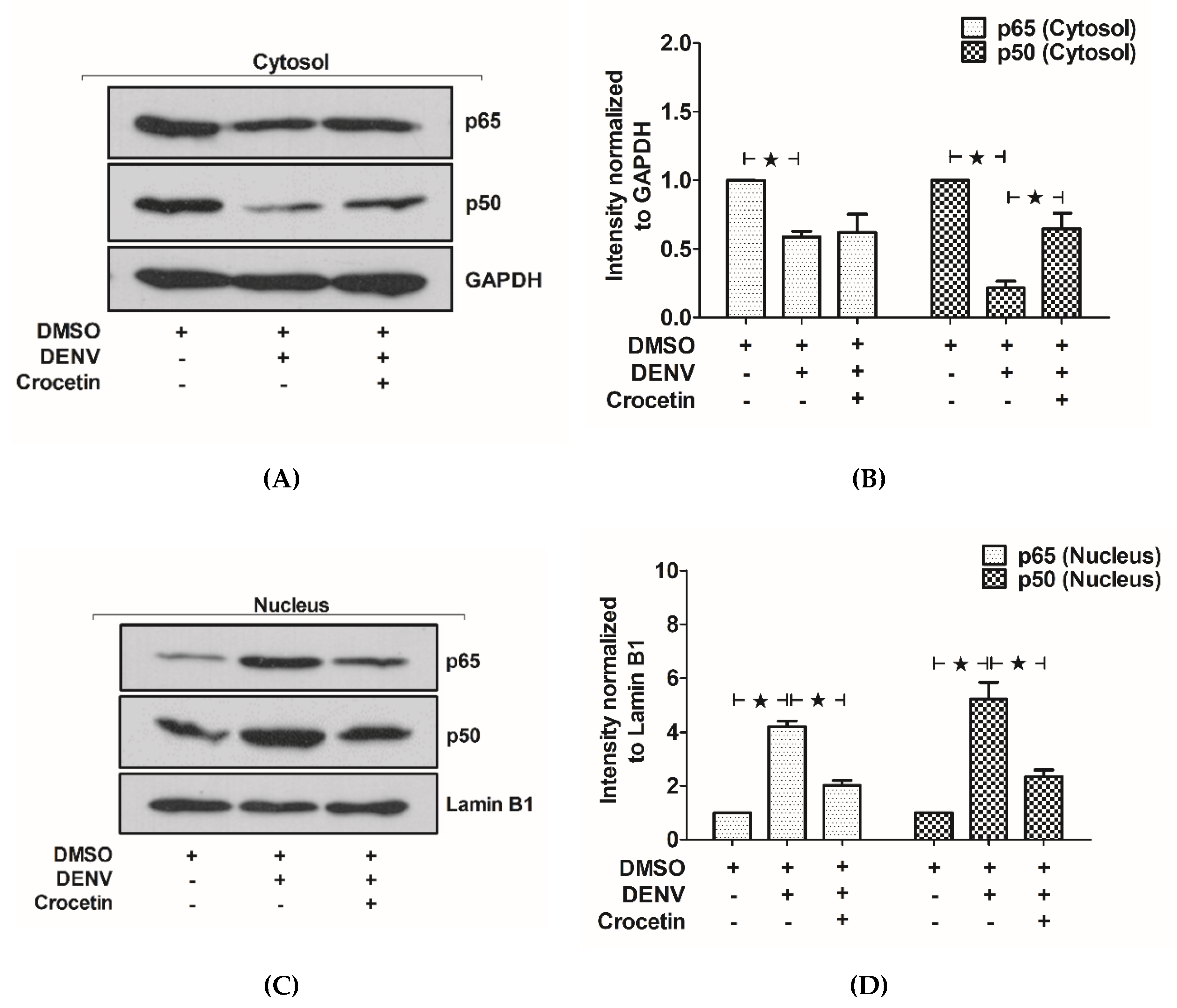
3.8. Crocetin Reduced Nuclear Localization of NF-kB in the Livers of DENV-Infected Mice
4. Discussion
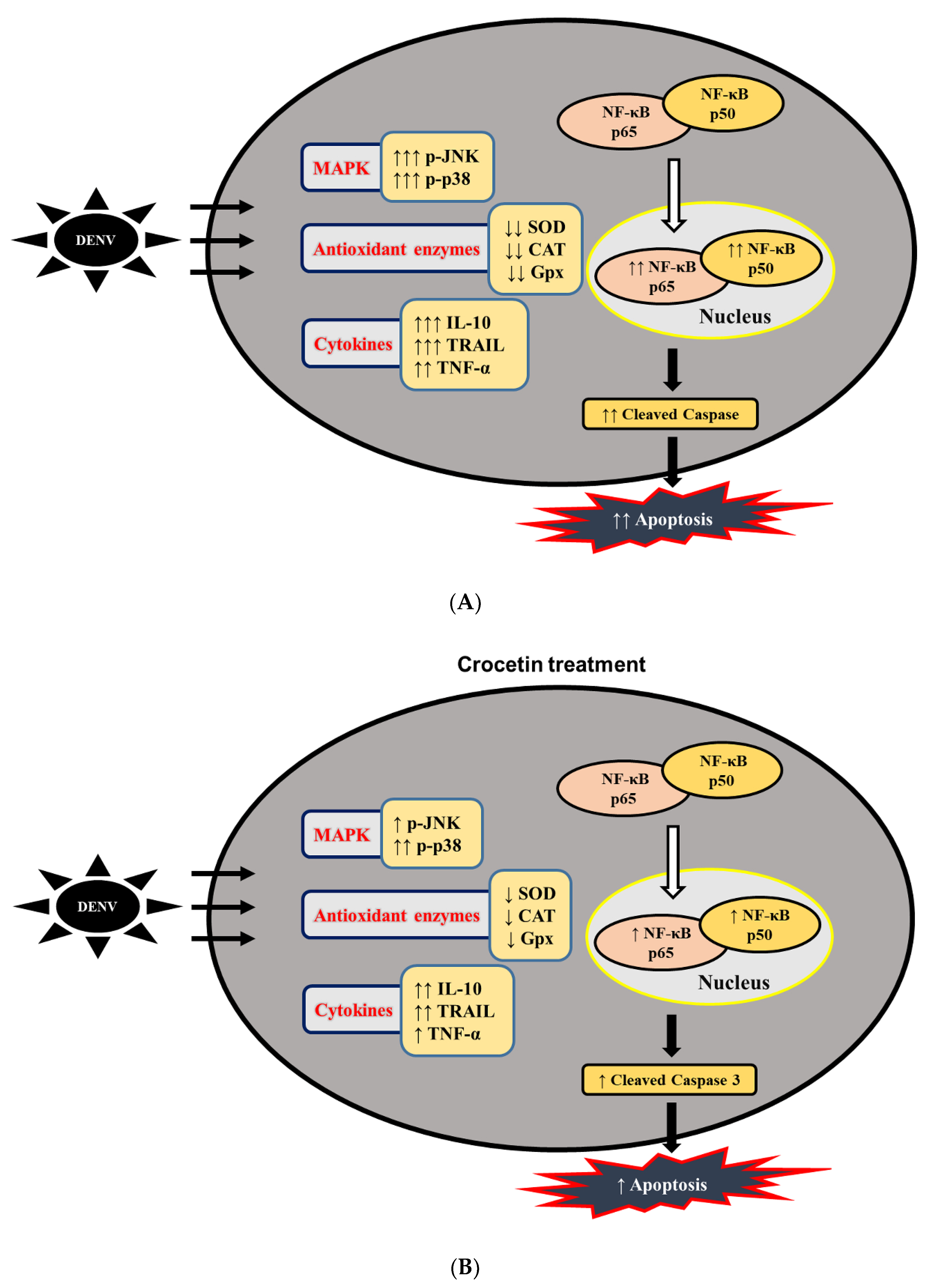
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. Emerging vector-borne flavivirus diseases: Are vaccines the solution? Expert Rev. Vaccines 2011, 10, 563–565. [Google Scholar] [CrossRef]
- Kalayanarooj, S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop. Med. Health 2011, 39 (Suppl. 4), 83–87. [Google Scholar] [CrossRef]
- Samanta, J.; Sharma, V. Dengue and its effects on liver. World J. Clin. Cases 2015, 3, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Franca, R.F.; Zucoloto, S.; Da Fonseca, B.A. A BALB/c mouse model shows that liver involvement in dengue disease is immune-mediated. Exp. Mol. Pathol. 2010, 89, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Win, M.M.; Charngkaew, K.; Punyadee, N.; Aye, K.S.; Win, N.; Chaisri, U.; Chomanee, N.; Avirutnan, P.; Yoksan, S.; Malasit, P. Ultrastructural Features of Human Liver Specimens from Patients Who Died of Dengue Hemorrhagic Fever. Trop. Med. Infect. Dis. 2019, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Khongphatthanayothin, A.; Lertsapcharoen, P.; Supachokchaiwattana, P.; Satupan, P.; Thongchaiprasit, K.; Poovorawan, Y.; Thisyakorn, C. Hepatosplanchnic circulatory dysfunction in acute hepatic infection: The case of dengue hemorrhagic fever. Shock 2005, 24, 407–411. [Google Scholar] [CrossRef]
- Jayaratne, S.D.; Atukorale, V.; Gomes, L.; Chang, T.; Wijesinghe, T.; Fernando, S.; Ogg, G.S.; Malavige, G.N. Evaluation of the WHO revised criteria for classification of clinical disease severity in acute adult dengue infection. BMC Res. Notes 2012, 5, 645. [Google Scholar] [CrossRef]
- Zompi, S.; Harris, E. Animal models of dengue virus infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Shresta, S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Chan, K.W.K.; Watanabe, S.; Kavishna, R.; Alonso, S.; Vasudevan, S.G. Animal models for studying dengue pathogenesis and therapy. Antivir. Res. 2015, 123, 5–14. [Google Scholar] [CrossRef]
- Sarathy, V.V.; White, M.; Li, L.; Gorder, S.R.; Pyles, R.B.; Campbell, G.A.; Milligan, G.N.; Bourne, N.; Barrett, A.D.T. A Lethal Murine Infection Model for Dengue Virus 3 in AG129 Mice Deficient in Type I and II Interferon Receptors Leads to Systemic Disease. J. Virol. 2015, 89, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.K.; Ng, J.K.W.; Trasti, S.L.; Schul, W.; Yip, G.; Alonso, S. A Non Mouse-Adapted Dengue Virus Strain as a New Model of Severe Dengue Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2010, 4, e672. [Google Scholar] [CrossRef]
- Paes, M.V.; Lenzi, H.L.; Nogueira, A.C.; Nuovo, G.J.; Pinhão, A.T.; Mota, E.M.; Basílio-de-Oliveira, C.A.; Schatzmayr, H.; Barth, O.M.; Alves, A.M. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab. Investig. 2009, 89, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, S.; Priya, S.P.; Kumari, S.; Amira, F.; Poorani, K.; Alsaeedy, H.; Ling, M.P.; Chee, H.Y.; Higuchi, A.; Alarfaj, A.A.; et al. Impact of dengue virus (serotype DENV-2) infection on liver of BALB/c mice: A histopathological analysis. Tissue Cell 2017, 49, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.A.; Amorim, J.F.S.; Paes, M.V.; Azevedo, A.S.; Gonçalves, A.J.S.; Costa, S.M.; Mantuano-Barradas, M.; Póvoa, T.F.; De Meis, J.; Basílio-de-Oliveira, C.A.; et al. Peripheral effects induced in BALB/c mice infected with DENV by the intracerebral route. Virology 2016, 489, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef]
- Limonta, D.; Capo, V.; Torres, G.; Perez, A.B.; Guzman, M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef]
- Couvelard, A.; Marianneau, P.; Bedel, C.; Drouet, M.T.; Vachon, F.; Henin, D.; Deubel, V. Report of a fatal case of dengue infection with hepatitis: Demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum. Pathol. 1999, 30, 1106–1110. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Yenchitsomanus, P.T.; Limjindaporn, T. Role of mitogen-activated protein kinase signaling in the pathogenesis of dengue virus infection. Cell Signal 2018, 48, 64–68. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A.; Cheunsuchon, B.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. JNK1/2 inhibitor reduces dengue virus-induced liver injury. Antivir. Res. 2017, 141, 7–18. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. SB203580 Modulates p38 MAPK Signaling and Dengue Virus-Induced Liver Injury by Reducing MAPKAPK2, HSP27, and ATF2 Phosphorylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Srisawat, C.; Morchang, A.; Malakar, S.; Thuwajit, P.; Kooptiwut, S.; Suttitheptumrong, A.; et al. Role of ERK1/2 signaling in dengue virus-induced liver injury. Virus Res. 2014, 188, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and its Main Constituents. Phytother. Res. 2016, 30, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.R.; Khashiadeh, M.; Roshankhah, S.; Kakabaraei, S.; Jalili, C. Protective effect of crocin on liver toxicity induced by morphine. Res. Pharm. Sci. 2016, 11, 120–129. [Google Scholar]
- Gao, K.; Liu, F.; Chen, X.; Chen, M.; Deng, Q.; Zou, X.; Guo, H. Crocetin protects against fulminant hepatic failure induced by lipopolysaccharide/D-galactosamine by decreasing apoptosis, inflammation and oxidative stress in a rat model. Exp. Ther. Med. 2019, 18, 3775–3782. [Google Scholar] [CrossRef]
- Garcia-Lastra, R.; San-Miguel, B.; Crespo, I.; Jorquera, F.; Alvarez, M.; Gonzalez-Gallego, J.; Tunon, M.J. Signaling pathways involved in liver injury and regeneration in rabbit hemorrhagic disease, an animal model of virally-induced fulminant hepatic failure. Vet. Res. 2010, 41, 2. [Google Scholar] [CrossRef]
- Kye Mon, K.; Nontprasert, A.; Kittitrakul, C.; Tangkijvanich, P.; Leowattana, W.; Poovorawan, K. Incidence and Clinical Outcome of Acute Liver Failure Caused by Dengue in a Hospital for Tropical Diseases, Thailand. Am. J. Trop. Med. Hyg. 2016, 95, 1338–1344. [Google Scholar] [CrossRef]
- Fernando, S.; Wijewickrama, A.; Gomes, L.; Punchihewa, C.T.; Madusanka, S.D.; Dissanayake, H.; Jeewandara, C.; Peiris, H.; Ogg, G.S.; Malavige, G.N. Patterns and causes of liver involvement in acute dengue infection. BMC Infect. Dis. 2016, 16, 319. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Alawattegama, A.T.M.; Gunrathne, M.; Tennakoon, S.; Kularatne, S.A.M.; Jayalath, T. Value of peripheral blood count for dengue severity prediction. BMC Res. Notes 2018, 11, 400. [Google Scholar] [CrossRef]
- Binh, P.T.; Matheus, S.; Huong, V.T.; Deparis, X.; Marechal, V. Early clinical and biological features of severe clinical manifestations of dengue in Vietnamese adults. J. Clin. Virol. 2009, 45, 276–280. [Google Scholar] [CrossRef]
- Azin, F.R.; Goncalves, R.P.; Pitombeira, M.H.; Lima, D.M.; Branco, I.C. Dengue: Profile of hematological and biochemical dynamics. Rev. Bras. Hematol. Hemoter. 2012, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Salomao, N.G.; Rabelo, K.; Povoa, T.F.; Alves, A.M.B.; Da Costa, S.M.; Goncalves, A.J.S.; Amorim, J.F.; Azevedo, A.S.; Nunes, P.C.G.; Basilio-de-Oliveira, C.A.; et al. BALB/c mice infected with DENV-2 strain 66985 by the intravenous route display injury in the central nervous system. Sci. Rep. 2018, 8, 9754. [Google Scholar] [CrossRef] [PubMed]
- Abdul Ahmad, S.A.; Palanisamy, U.D.; Khoo, J.J.; Dhanoa, A.; Syed Hassan, S. Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol. J. 2019, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.B.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Iran J. Basic Med. Sci. 2017, 20, 110–121. [Google Scholar] [PubMed]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran J. Basic Med. Sci. 2019, 22, 334–344. [Google Scholar]
- Amin, B.; Nakhsaz, A.; Hosseinzadeh, H. Evaluation of the antidepressant-like effects of acute and sub-acute administration of crocin and crocetin in mice. Avicenna J. Phytomed. 2015, 5, 458–468. [Google Scholar]
- Lee, L.K.; Gan, V.C.; Lee, V.J.; Tan, A.S.; Leo, Y.S.; Lye, D.C. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl. Trop. Dis. 2012, 6, e1676. [Google Scholar] [CrossRef]
- Chen, P.; Chen, Y.; Wang, Y.; Cai, S.; Deng, L.; Liu, J.; Zhang, H. Comparative Evaluation of Hepatoprotective Activities of Geniposide, Crocins and Crocetin by CCl4-Induced liver Injury in Mice. Biomol. Ther. 2016, 24, 156–162. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Panaampon, J.; Suttitheptumrong, A.; Chuncharunee, A.; Bootkunha, J.; Yenchitsomanus, P.T.; Limjindaporn, T. Drug repurposing of N-acetyl cysteine as antiviral against dengue virus infection. Antivir. Res. 2019, 166, 42–55. [Google Scholar] [CrossRef]
- Ceballos-Olvera, I.; Chavez-Salinas, S.; Medina, F.; Ludert, J.E.; Del Angel, R.M. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology 2010, 396, 30–36. [Google Scholar] [CrossRef]
- Tongmuang, N.; Yasamut, U.; Noisakran, S.; Sreekanth, G.P.; Yenchitsomanus, P.T.; Limjindaporn, T. Suppression of micro1 subunit of the adaptor protein complex 2 reduces dengue virus release. Virus Genes 2020, 56, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Kochel, T.J. The battle between infection and host immune responses of dengue virus and its implication in dengue disease pathogenesis. Sci. World J. 2013, 2013, 843469. [Google Scholar] [CrossRef] [PubMed]
- Manickam, C.; Shah, S.V.; Lucar, O.; Ram, D.R.; Reeves, R.K. Cytokine-Mediated Tissue Injury in Non-human Primate Models of Viral Infections. Front. Immunol. 2018, 9, 2862. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; Seneviratne, S.L. Liver involvement in dengue viral infections. Rev. Med. Virol. 2018, 28. [Google Scholar] [CrossRef]
- De Azeredo, E.L.; Monteiro, R.Q.; De-Oliveira Pinto, L.M. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediat. Inflamm. 2015, 2015, 313842. [Google Scholar] [CrossRef]
- Olagnier, D.; Peri, S.; Steel, C.; Van Montfoort, N.; Chiang, C.; Beljanski, V.; Slifker, M.; He, Z.; Nichols, C.N.; Lin, R.; et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014, 10, e1004566. [Google Scholar] [CrossRef]
- Jan, J.T.; Chen, B.H.; Ma, S.H.; Liu, C.I.; Tsai, H.P.; Wu, H.C.; Jiang, S.Y.; Yang, K.D.; Shaio, M.F. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: Arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J. Virol. 2000, 74, 8680–8691. [Google Scholar] [CrossRef]
- Liao, H.; Xu, J.; Huang, J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J. Med. Virol. 2010, 82, 1392–1399. [Google Scholar] [CrossRef]
- Yang, R.; Vernon, K.; Thomas, A.; Morrison, D.; Qureshi, N.; Van Way, C.W., 3rd. Crocetin reduces activation of hepatic apoptotic pathways and improves survival in experimental hemorrhagic shock. J. Parenter Enteral Nutr. 2011, 35, 107–113. [Google Scholar] [CrossRef]
- Cherupanakkal, C.; Samadanam, D.M.; Muthuraman, K.R.; Ramesh, S.; Venkatesan, A.; Balakrishna Pillai, A.K.; Rajendiran, S. Lipid peroxidation, DNA damage, and apoptosis in dengue fever. IUBMB Life 2018, 70, 1133–1143. [Google Scholar] [CrossRef]
- Morchang, A.; Lee, R.C.H.; Yenchitsomanus, P.T.; Sreekanth, G.P.; Noisakran, S.; Chu, J.J.H.; Limjindaporn, T. RNAi screen reveals a role of SPHK2 in dengue virus-mediated apoptosis in hepatic cell lines. PLoS ONE 2017, 12, e0188121. [Google Scholar] [CrossRef]
- Soundravally, R.; Sankar, P.; Bobby, Z.; Hoti, S.L. Oxidative stress in severe dengue viral infection: Association of thrombocytopenia with lipid peroxidation. Platelets 2008, 19, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Valero, N.; Mosquera, J.; Anez, G.; Levy, A.; Marcucci, R.; De Mon, M.A. Differential oxidative stress induced by dengue virus in monocytes from human neonates, adult and elderly individuals. PLoS ONE 2013, 8, e73221. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Martinez, G.; Tapanes, R.; Castro, O.; Gonzalez, D.; Bernardo, L.; Vazquez, S.; Kouri, G.; Guzman, M.G. Oxidative stress in adult dengue patients. Am. J. Trop. Med. Hyg. 2004, 71, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, L.G.; Peiris, H.; Kamani, J.; Wanigasuriya, P.; Jayaratne, S.D.; Wijayasiri, W.A.; Wijesekara, G.U. Antioxidants in patients with dengue viral infection. Southeast Asian J. Trop. Med. Public Health 2014, 45, 1015–1022. [Google Scholar] [PubMed]
- Wang, J.; Chen, Y.; Gao, N.; Wang, Y.; Tian, Y.; Wu, J.; Zhang, J.; Zhu, J.; Fan, D.; An, J. Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS ONE 2013, 8, e55407. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Ge, Z. Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed. Pharmacother. 2017, 93, 376–382. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef]
- Bian, J.; Wang, K.; Kong, X.; Liu, H.; Chen, F.; Hu, M.; Zhang, X.; Jiao, X.; Ge, B.; Wu, Y.; et al. Caspase- and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus. Arch. Virol. 2011, 156, 1335–1344. [Google Scholar] [CrossRef]
- Arias, J.; Valero, N.; Mosquera, J.; Montiel, M.; Reyes, E.; Larreal, Y.; Alvarez-Mon, M. Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology 2014, 452–453, 42–51. [Google Scholar] [CrossRef]
- Valero, N.; Mosquera, J.; Levy, A.; Anez, G.; Marcucci, R.; Alvarez-Mon, M. Differential induction of cytokines by human neonatal, adult, and elderly monocyte/macrophages infected with dengue virus. Viral Immunol. 2014, 27, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Warke, R.V.; Martin, K.J.; Giaya, K.; Shaw, S.K.; Rothman, A.L.; Bosch, I. TRAIL is a novel antiviral protein against dengue virus. J. Virol. 2008, 82, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Gomes, L.; Alles, L.; Chang, T.; Salimi, M.; Fernando, S.; Nanayakkara, K.D.; Jayaratne, S.; Ogg, G.S. Serum IL-10 as a marker of severe dengue infection. BMC Infect. Dis. 2013, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Jeewandara, C.; Alles, K.M.; Salimi, M.; Gomes, L.; Kamaladasa, A.; Jayaratne, S.D.; Ogg, G.S. Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Negl. Trop. Dis. 2013, 7, e2409. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Jain, A.; Garg, R.K.; Kumar, R.; Agrawal, O.P.; Lakshmana Rao, P.V. Serum levels of IL-8, IFNgamma, IL-10, and TGF beta and their gene expression levels in severe and non-severe cases of dengue virus infection. Arch. Virol. 2015, 160, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Limonta, D.; Torrentes-Carvalho, A.; Marinho, C.F.; De Azeredo, E.L.; De Souza, L.J.; Motta-Castro, A.R.; Da Cunha, R.V.; Kubelka, C.F.; Nogueira, R.M.; De-Oliveira-Pinto, L.M. Apoptotic mediators in patients with severe and non-severe dengue from Brazil. J. Med. Virol. 2014, 86, 1437–1447. [Google Scholar] [CrossRef]
- Zain, N.; Putra, S.T.; Zein, U.; Hariman, H. Soluble Fas Ligand as a Potential Marker of Severity of Dengue Infection. Malays. J. Med. Sci. 2017, 24, 28–32. [Google Scholar] [CrossRef]
- Torrentes-Carvalho, A.; Azeredo, E.L.; Reis, S.R.; Miranda, A.S.; Gandini, M.; Barbosa, L.S.; Kubelka, C.F. Dengue-2 infection and the induction of apoptosis in human primary monocytes. Mem. Inst. Oswaldo Cruz 2009, 104, 1091–1099. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Medina, E.; Anders, D.; Chhatwal, G.S. Induction of NF-kappaB nuclear translocation in human respiratory epithelial cells by group A streptococci. Microb. Pathog. 2002, 33, 307–313. [Google Scholar] [CrossRef]
- Papa, S.; Bubici, C.; Zazzeroni, F.; Franzoso, G. Mechanisms of liver disease: Cross-talk between the NF-kappaB and JNK pathways. Biol. Chem. 2009, 390, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Dong, X.; Liu, J. Crocetin attenuates inflammation and amyloid-beta accumulation in APPsw transgenic mice. Immun. Ageing 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Khunchai, S.; Junking, M.; Suttitheptumrong, A.; Kooptiwut, S.; Haegeman, G.; Limjindaporn, T.; Yenchitsomanus, P.T. NF-kappaB is required for dengue virus NS5-induced RANTES expression. Virus Res. 2015, 197, 92–100. [Google Scholar] [CrossRef]
- Lin, C.K.; Tseng, C.K.; Wu, Y.H.; Liaw, C.C.; Lin, C.Y.; Huang, C.H.; Chen, Y.H.; Lee, J.C. Cyclooxygenase-2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents. Sci. Rep. 2017, 7, 44701. [Google Scholar] [CrossRef]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Gonzalez, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′–3′) | Reverse Primer (3′–5′) |
|---|---|---|
| TNF-α | CCCCCAGTCTGTATCCTTCT | TTTGAGTCCTTGATGGTGGT |
| Fas | TGTGAACATGGAACCCTTGA | TTCAGGGTCATCCTGTCTCC |
| Fas-L | CATCACAACCACTCCCACTG | GTTCTGCCAGTTCCTTCTGC |
| IL-10 | CCAAGCCTTATCGGAAATGA | TTTTCACAGGGGAGAAATCG |
| IL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| TRAIL | GATGTTGGTGCCTGGAGTTT | AAGCAAAGGGCAGAAAGTCA |
| HO-1 | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGCA |
| COX-2 | GCGAGCTAAGAGCTTCAGGA | TCATACATTCCCCACGGTTT |
| GAPDH | TGAATACGGCTACAGCAACA | AGGCCCCTCCTGTTATTATG |
| DENV NS1 | CCGGCCAGATCTGGAGACATCAAAGGAATC | GCCATCAATGAGAAAGGTCTGG |
| Gene | Gene Description | mRNA Expression | ||
|---|---|---|---|---|
| Il10 | Interleukin 10 | 1 | 21.6818 | 11.7071 |
| Fadd | Fas (TNFRSF6)-associated via death domain | 1 | 9.9330 | 2.9395 |
| Fasl | Fas ligand (TNF superfamily, member 6) | 1 | 9.5798 | 3.9933 |
| Tnfsf10 | Tumor necrosis factor (ligand) superfamily, member 10 | 1 | 8.2658 | 3.8906 |
| Casp8 | Caspase-8 | 1 | 7.7320 | 2.1019 |
| Apaf1 | Apoptotic peptidase activating factor 1 | 1 | 6.7321 | 2.7132 |
| Cd40 | CD40 antigen | 1 | 4.9866 | 2.8089 |
| Fas | Fas (TNF receptor superfamily member 6) | 1 | 4.7654 | 1.3851 |
| Dapk1 | Death-associated protein kinase 1 | 1 | 4.5824 | 1.6507 |
| Pycard | PYD and CARD domain containing | 1 | 4.4948 | 1.4728 |
| Casp3 | Caspase-3 | 1 | 4.1892 | 2.0943 |
| Aifm1 | Apoptosis-inducing factor, mitochondrion-associated 1 | 1 | 4.0007 | 2.5176 |
| Tnf | Tumor necrosis factor | 1 | 3.9013 | 1.6363 |
| Traf1 | Tnf receptor-associated factor 1 | 1 | 3.5476 | 2.0208 |
| Casp9 | Caspase-9 | 1 | 3.3287 | 1.2058 |
| Casp7 | Caspase-7 | 1 | 2.7851 | 1.2013 |
| Cd40lg | CD40 ligand | 1 | 2.8420 | 1.4075 |
| Nfkb1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1, p105 | 1 | 2.5692 | 1.3358 |
| Traf2 | Tnf receptor-associated factor 2 | 1 | 1.8351 | 1.3586 |
| Api5 | Apoptosis inhibitor 5 | 1 | 0.4351 | 0.7965 |
| Dad1 | Defender against cell death 1 | 1 | 0.3359 | 0.8467 |
| Naip2 | NLR family, apoptosis inhibitory protein 2 | 1 | 0.1019 | 0.7728 |
| DMSO DENV Crocetin | + | + | + | |
| − | + | + | ||
| − | − | + | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreekanth, G.P.; Chuncharunee, A.; Yenchitsomanus, P.-t.; Limjindaporn, T. Crocetin Improves Dengue Virus-Induced Liver Injury. Viruses 2020, 12, 825. https://doi.org/10.3390/v12080825
Sreekanth GP, Chuncharunee A, Yenchitsomanus P-t, Limjindaporn T. Crocetin Improves Dengue Virus-Induced Liver Injury. Viruses. 2020; 12(8):825. https://doi.org/10.3390/v12080825
Chicago/Turabian StyleSreekanth, Gopinathan Pillai, Aporn Chuncharunee, Pa-thai Yenchitsomanus, and Thawornchai Limjindaporn. 2020. "Crocetin Improves Dengue Virus-Induced Liver Injury" Viruses 12, no. 8: 825. https://doi.org/10.3390/v12080825
APA StyleSreekanth, G. P., Chuncharunee, A., Yenchitsomanus, P.-t., & Limjindaporn, T. (2020). Crocetin Improves Dengue Virus-Induced Liver Injury. Viruses, 12(8), 825. https://doi.org/10.3390/v12080825






