Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture and Viruses
2.3. Hemoadsorption in Swine Macrophages
2.4. Virus Titration
2.5. Extraction of ASFV DNA and RtPCR of ASFV
2.6. Immunoproxidase Staining for ASFV
3. Results
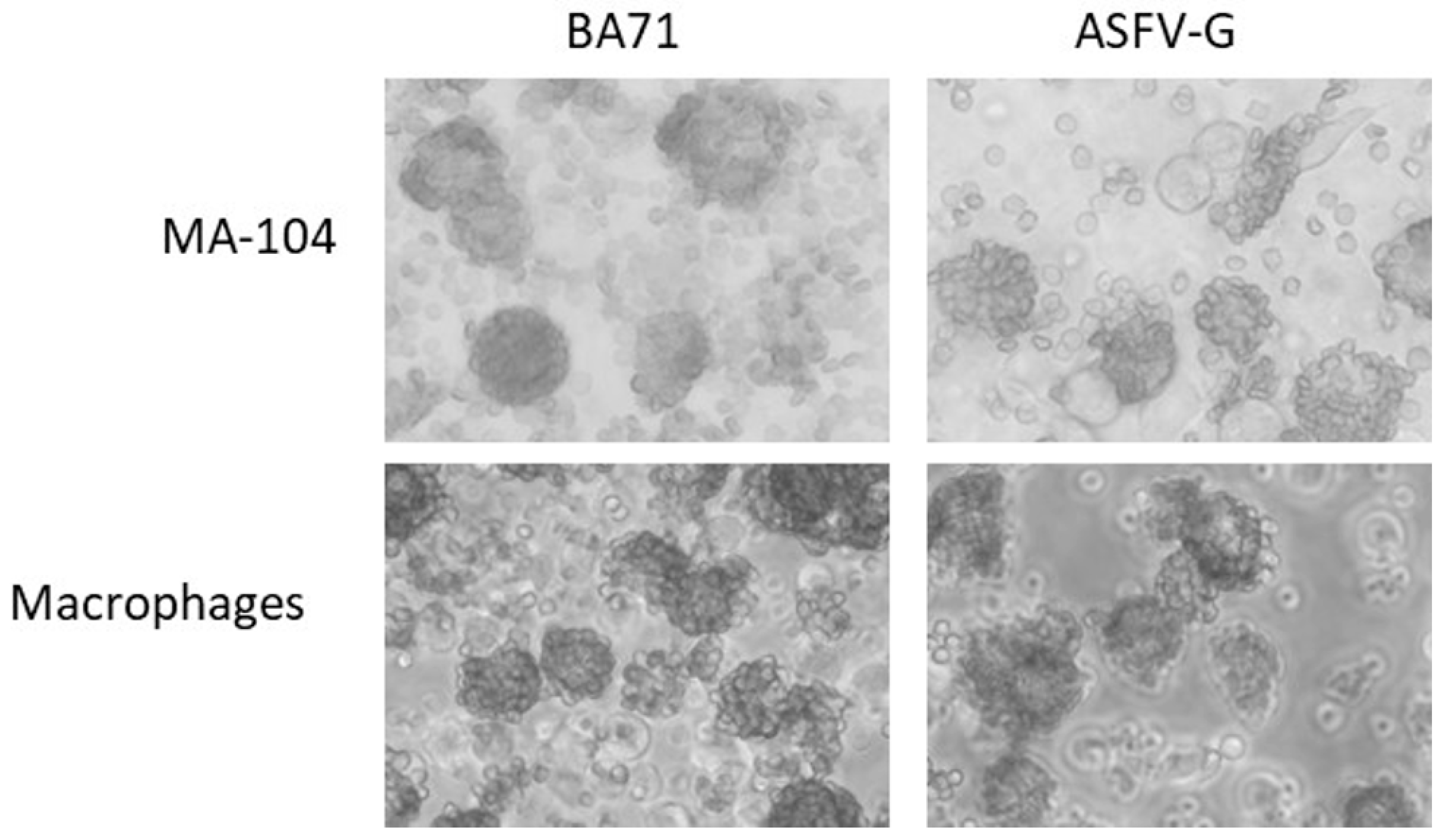
3.1. ASFV-Infected MA-104 Cells Can Be Detected by Hemadsorption (HA)
3.2. ASFV-Infected MA-104 Cells Can Be Specifically Stained by Immunocytochemistry Using an Anti-ASFV p30 Monoclonal Antibody
3.3. Relative Sensitivity of MA-104 Cells to Detect Infectious ASFV from Different Field Isolates Compared to that of Primary Swine Macrophages or Real-Time PCR
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rock, D.L. Challenges for African swine fever vaccine development—“… perhaps the end of the beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Transact. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Chitko-McKown, C.G.; Chapes, S.K.; Miller, L.C.; Riggs, P.K.; Ortega, M.T.; Green, B.T.; McKown, R.D. Development and characterization of two porcine monocyte-derived macrophage cell lines. Results Immunol. 2013, 3, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The progressive adaptation of a georgian isolate of African swine fever virus to vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J. Virol. 2015, 89, 2324–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, S.; Ribeiro, G.; Costa, J.V. Sequence and organization of the left multigene family 110 region of the Vero-adapted L60V strain of African swine fever virus. Virus Genes 1997, 15, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Tabares, E.; Olivares, I.; Santurde, G.; Garcia, M.J.; Martin, E.; Carnero, M.E. African swine fever virus DNA: Deletions and additions during adaptation to growth in monkey kidney cells. Arch. Virol. 1987, 97, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Berggren, K.A.; Ramirez-Medina, E.; Vuono, E.A.; Gladue, D.P. CRISPR/cas gene editing of a large DNA virus: African swine fever virus. Bio Protoc. 2018, 8, e2978. [Google Scholar] [CrossRef]
- Lacasta, A.; Monteagudo, P.L.; Jimenez-Marin, A.; Accensi, F.; Ballester, M.; Argilaguet, J.; Galindo-Cardiel, I.; Segales, J.; Salas, M.L.; Dominguez, J.; et al. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in viral pathogenesis and immune protection. Vet. Res. 2015, 46, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesley, R.D.; Pan, I.C. African swine fever virus DNA: Restriction endonuclease cleavage patterns of wild-type, Vero cell-adapted and plaque-purified virus. J. Gen. Virol. 1982, 63, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Carrillo, C.; Zsak, L.; Laegreid, W.W.; Kutish, G.F.; Neilan, J.G.; Burrage, T.G.; Rock, D.L. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 1998, 72, 2881–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borca, M.V.; O’Donnell, V.; Holinka, L.G.; Risatti, G.R.; Ramirez-Medina, E.; Vuono, E.A.; Shi, J.; Pruitt, S.; Rai, A.; Silva, E.; et al. Deletion of CD2-like gene from the genome of African swine fever virus strain Georgia does not attenuate virulence in swine. Sci. Rep. 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Yanez, R.J.; Almazan, F.; Vinuela, E.; Rodriguez, J.F. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J. Virol. 1993, 67, 5312–5320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernandez-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simon, A.; et al. Assessment of African swine fever diagnostic techniques as a response to the epidemic outbreaks in Eastern European Union countries: How to improve surveillance and control programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Organisation for Animal Health. African Swine Fever (Infection with African Swine Fever Virus). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 22 July 2020).
- MA-104 Clone 1 (ATCC® CRL-2378.1™). Available online: https://www.atcc.org/Products/All/CRL-2378.1.aspx (accessed on 2 June 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, A.; Pruitt, S.; Ramirez-Medina, E.; Vuono, E.A.; Silva, E.; Velazquez-Salinas, L.; Carrillo, C.; Borca, M.V.; Gladue, D.P. Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples. Viruses 2020, 12, 820. https://doi.org/10.3390/v12080820
Rai A, Pruitt S, Ramirez-Medina E, Vuono EA, Silva E, Velazquez-Salinas L, Carrillo C, Borca MV, Gladue DP. Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples. Viruses. 2020; 12(8):820. https://doi.org/10.3390/v12080820
Chicago/Turabian StyleRai, Ayushi, Sarah Pruitt, Elizabeth Ramirez-Medina, Elizabeth A. Vuono, Ediane Silva, Lauro Velazquez-Salinas, Consuelo Carrillo, Manuel V. Borca, and Douglas P. Gladue. 2020. "Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples" Viruses 12, no. 8: 820. https://doi.org/10.3390/v12080820
APA StyleRai, A., Pruitt, S., Ramirez-Medina, E., Vuono, E. A., Silva, E., Velazquez-Salinas, L., Carrillo, C., Borca, M. V., & Gladue, D. P. (2020). Identification of a Continuously Stable and Commercially Available Cell Line for the Identification of Infectious African Swine Fever Virus in Clinical Samples. Viruses, 12(8), 820. https://doi.org/10.3390/v12080820






