Virus Pathogens in Australian Vineyards with an Emphasis on Shiraz Disease †
Abstract
1. Introduction
2. Grapevine Viruses in Australian Vineyards
3. Virus Transmission in Vineyards
3.1. Primary Transmission
3.2. Secondary Transmission
4. Symptomatology
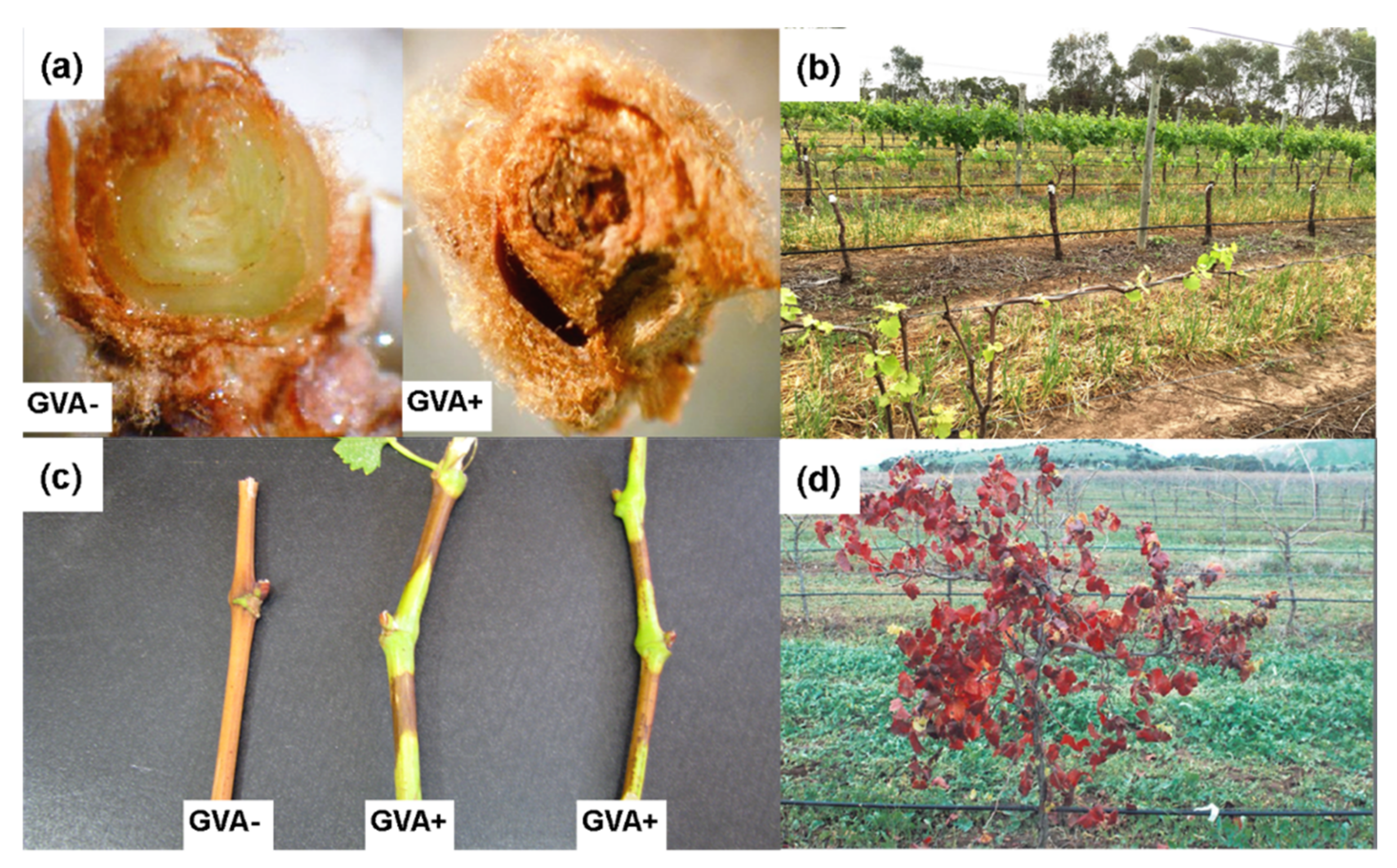
4.1. Shiraz Disease
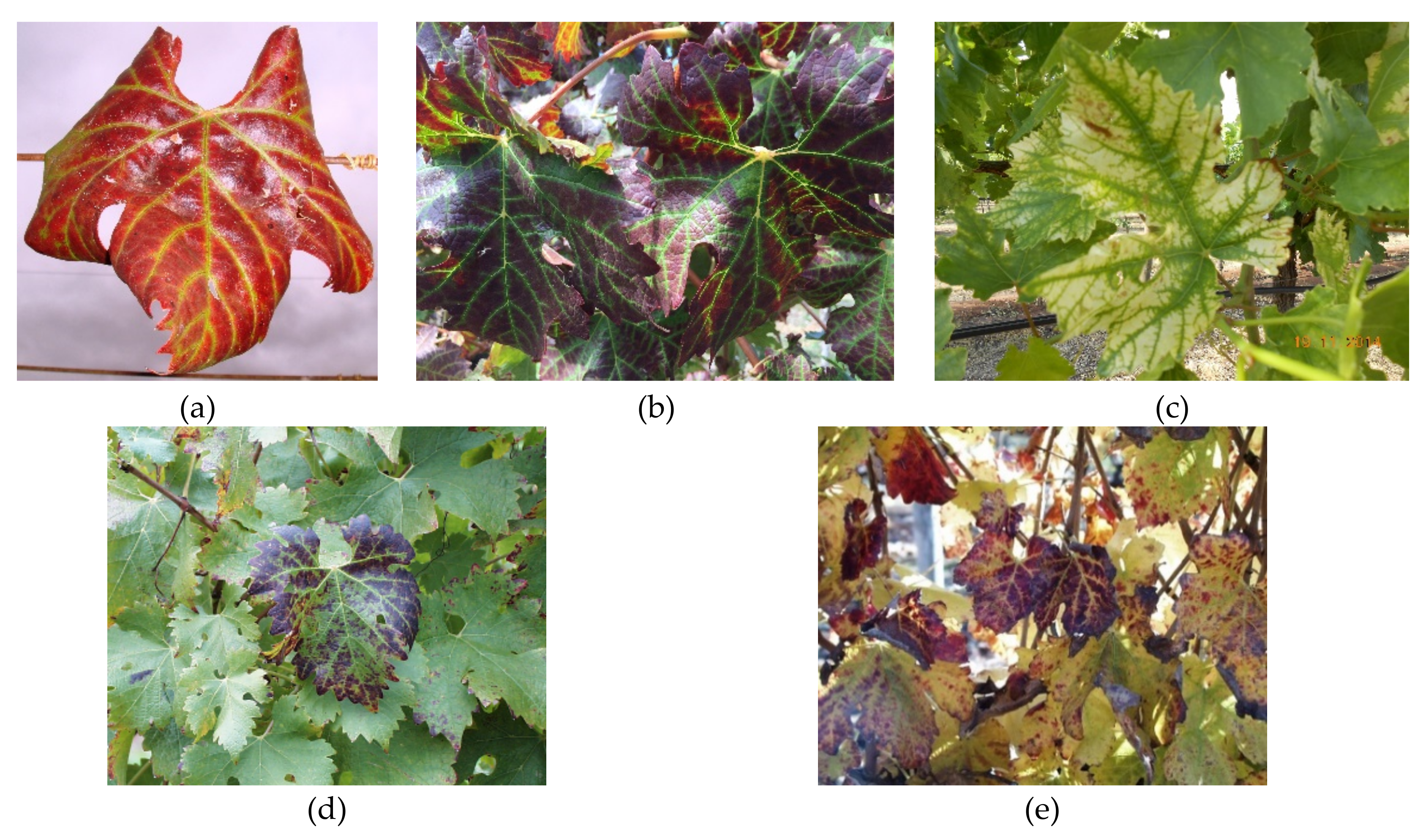
4.2. Leafroll Disease
5. High Throughput Sequencing (HTS) and Phylogenetic Analysis of Viruses Associated with Shiraz Disease
5.1. High Throughput Sequencing (HTS)
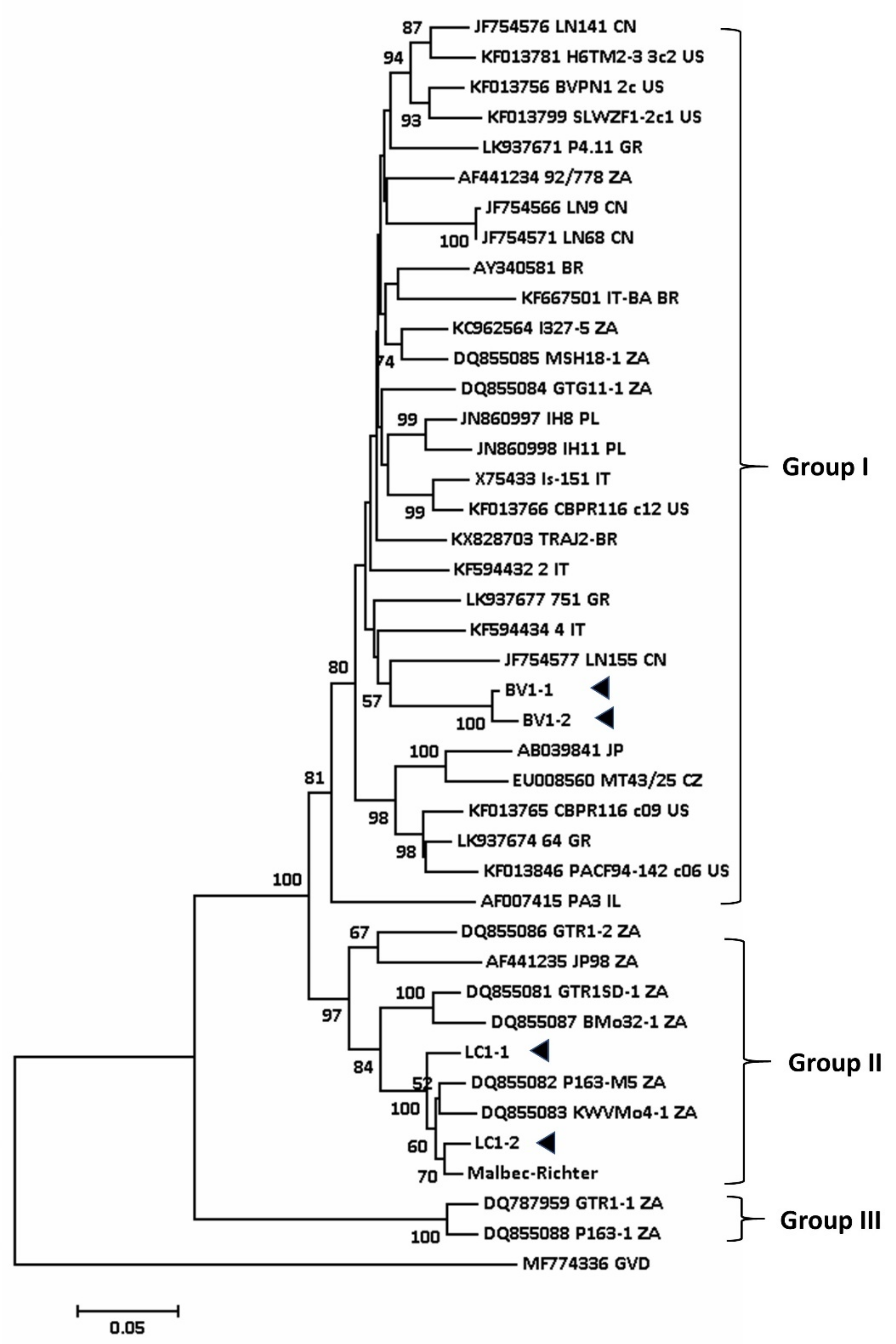
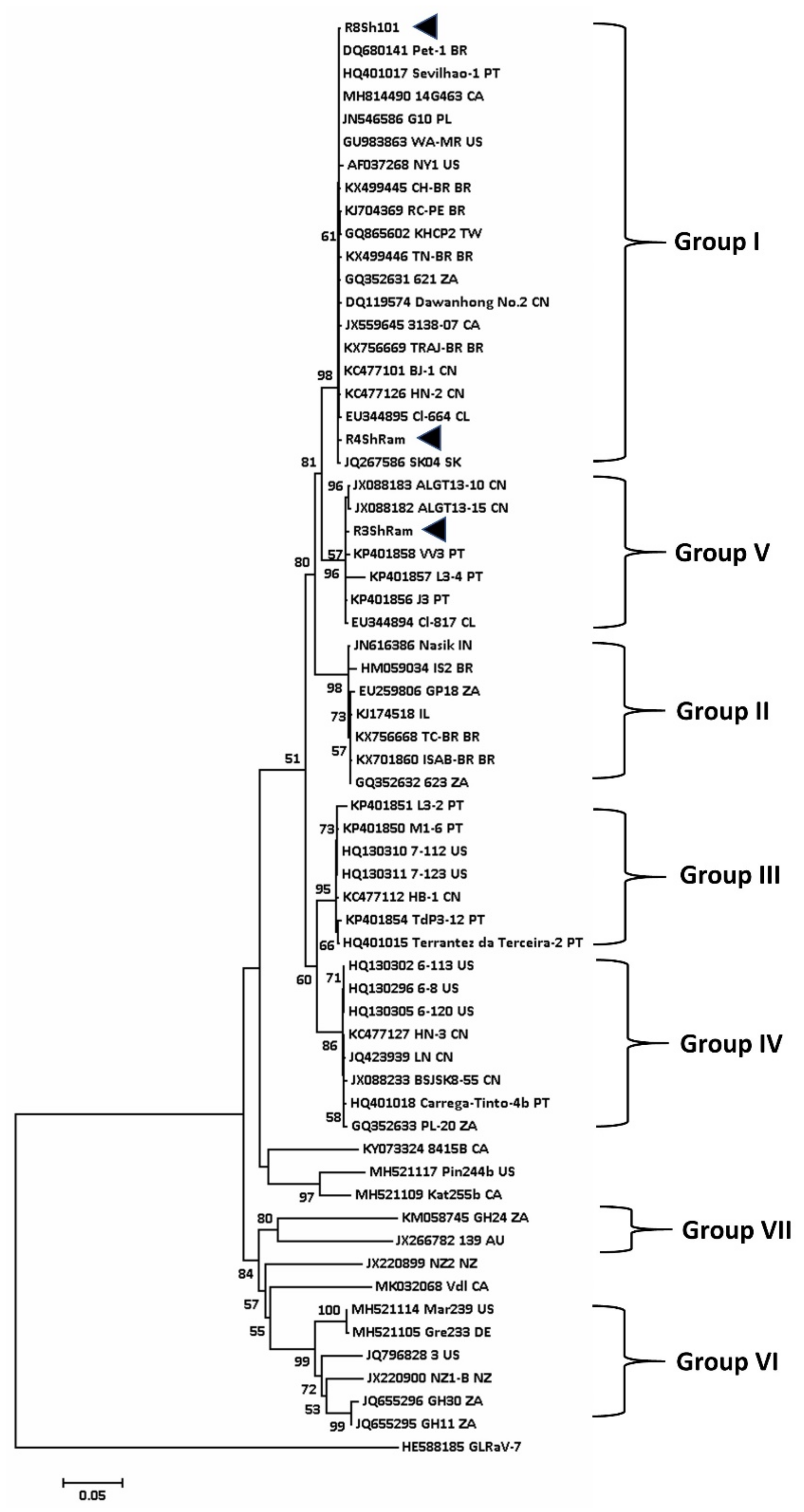
5.2. Phylogenetic Analysis of Viruses Associated with Shiraz Disease
6. Virus Effects on Vine Physiology and Fruit Composition
7. Economic Impacts of GVA and GLRaV-3
8. Management of Shiraz Disease and Grapevine Leafroll Disease in Australian Vineyards
9. Observations of Shiraz Disease in South Australian Vineyards
10. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020. [Google Scholar] [CrossRef]
- Du Preez, J.; Stephan, D.; Mawassi, M.; Burger, J.T. The grapevine-infecting vitiviruses, with particular reference to grapevine virus A. Arch. Virol. 2011, 156, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Constable, F.E.; Nicholas, P.; Rodoni, B.C. Development and Validation of Diagnostic Protocols for the Detection of Endemic and Exotic Pathogens of Grapevines; Final Report to Grape and Wine Research & Development Corporation; Wine Australia: Adelaide, Australia, 2010; p. 283. Available online: http://www.gwrdc.com.au/wp-content/uploads/2012/09/DPI-05-04.pdf (accessed on 14 May 2020).
- Constable, F.; Tassie, E.; McLoughlin, S. A Comprehensive Review of Grapevine Pinot Gris Virus (GPGV). Final Report to Wine Australia; Project No. VHA 1701; 2019. Available online: https://www.wineaustralia.com/getmedia/62a813e4-b27f-4a27-a32e-9b75c2c23103/VHA-1701-FINAL-REPORT.pdf (accessed on 23 June 2020).
- IPPC. Official Pest Reports—Australia (2015-06-25) Absence of Grapevine Fanleaf Virus from Australia. Available online: https://www.ippc.int/en/countries/australia/pestreports/2015/06/absence-of-grapevine-fanleaf-virus-from-australia/ (accessed on 10 July 2020).
- Meng, B.; Martelli, G.P.; Golino, D.; Fuchs, M. Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; p. 698. [Google Scholar]
- Habili, N.; Wu, Q.; Pagay, V. Virus-associated Shiraz Disease may lead Shiraz to become an endangered variety in Australia. Wine Vitic. J. 2016, 31, 47–50. [Google Scholar]
- Alabi, O.J.; Casassa, F.; Gutha, L.R.; Larsen, R.C.; Henick-Kling, T.; Harbertson, J.F.; Naidu, R.A. Impacts of grapevine leafroll disease on fruit yield and grape and wine chemistry in a wine grape (Vitis vinifera L.) cultivar. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Goszczynski, D.E.; Habili, N. Grapevine virus A variants of group II associated with Shiraz disease in South Africa are present in plants affected by Australian Shiraz disease, and have also been detected in the USA. Plant Pathol. 2012, 61, 205–214. [Google Scholar] [CrossRef]
- Corbett, M.K.; Wiid, J. Closterovirus-like particles in extracts from diseased grapevines. In Proceedings of the 8th Meeting of the International Council for the study of Viruses and Virus Diseases of the Grapevine, Bari, Italy, 3–7 September 1984; pp. 91–100. [Google Scholar]
- Goszczynski, D.E.; Jooste, A.E.C. Identification of divergent variants of Grapevine virus A. Eur. J. Plant Pathol. 2003, 109, 397–403. [Google Scholar] [CrossRef]
- Habili, N. Australian Shiraz Disease: An emerging virus disease of Vitis vinifera cv. Shiraz. Wine Vitic. J. 2013, 28, 59–61. [Google Scholar]
- Grenan, S.; Renault-Spilmont, A.S.; Boursiquot, J.-M. Syrah decline in France: Historical background and first approaches. In Proceedings of the Syrah Vine Health Symposium, University of California, Davis, CA, USA, 6 November 2007; p. 3. [Google Scholar]
- Puckett, J.M.; Dangl, G.; Golino, D.; Al Rwahnih, M. Evidence to support Syrah Decline is a non-infectious genetic syndrome in several Syrah selections. In Proceedings of the 19th Congress of ICVG, Santiago, Chile, 9–12 April 2018; pp. 78–79. [Google Scholar]
- Constable, F.; Drew, C. Review of vine health parameters, implementation priorities and capabilities for vine improvement groups and accredited nurseries. In Final Report to the Grape and Wine Research & Development Corporation; Project number NVH 03/01; Scholefield Robinson Horticultural Services Pty. Ltd.: Adelaide, Australia, 2004; p. 105. Available online: https://www.wineaustralia.com/getmedia/0807646b-1fb9-4cfd-80df-61b96d76809d/NVH-03-01 (accessed on 12 January 2020).
- Martin, R.R.; Constable, F.; Tzanetakis, I.E. Quarantine regulations and the impact of modern detection methods. Ann. Rev. Phytopathol. 2016, 54, 189–205. [Google Scholar] [CrossRef]
- Rakimov, A.; Ben-Dov, Y.; White, V.; Hoffmann, A.A. Soft scale insects (Hemiptera: Coccoidea: Coccidae) on grapevines in Australia. Aust. J. Entomol. 2013, 52, 371–378. [Google Scholar] [CrossRef]
- PGIBSA. A Growers’ Guide to Top.-Working Grapevines; Phylloxera and Grape Industry Board of South Australia: Adelaide, SA, Australia, 2004; p. 20. [Google Scholar]
- Tsai, C.W.; Chau, J.; Fernandez, L.; Bosco, D.; Daane, K.M.; Almeida, R.P.P. Transmission of Grapevine leafroll-associated virus 3 by the vine mealybug (Planococcus ficus). Phytopathology 2008, 98, 1093–1098. [Google Scholar] [CrossRef]
- Krueger, K.; Douglas-Smit, N. Grapevine leafroll-associated virus 3 (GLRaV-3) transmission by three soft scale insect species (Hemiptera: Coccidae) with notes on their biology. Afr. Entomol. 2013, 21, 1–8. [Google Scholar] [CrossRef]
- Golino, D.; Sim, S.; Gill, R.; Rowhani, A. California mealybugs can spread grapevine leafroll disease. Calif. Agric. 2002, 56, 196–201. [Google Scholar] [CrossRef]
- La Notte, P.; Buzkan, N.; Choueiri, E.; Minafra, A.; Martelli, G.P. Acquisition and transmission of grapevine virus A by the mealybug Pseudococcus longispinus. J. Plant Pathol. 1997, 79, 79–85. [Google Scholar]
- Rosciglione, B.; Castellano, M.A.; Martelli, G.P.; Savino, V.; Cannizzaro, G. Mealybug transmission of Grapevine Virus-A. Vitis 1983, 22, 331–347. [Google Scholar]
- Naidu, R.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Daane, K.M.; Bell, V.A.; Blaisdell, G.K.; Cooper, M.L.; Herrbach, E.; Pietersen, G. Ecology and management of grapevine leafroll disease. Front. Microbiol. 2013, 4, 94. [Google Scholar] [CrossRef]
- Le Maguet, J.; Fuchs, J.J.; Chadoeuf, J.; Beuve, M.; Herrbach, E.; Lemaire, O. The role of the mealybug Phenacoccus aceris in the spread of Grapevine leafroll-associated virus-1 (GLRaV-1) in two French vineyards. Eur. J. Plant Pathol. 2013, 135, 415–427. [Google Scholar] [CrossRef]
- Habili, N.; Nutter, F.W., Jr. Temporal and spatial analysis of grapevine leafroll-associated virus 3 in Pinot Noir grapevines in Australia. Plant Dis. 1997, 81, 625–628. [Google Scholar] [CrossRef]
- Herrbach, E.; Alliaume, A.; Prator, C.A.; Daane, K.M.; Cooper, M.L.; Almeida, R.P.P. Vector transmission of grapevine leafroll-associated viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 483–503. [Google Scholar]
- Wistrom, C.M.; Blaisdell, G.K.; Wunderlich, L.R.; Almeida, R.P.P.; Daane, K.M. Ferrisia gilli Gullan (Hemiptera: Pseudococcidae) transmits grapevine leafroll associated viruses. J. Econ. Entomol. 2016, 109, 1519–1523. [Google Scholar] [CrossRef]
- Bertin, S.; Cavalieri, V.; Gribaudo, I.; Sacco, D.; Marzachi, C.; Bosco, D. Transmission of Grapevine virus A and Grapevine leafroll-associated virus 1 and 3 by Heliococcus bohemicus (Hemiptera: Pseudococcidae) nymphs from plants with mixed infections. J. Econ. Entomol. 2016, 109, 1504–1511. [Google Scholar] [CrossRef]
- Bertin, S.; Pacifico, D.; Cavalieri, V.; Marzachi, C.; Bosco, D. Transmission of Grapevine virus A and Grapevine leafroll-associated viruses 1 and 3 by Planococcus ficus and Planococcus citri fed on mixed-infected plants. Ann. Appl. Biol. 2016, 169, 53–63. [Google Scholar] [CrossRef]
- Sforza, R.; Boudon-Padieu, E.; Greif, C. New mealybug species vectoring Grapevine leafroll-associated viruses-1 and-3 (GLRaV-1 and-3). Eur. J. Plant Pathol. 2003, 109, 975–981. [Google Scholar] [CrossRef]
- Zorloni, A.; Prati, S.; Bianco, P.A.; Belli, G. Transmission of Grapevine virus A and Grapevine leafroll-associated virus 3 by Heliococcus bohemicus. J. Plant Pathol. 2006, 88, 325–328. [Google Scholar]
- Le Maguet, J.; Beuve, M.; Herrbach, E.; Lemaire, O. Transmission of six ampeloviruses and two vitiviruses to grapevine by Phenacoccus aceris. Phytopathology 2012, 102, 717–723. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Some characteristics of the transmission of grapevine leafroll associated virus 3 by Planococcus citri Risso. Eur. J. Plant Pathol. 1997, 103, 373–378. [Google Scholar] [CrossRef]
- Cabaleiro, C.; Segura, A. Field transmission of grapevine leafroll associated virus 3 (GLRaV-3) by the mealybug Planococcus citri. Plant Dis. 1997, 81, 283–287. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Digiaro, M.; Dhouibi, M.H. Transmission of grapevine leafroll viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 2009, 93, 999–1002. [Google Scholar] [CrossRef]
- Engelbrecht, D.; Kasdorf, G. Transmission of grapevine leafroll disease and associated closteroviruses by the vine mealybug, Planococcus ficus. Phytophylactica 1990, 22, 341–346. [Google Scholar]
- Garau, R.; Prota, V.A.; Boscia, D.; Fiori, M.; Prota, U. Pseudococcus affinis mask, a new vector of grapevine Trichovirus-A and Triochovirus-B. Vitis 1995, 34, 67–68. [Google Scholar]
- Nakaune, R.; Toda, S.; Mochizuki, M.; Nakano, M. Identification and characterization of a new vitivirus from grapevine. Arch. Virol. 2008, 153, 1827–1832. [Google Scholar] [CrossRef]
- Fuchs, M.; Marsella-Herrick, P.; Loeb, G.M.; Martinson, T.E.; Hoch, H.C. Diversity of ampeloviruses in mealybug and soft scale vectors and in grapevine hosts from leafroll-affected vineyards. Phytopathology 2009, 99, 1177–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petersen, C.L.; Charles, J.G. Transmission of grapevine leafroll-associated closteroviruses by Pseudococcus longispinus and P. calceolariae. Plant Pathol. 1997, 46, 509–515. [Google Scholar] [CrossRef]
- Douglas, N.; Kruger, K. Transmission efficiency of Grapevine leafroll-associated virus 3 (GLRaV-3) by the mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae). Eur. J. Plant Pathol. 2008, 122, 207–212. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Opin. Virol. 2015, 15, 48–55. [Google Scholar] [CrossRef]
- Zhou, J.S.; Drucker, M.; Ng, J.C.K. Direct and indirect influences of virus-insect vector-plant interactions on non-circulative, semi-persistent virus transmission. Curr. Opin. Virol. 2018, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Rowhani, A.; Golino, D.A.; Daane, K.M.; Almeida, R.P.P. Mealybug transmission of grapevine leafroll viruses: An analysis of virus-vector specificity. Phytopathology 2010, 100, 830–834. [Google Scholar] [CrossRef]
- Blaisdell, G.K.; Zhang, S.; Rowhani, A.; Klassen, V.; Cooper, M.L.; Daane, K.M.; Almeida, R.P.P. Trends in vector-borne transmission efficiency from coinfected hosts: Grapevine leafroll-associated virus-3 and Grapevine Virus. A. Eur. J. Plant Pathol. 2020, 156, 1163–1167. [Google Scholar] [CrossRef]
- Hommay, G.; Komar, V.; Lemaire, O.; Herrbach, E. Grapevine virus A transmission by larvae of Parthenolecanium corni. Eur. J. Plant Pathol. 2008, 121, 185–188. [Google Scholar] [CrossRef]
- Daane, K.M.; Almeida, R.P.P.; Bell, V.A.; Walker, J.T.S.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; et al. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 271–307. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Daane, K.M.; Ponti, L.; Walton, V.M.; Ellis, C.K. Prospective evaluation of the biological control of vine mealybug: Refuge effects and climate. J. Appl. Ecol. 2008, 45, 524–536. [Google Scholar] [CrossRef]
- Burger, J.T.; Maree, H.J.; Gouveia, P.; Naidu, R.A. Grapevine leafroll-associated virus3. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 167–195. [Google Scholar]
- Tanne, E.; Ben-Dov, Y.; Raccah, B.; Dov, Y.B. Transmission of the corky-bark disease by the mealybug Planococcus ficus. Phytoparasitica 1989, 17, 55. [Google Scholar] [CrossRef]
- Barras, I.C.; Jerie, P.; Ward, S.A. Aerial dispersal of first- and second-instar longtailed mealybug, Pseudococcus longispinus (Targioni-Tozzetti) (Pseudococcidae: Hemiptera). Aust. J. Exp. Agric. 1994, 34, 1205–1208. [Google Scholar] [CrossRef]
- Hommay, G.; Wiss, L.; Chadoeuf, J.; Maguet, J.l.; Beuve, M.; Herrbach, E.; Le Maguet, J. Gone with the wind: Aerial dispersal of Parthenolecanium corni crawlers in a newly planted grapevine plot. Ann. Appl. Biol. 2019, 174, 372–387. [Google Scholar] [CrossRef]
- Habili, N.; Kominek, P.; Little, A. Grapevine leafroll-associated virus 1 as a common grapevine pathogen. Plant Viruses 2007, 1, 63–68. [Google Scholar]
- Hayes, A.; Neeman, T.; Cooper, P.D. Overwintering survival of grapevine scale Parthenolecanium persicae (Hemiptera: Coccidae) in the Canberra region of Australia. Aust. Entomol. 2019, 58, 346–353. [Google Scholar] [CrossRef]
- Simbiken, N.A. Feeding and Ecology of Grapevine Scales, Parthenolecanium Persicae and P. pruinosum, on Different Varieties of Grapevine. Master’s Thesis, Australian National University, Canberra, Australia, 2014. [Google Scholar]
- Simbiken, N.A. Development and feeding effect of frosted scale Parthenolecanium pruinosum Cocquillet (Hemiptera: Coccidae) on selected Vitis vinifera L. cultivars. Aust. J. Grape Wine Res. 2015, 21, 451–457. [Google Scholar] [CrossRef]
- Habili, N.; Wu, Q. Simultaneous Transmission of Grapevine Leafroll-Assocaited Virus 3 and Grapevine Virus A by the Scale Insect Parthenolecanium persicae in Australia 2015; Report number: 20151225.3890790. Available online: http://www.promedmail.org/post/3890790 (accessed on 23 May 2020).
- Belli, G.; Fortusini, A.; Casati, P.; Belli, L.; Bianco, P.A.; Prati, S. Transmission of a grapevine leafroll associated closterovirus by the scale insect Pulvinaria vitis L. Riv. Di Patol. Veg. 1994, 4, 105–108. [Google Scholar]
- Fuchs, M.; Martinson, T.E.; Loeb, G.M.; Hoch, H.C. Survey for the three major leafroll disease-associated viruses in Finger Lakes vineyards in New York. Plant Dis. 2009, 93, 395–401. [Google Scholar] [CrossRef]
- Predajna, L.; Glasa, M. Partial Sequence Analysis of Geographically Close Grapevine virus A Isolates Reveals their High Regional Variability and an Intra-Isolate Heterogeneity. J. Phytopathol. 2016, 164, 427–431. [Google Scholar] [CrossRef]
- Glasa, M.; Predajna, L.; Soltys, K.; Sihelska, N.; Nagyova, A.; Wetzel, T.; Sabanadzovic, S. Analysis of Grapevine rupestris stem pitting-associated virus in Slovakia reveals differences in intra-host population diversity and naturally occurring recombination events. Plant Pathol. J. 2017, 33, 34–42. [Google Scholar] [CrossRef][Green Version]
- Hily, J.M.; Beuve, M.; Vigne, E.; Demangeat, G.; Candresse, T.; Lemaire, O. A genome-wide diversity study of grapevine rupestris stem pitting-associated virus. Arch. Virol. 2018, 163, 3105–3111. [Google Scholar] [CrossRef]
- Puckett, J.M.; Al Rwahnih, M.; Klassen, V.; Golino, D. The Davis grapevine virus collection—A current perspective. Proceedings of 19th Congress of ICVG, Santiago, Chile, 9–12 April 2018. [Google Scholar]
- Krake, L.R.; Scott, N.S.; Rezaian, M.A.; Taylor, R.H. Graft-Transmitted Diseases of Grapevines; CSIRO Publishing: Melbourne, Australia, 1999. [Google Scholar]
- Martelli, G.P. Directory of virus and virus-like diseases of the grapevine and their agents. J. Plant Pathol. 2014, 96, 1–136. [Google Scholar]
- Constable, F.E.; Connellan, J.; Nicholas, P.; Rodoni, B.C. Comparison of enzyme-linked immunosorbent assays and reverse transcription-polymerase chain reaction for the reliable detection of Australian grapevine viruses in two climates during three growing seasons. Aust. J. Grape Wine Res. 2012, 18, 239–244. [Google Scholar] [CrossRef]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine leafroll disease and associated viruses: A unique pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Habili, N.; Randles, J.W. Descriptors for Grapevine virus A-associated syndrome in Shiraz, Merlot and Ruby Cabernet in Australia, and its similarity to Shiraz Disease in South Africa. Aust. New Zealand Grapegrow. Winemak. 2004, 488, 71–74. [Google Scholar]
- Goszczynski, D.E.; du Preez, J.; Burger, J.T. Molecular divergence of Grapevine virus A (GVA) variants associated with Shiraz disease in South Africa. Virus Res. 2008, 138, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Saldarelli, P.; Giampetruzzi, A.; Maree, H.J.; Al Rwahnih, M. High-Throughput Sequencing: Advantages beyond virus identification. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 625–642. [Google Scholar]
- Maree, H.J.; Almeida, R.P.P.; Bester, R.; Chooi, K.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.C.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lara, A.; Klaassen, V.; Stevens, K.; Sudarshana, M.R.; Rowhani, A.; Maree, H.J.; Chooi, K.M.; Blouin, A.G.; Habili, N.; Song, Y.; et al. Characterization of grapevine leafroll-associated virus 3 genetic variants and application towards RT-qPCR assay design. PLoS ONE 2018, 13, e208862. [Google Scholar] [CrossRef] [PubMed]
- Endeshaw, S.T.; Sabbatini, P.; Romanazzi, G.; Schilder, A.C.; Neri, D. Effects of grapevine leafroll associated virus 3 infection on growth, leaf gas exchange, yield and basic fruit chemistry of Vitis vinifera L. cv. Cabernet Franc. Sci. Hortic. 2014, 170, 228–236. [Google Scholar] [CrossRef]
- Martinson, T.E.; Fuchs, M.; Loeb, G.; Hoch, H.C. Grapevine leafroll: An increasing problem in the Finger Lakes, the US and the world. Finger Lakes Vineyard Notes 2008, 6, 6–11. [Google Scholar]
- Guidoni, S.; Mannini, F.; Ferrandino, A.; Argamante, N.; Di Stefano, R. Effect of virus status on leaf and berry phenolic compounds in two wine grapevine vitis vinifera cultivars. Acta Hortic. 2000, 445–452. [Google Scholar] [CrossRef]
- Komar, V.; Vigne, E.; Demangeat, G.; Lemaire, O.; Fuchs, M. Comparative performance of virus-infected Vitis vinifera cv. Savagnin rose grafted onto three rootstocks. Am. J. Enol. Vitic. 2010, 61, 68–73. [Google Scholar]
- Kovacs, L.G.; Hanami, H.; Fortenberry, M.; Kaps, M.L. Latent infection by leafroll agent GLRaV-3 is linked to lower fruit quality in French-American hybrid grapevines Vidal blanc and St Vincent. Am. J. Enol. Vitic. 2001, 52, 254–259. [Google Scholar]
- Lee, J.M.; Martin, R.R. Influence of grapevine leafroll associated viruses (GLRaV-2 and -3) on the fruit composition of Oregon Vitis vinifera L. cv. Pinot noir: Phenolics. Food Chem. 2009, 112, 889–896. [Google Scholar] [CrossRef]
- Tomazic, I.; Korosec-Koruza, Z.; Petrovic, N. Sanitary status of Slovenian indigenous grapevine cultivar refosk. J. Int. Sci. Vigne Vin. 2005, 39, 19–22. [Google Scholar] [CrossRef]
- Credi, R.; Babini, A.R. Effect of virus and virus-like infections on the growth of grapevine rootstocks. Adv. Hortic. Sci. 1996, 10, 95–98. [Google Scholar]
- Vega, A.; Gutierrez, R.A.; Pena-Neira, A.; Cramer, G.R.; Arce-Johnson, P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar] [CrossRef]
- Flamini, R.; Vedova, A.D.; Panighel, A.; Biscaro, S.; Borgo, M.; Calo, A. Characterization of Torbato (V. vinifera) aroma and study of leaf-roll effects on the grape aroma compounds Caratterizzazione aromatica del Torbato (V. vinifera) e studio degli effetti dell’accartocciamento fogliare sui composti aromatici delle sue uve. Riv. Vitic. Enol. 2006, 59, 13–26. [Google Scholar]
- Bertamini, M.; Malossini, U.; Krishnasamy, M.; Namachevayam, N.; Muthuchelian, K.; Nedunchezhian, N. Physiological response of field grown grapevine (Vitis vinifera L. cv. Marzemino) to grapevine leafroll-associated virus (GLRaV-1). Phytopathol. Mediterr. 2005, 44, 256–265. [Google Scholar]
- Clingeleffer, P.R.; Krake, L.R. Light (minimal) pruning enhances expression of higher yield from clones of Vitis vinifera L cv Sultana following thermotherapy for virus attenuation. Aust. J. Grape Wine Res. 2002, 8, 95–100. [Google Scholar] [CrossRef]
- Brar, H.S.; Zora, S.; Swinny, E.; Cameron, I.; Singh, Z. Girdling and grapevine leafroll associated viruses affect berry weight, colour development and accumulation of anthocyanins in ‘Crimson Seedless’ grapes during maturation and ripening. Plant Sci. 2008, 175, 885–897. [Google Scholar] [CrossRef]
- Cameron, I.J. Emperor clonal selection and the effect of leafroll virus on table grape quality. In Proceedings of the First Table Grape Industry Technical Conference; Department of Agriculture: Mildura, VIC, Australia, 1984; pp. 43–47. [Google Scholar]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Webb, L.B.; Whetton, P.H.; Bhend, J.; Darbyshire, R.; Briggs, P.R.; Barlow, E.W.R. Earlier wine-grape ripening driven by climatic warming and drying and management practices. Nat. Clim. Chang. 2012, 2, 259–264. [Google Scholar] [CrossRef]
- Scholefield, P.; Morison, J. Assessment of Economic Cost of Endemic Pests and Diseases on the Australian Grape and Wine Industry; Final Report to the GWRDC, Project GWR 08/04; 2010. Available online: https://www.wineaustralia.com/getmedia/3ca22df5-54be-4c82-a711-bcf8d00ed698/GWR-08-04 (accessed on 24 June 2020).
- Atallah, S.S.; Gomez, M.I.; Conrad, J.M. Specification of Spatial-Dynamic Externalities and Implications for Strategic Behavior in Disease Control. Land Econ. 2017, 93, 209–229. [Google Scholar] [CrossRef]
- Naidu, R.A. Grapevine leafroll-associated virus 1. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 127–139. [Google Scholar] [CrossRef]
- Lucchi, A.; Ricciardi, R.; Benelli, G.; Bagnoli, B. What do we really know on the harmfulness of Cryptoblabes gnidiella (Milliere) to grapevine? From ecology to pest management. Phytoparasitica 2019, 47, 1–15. [Google Scholar] [CrossRef]
- Martinson, T.; Williams III, L.; English-Loeb, G. Compatibility of chemical disease and insect management practices used in New York vineyards with biological control by Anagrus spp.(Hymenoptera: Mymaridae), parasitoids of Erythroneura leafhoppers. Biol. Control. 2001, 22, 227–234. [Google Scholar] [CrossRef][Green Version]
- Rakimov, A.; Hoffmann, A.A.; Malipatil, M.B. Natural enemies of soft scale insects (Hemiptera: Coccoidea: Coccidae) in Australian vineyards. Aust. J. Grape Wine Res. 2015, 21, 302–310. [Google Scholar] [CrossRef]
- Cotsaris, D.; Burne, P.; Pietsch, A. Vineyard Scale. In CCW Factsheet No. 3.; CCW Cooperative, Ltd.: Berri, SA, Australia, 2009; Available online: http://www.ccwcoop.com.au/__files/f/4381 (accessed on 12 September 2019).
- Pietersen, G.; Spreeth, N.; Oosthuizen, T.; Rensburg, A.v.; Rensburg, M.v.; Lottering, D.; Rossouw, N.; Tooth, D.; van Rensburg, A.; van Rensburg, M. Control of grapevine leafroll disease spread at a commercial wine estate in South Africa: A case study. Am. J. Enol. Vitic. 2013, 64, 296–305. [Google Scholar] [CrossRef]
- Daane, K.M.; Bentley, W.J.; Walton, V.; Malakar-Kuenen, R.; Millar, J.G.; Ingels, C.; Weber, E.; Gispert, C. New controls investigated for vine mealybug. Calif. Agric. 2006, 60, 31–38. [Google Scholar] [CrossRef]
- Mani, M.; Shivaraju, C. Mealybugs and Their Management in Agricultural and Horticultural Crops; Springer: New Delhi, India, 2016. [Google Scholar]
- Bonfiglioli, R.; Hoskins, N. Managing virus in New Zealand vineyards. Aust. New Zealand Grapegrow. Winemak. 2006, 45, 43–46. [Google Scholar]
- Bell, V.A.; Hedderley, D.I.; Pietersen, G.; Lester, P.J. Vineyard-wide control of grapevine leafroll-associated virus 3 requires an integrated response. J. Plant Pathol. 2018, 100, 399–408. [Google Scholar] [CrossRef]
- Atallah, S.S.; Gomez, M.I.; Conrad, J.M.; Nyrop, J.P. A plant-level, spatial, bioeconomic model of plant disease diffusion and control: Grapevine leafroll disease. Am. J. Agric. Econ. 2015, 97, 199–218. [Google Scholar] [CrossRef]
- Meng, B.; Rowhani, A. Grapevine ruperstris stem pitting-associated virus. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 257–287. [Google Scholar]
- Reynolds, A.G.; Lanterman, W.S.; Wardle, D.A. Yield and berry composition of five Vitis cultivars as affected by rupestris stem pitting virus. Am. J. Enol. Vitic. 1997, 48, 449–458. [Google Scholar]
- Naidu, R.A.; Perry, E.M.; Pierce, F.J.; Mekuria, T. The potential of spectral reflectance technique for the detection of Grapevine leafroll-associated virus-3 in two red-berried wine grape cultivars. Comput. Electron. Agric. 2009, 66, 38–45. [Google Scholar] [CrossRef]
- Pagay, V.; Habili, N.; Wu, Q.; Coleman, D. Rapid and non-destructive detection of Shiraz Disease and Grapevine Leafroll Disease on asymptomatic grapevines in Australian vineyards. In Proceedings of the 19th Congress of ICVG, Santiago, Chile, 9–12 April 2018. [Google Scholar]
- MacDonald, S.L.; Staid, M.; Staid, M.; Cooper, M.L. Remote hyperspectral imaging of grapevine leafroll-associated virus 3 in cabernet sauvignon vineyards. Comput. Electron. Agric. 2016, 130, 109–117. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Navarro, B.; Di Serio, F.; Stevens, K.; Hwang, M.S.; Kohl, J.; Vu, S.T.; Falk, B.W.; Golino, D.; Al Rwahnih, M. Two novel negative-sense RNA viruses infecting grapevine are members of a newly proposed genus within the family Phenuiviridae. Viruses 2019, 11, 685. [Google Scholar] [CrossRef]
| Family/Genome | Genus | Species 1 | Associated Disease | Vector |
|---|---|---|---|---|
| Betaflexiviridae Monopartite linear ssRNA (+) | Vitivirus | grapevine virus A (GVA) | Shiraz Disease, Kober Stem Grooving | Mealybug/scale |
| grapevine virus B (GVB) | Corky Bark | Mealybug/scale | ||
| Foveavirus | grapevine rupestris stem pitting associated virus (GRSPaV) | Asymptomatic in most, stem pitting | Unknown | |
| Trichovirus | grapevine Pinot gris virus (GPGV) | Leaf mottling and deformation, symptomless | Colomerus vitis | |
| Closteroviridae Monopartite linear ssRNA (+) | Ampelovirus—Subgroup I | grapevine leafroll associated virus (GLRaV)-1 and GLRaV-3 | Leafroll disease | Mealybug/scale |
| Ampelovirus—Subgroup II | GLRaV-4 and its strains: 5, 6, 9 2 | Leafroll disease | Mealybug/scale | |
| Closterovirus | GLRaV-2 | Leafroll disease, Graft incompatibility | Unknown | |
| Secoviridae Bipartite, linear ssRNA (+) | Nepovirus | grapevine fanleaf virus (GFLV) 3 | Fanleaf, degeneration, decline, chlorosis | Xiphinema index; X. diversicaudatum |
| Tymoviridae Monopartite, linear ssRNA (+) | Maculavirus | grapevine fleck virus (GFkV) | Fleck on V. rupestris, Asymptomatic in other Vitis sp. | Unknown |
| Marafivirus | grapevine rupestris vein feathering virus (GRVFV) | Asymptomatic | Unknown |
| Mealybugs | Common Name | Transmitted Viruses | Presence in Australia | References |
|---|---|---|---|---|
| Ferrisia gilli (Gullan) | Gill’s mealybug | GLRaV-3,4 | No | [29] |
| Heliococcus bohemicus | Bohemian mealybug | GLRaV-1,3; GVA | No | [30,31,32,33] |
| Phenacoccus aceris | Apple mealybug | GLRaV-1,3,4; GVA; GVB | No | [26,32,34] |
| Planococcus citri | Citrus mealybug | GLRaV-1,3; GVA | Yes | [31,35,36] |
| Planococcus ficus | Grapevine mealybug | GLRaV-1,3, 4; GVA | No | [19,31,37,38] |
| Pseudococcus viburni | Obscure mealybug, tuber mealybug | GLRaV-3; GVA; GVB | Yes | [39] |
| Pseudococcus comstocki | Comstock mealybug | GVE | No | [40] |
| Pseudococcus maritimus | Grape mealybug | GLRaV-1,3 | No | [41] |
| Pseudococcus calceolariae | Citrophilus mealybug, scarlet mealybug | GLRaV-3 | Yes | [42] |
| Pseudococcus longispinus | Long-tailed mealybug | GLRaV-1,3; GVA | Yes | [22,42,43] |
| Scale Insects | Common Name | Transmitted Viruses | Presence in Australia | References |
|---|---|---|---|---|
| Ceroplastes rusci | Fig wax scale | GLRaV-3,4 strains 5 | Yes | [37] |
| Coccus hesperidium | Brown soft scale | [17] | ||
| Coccus longulus | Long brown scale | GLRaV-3 | Yes | [20] |
| Parasaissetia nigra | Nigra scale | GLRaV-3 | Yes | [20] |
| Parthenolecanium corni | Brown scale, European fruit lecanium scale | GLRaV-1,3; GVA | Yes | [32,34,48,54,55] |
| Parthenolecanium persicae | Grapevine scale | GLRaV-3; GVA | Yes | [56,57,58,59] |
| Parthenolecanium pruinosum | Frosted scale | Unknown | Yes | [57,58] |
| Pulvinaria vitis* | Wooly vine scale | GLRaV-3 | Yes | [60] |
| Pulvinaria innumerabilis | Wooly maple scale | GLRaV-1,3 | No | [61] |
| Neopulvinaria innumerabilis* | Soft scale | GLRaV-1 | Yes | [55] |
| Saissetia sp. | Soft scale | GLRaV-3 | Yes | [20] |
| Cultivar | Region | Sampling Year | Sample ID | Isolate Name | SD Symptoms | Viruses Identified | GVA Group |
|---|---|---|---|---|---|---|---|
| Shiraz | Barossa Valley | 2018 | BV1 | Isolate 1 | No | GRSPaV, GRVFV, GLRaV-1, GVA, GYSVd-1, HSVd | I |
| Shiraz | Barossa Valley | 2018 | BV4 | Isolate 2 | No | GRSPaV, GRVFV, GYSVd-1, HSVd | - 1 |
| Shiraz | Langhorne Creek | 2018 | LC1 | Isolate 1 | Yes | GRSPaV, GVA, GLRaV-9, GRVFV, GYSVd-1, HSV | II |
| Shiraz | Langhorne Creek | 2018 | LC16 | Isolate 2 | No | GRSPaV, GRVFV, GYSVd-1, HSVd | - 1 |
| Malbec | Padthaway | 2016 | Malbec | Malbec-Richter 2 | Yes | GVA, GLRaV-3 (4 and its strains 5, 6 & 9), GRSPaV | II |
| Virus | Variety/Rootstock | Sample ID (Isolate) | Accession# | Sequence Length (bp) | Location | Symptom on Shiraz | Group |
|---|---|---|---|---|---|---|---|
| GVA | Shiraz | BV1-1 | MT070961 | 2751 | Barossa Valley | None | I |
| GVA | Shiraz | BV1-2 | MT070960 | 597 | Barossa Valley | None | I |
| GVA | Shiraz | LC1-1 | MT070963 | 7363 | Langhorne Creek | SD | II |
| GVA | Shiraz | LC1-2 | MT070962 | 7052 | Langhorne Creek | SD | II |
| GVA | Malbec on Richter | Malbec-Richter | MT070959 | 598 | Padthaway | SD | II |
| GLRaV-3 | Shiraz on Ramsey | R3ShRam | MN984352 | 942 | Riverland | SD | V |
| GLRaV-3 | Shiraz on Ramsey | R4ShRam | MN984353 | 934 | Riverland | SD | I |
| GLRaV-3 | Shiraz on 101-14 | R8Sh101 | MN984354 | 941 | Riverland | SD | I |
| GLRaV-3 | Malbec on Richter | Malbec-Richter | N/A | N/A | Padthaway | SD | I 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Habili, N.; Constable, F.; Al Rwahnih, M.; Goszczynski, D.E.; Wang, Y.; Pagay, V. Virus Pathogens in Australian Vineyards with an Emphasis on Shiraz Disease. Viruses 2020, 12, 818. https://doi.org/10.3390/v12080818
Wu Q, Habili N, Constable F, Al Rwahnih M, Goszczynski DE, Wang Y, Pagay V. Virus Pathogens in Australian Vineyards with an Emphasis on Shiraz Disease. Viruses. 2020; 12(8):818. https://doi.org/10.3390/v12080818
Chicago/Turabian StyleWu, Qi, Nuredin Habili, Fiona Constable, Maher Al Rwahnih, Darius E. Goszczynski, Yeniu Wang, and Vinay Pagay. 2020. "Virus Pathogens in Australian Vineyards with an Emphasis on Shiraz Disease" Viruses 12, no. 8: 818. https://doi.org/10.3390/v12080818
APA StyleWu, Q., Habili, N., Constable, F., Al Rwahnih, M., Goszczynski, D. E., Wang, Y., & Pagay, V. (2020). Virus Pathogens in Australian Vineyards with an Emphasis on Shiraz Disease. Viruses, 12(8), 818. https://doi.org/10.3390/v12080818







