Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viruses
2.3. Antibodies and Reagents
2.4. Cell Viability Assay
2.5. Immunofluorescence Microscopy
2.6. β-Galactosidase Assay
2.7. Electron Microscopy
2.8. Immunoblot Analysis
2.9. Real-Time Quantitative RT-PCR Assay
2.10. Statistical Analysis
3. Results
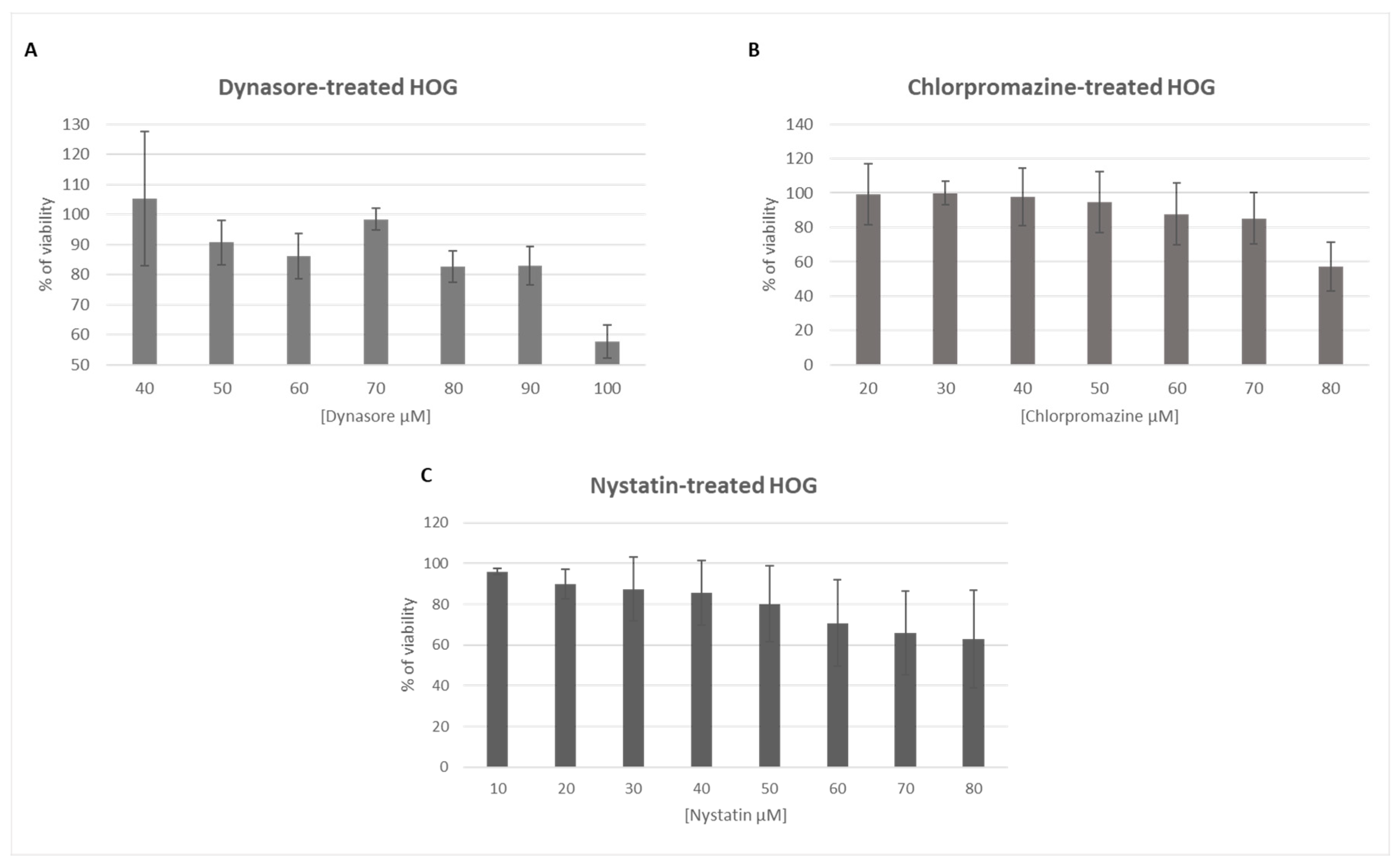
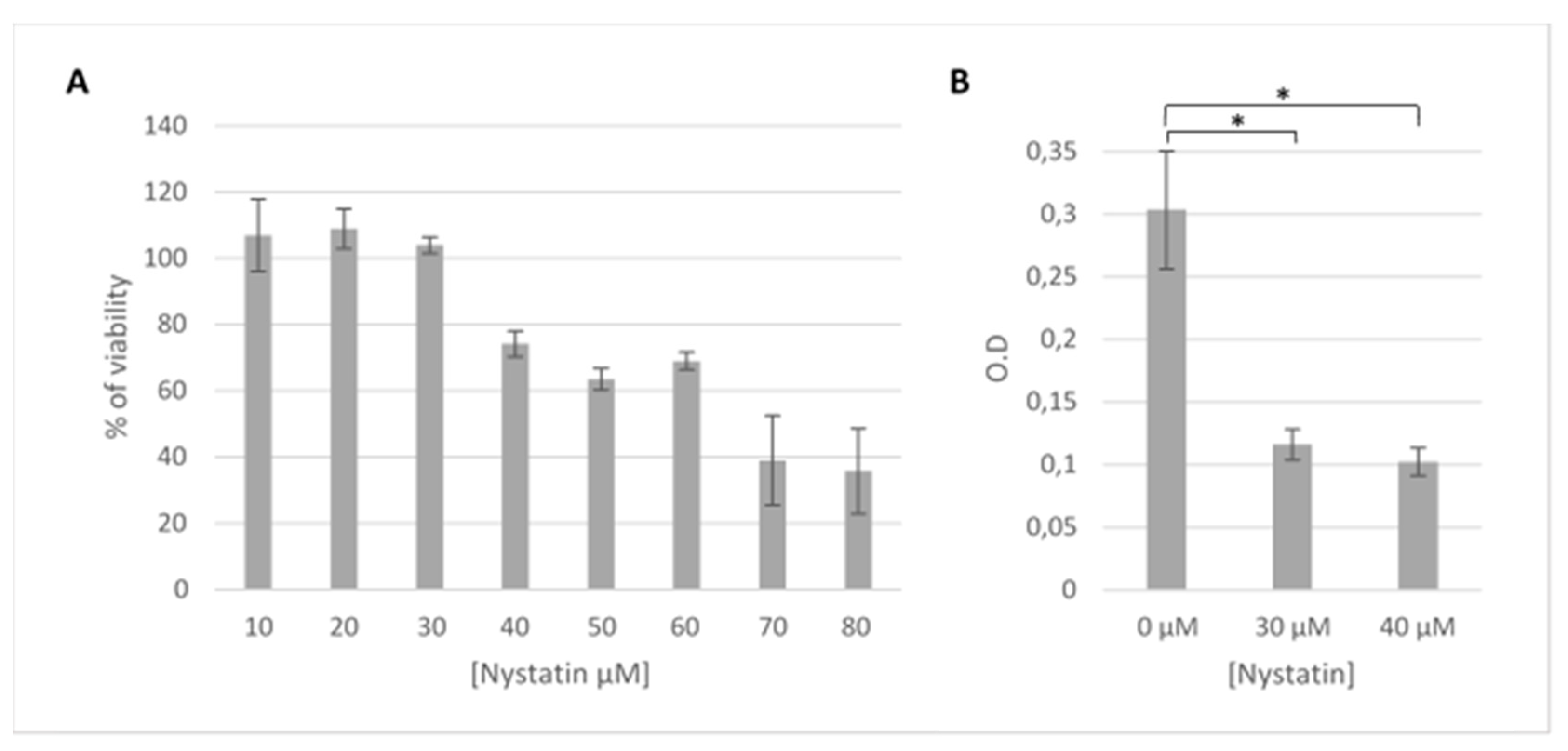
3.1. The Endocytosis Inhibitors Dynasore, Chlorpromazine and Nystatin are Nontoxic in HOG Cells at Established Concentrations
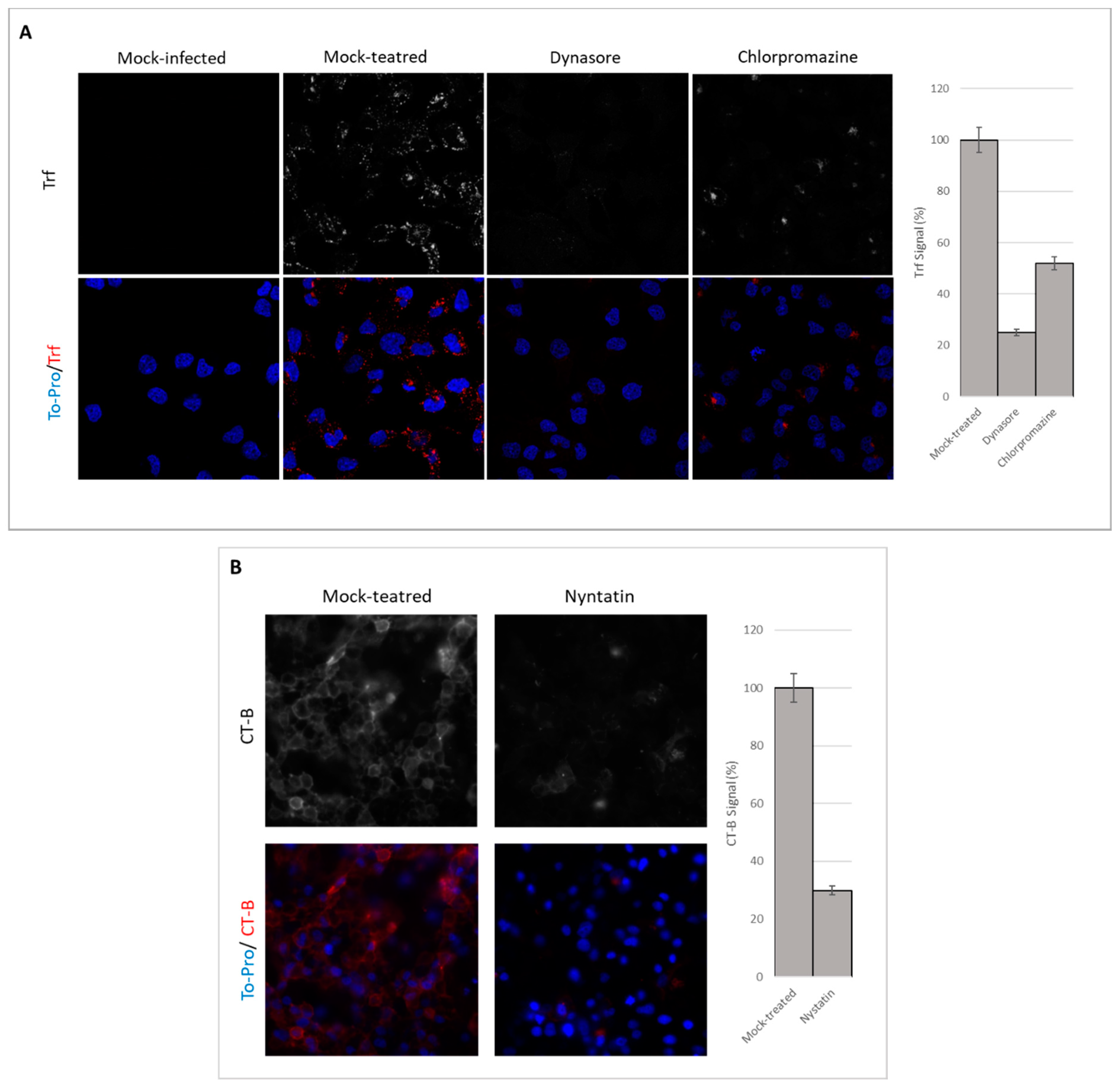
3.2. Endocytosis Assay
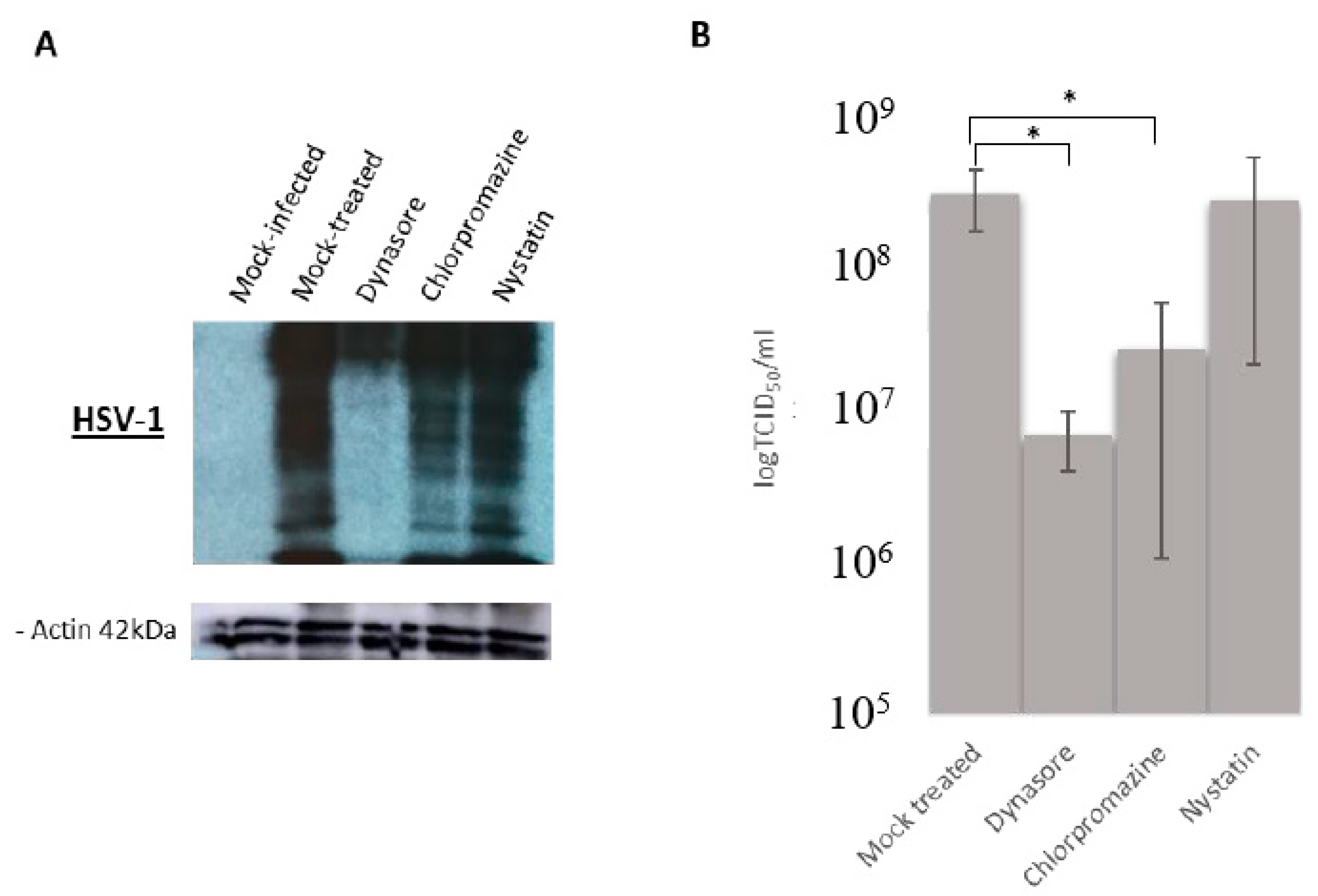
3.3. Effect of Endocytosis Inhibition on HSV-1 Infection
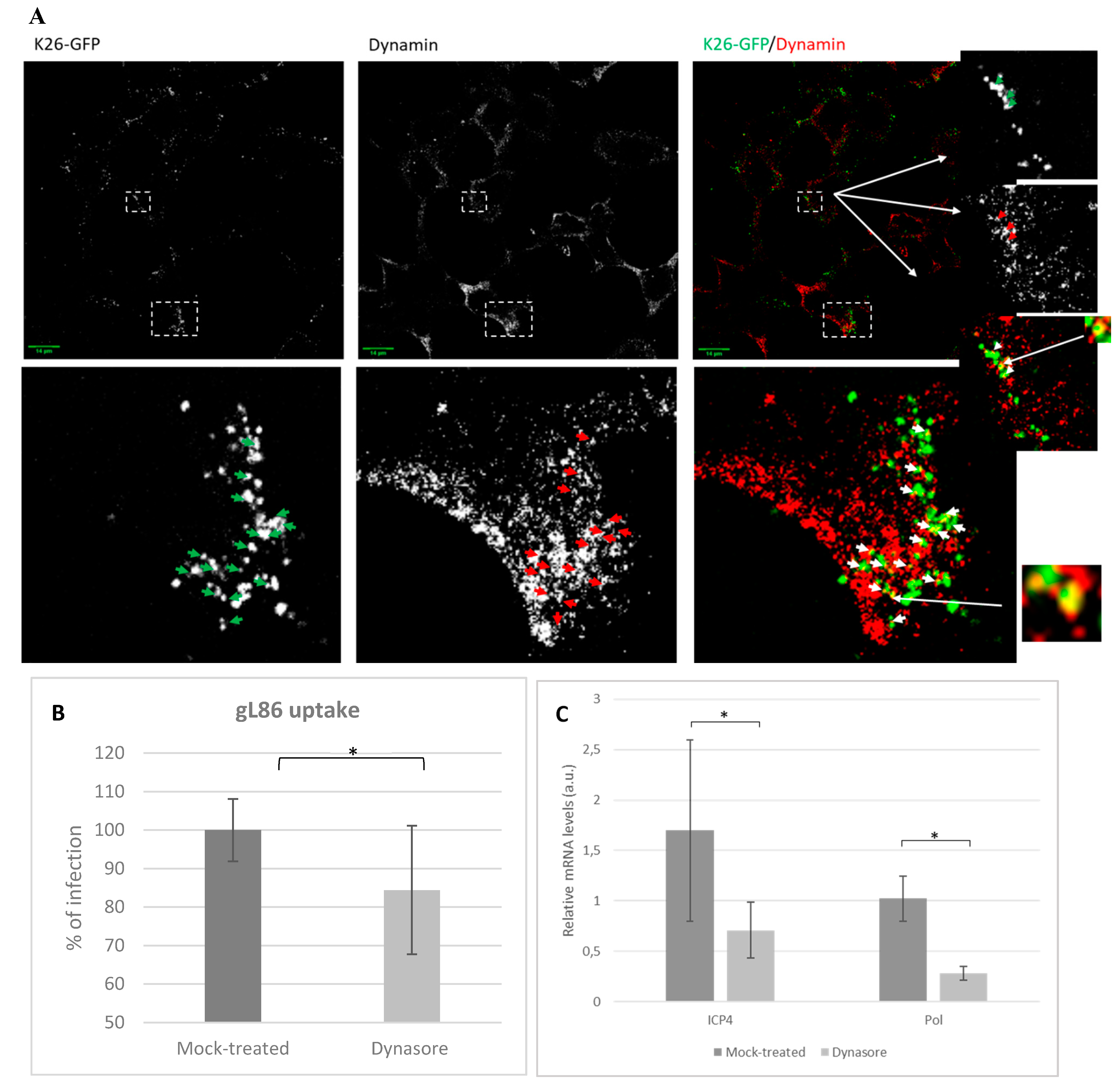
3.4. Role of Dynamin in HSV-1 Infections
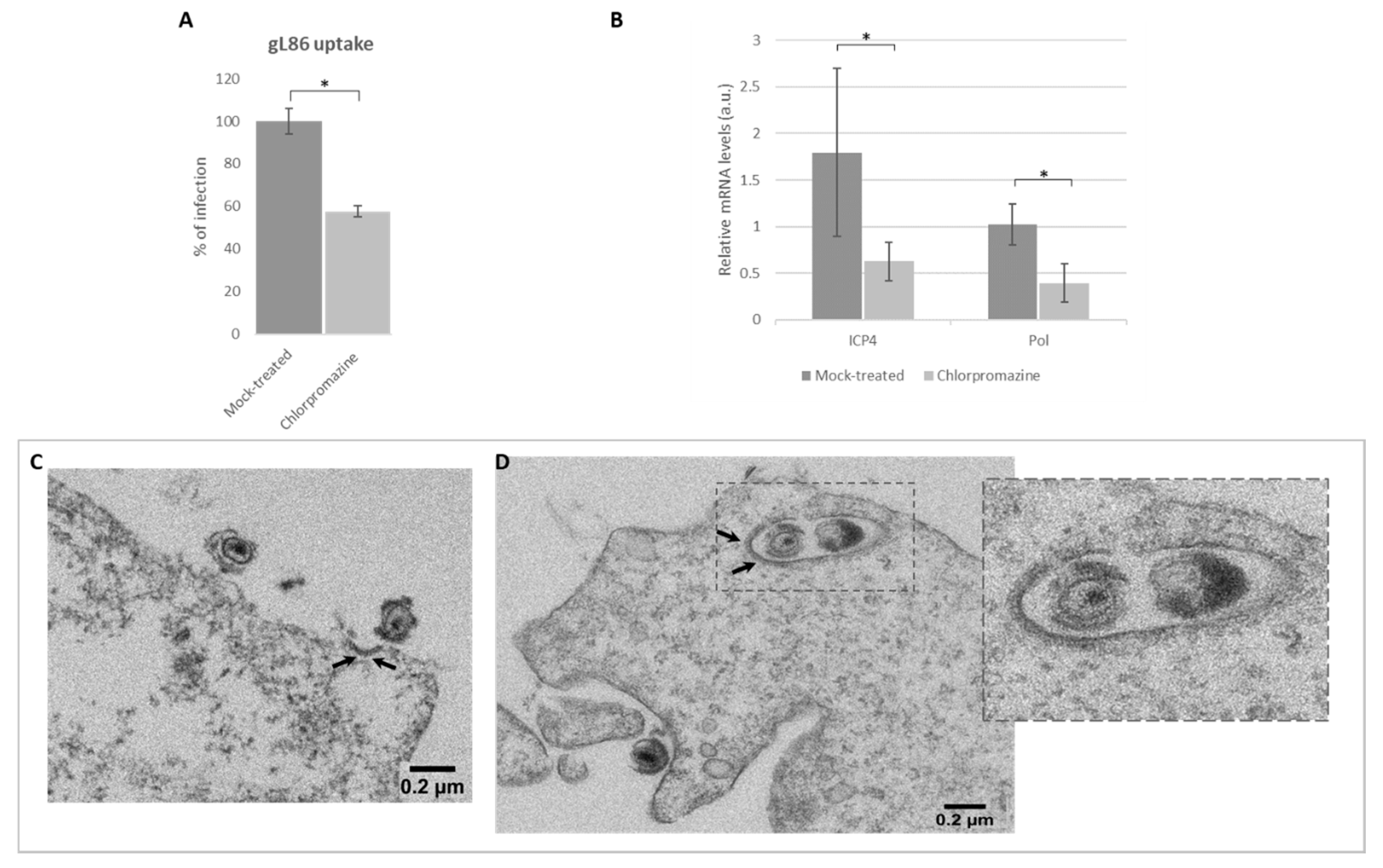
3.5. Role of Clathrin in Viral Infection by Endocytosis
3.6. HSV-1 Entry by Lipid Raft-Mediated Endocytosis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wutzler, P.; Doerr, H.W.; Farber, I.; Eichhorn, U.; Helbig, B.; Sauerbrei, A.; Brandstadt, A.; Rabenau, H.F. Seroprevalence of Herpes Simplex Virus Type 1 and Type 2 in Selected German Populations-Relevance for the Incidence of Genital Herpes. J. Med. Virol. 2000, 61, 201–207. [Google Scholar] [CrossRef]
- World Health Organization. Herpes Simplex Virus. Available online: http://www.who.int/mediacentre/factsheets/fs400/en/ (accessed on 1 March 2020).
- Karasneh, G.A.; Shukla, D. Herpes Simplex Virus Infects most Cell Types in Vitro: Clues to its Success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.S.; Nicola, A.V.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H. Glycoprotein D Receptor-Dependent, Low-pH-Independent Endocytic Entry of Herpes Simplex Virus Type 1. J. Virol. 2005, 79, 6655–6663. [Google Scholar] [CrossRef] [PubMed]
- Sayers, C.L.; Elliott, G. Herpes Simplex Virus 1 Enters Human Keratinocytes by a Nectin-1-Dependent, Rapid Plasma Membrane Fusion Pathway that Functions at Low Temperature. J. Virol. 2016, 90, 10379–10389. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Shukla, D. Viral Entry Mechanisms: Cellular and Viral Mediators of Herpes Simplex Virus Entry. FEBS J. 2009, 276, 7228–7236. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.; Knipe, D.; Howley, P. Fields Virology, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2479–2602. [Google Scholar]
- Sathiyamoorthy, K.; Chen, J.; Longnecker, R.; Jardetzky, T.S. The COMPLEXity in Herpesvirus Entry. Curr. Opin. Virol. 2017, 24, 97–104. [Google Scholar] [CrossRef]
- El-Sayed, A.; Harashima, H. Endocytosis of Gene Delivery Vectors: From Clathrin-Dependent to Lipid Raft-Mediated Endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms 2019, 7, 429. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus Entry by Endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Barrow, E.; Nicola, A.V.; Liu, J. Multiscale Perspectives of Virus Entry Via Endocytosis. Virol. J. 2013, 10, 1–11. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Zhao, M.; Liu, E.; Duan, M.; Guan, Z.; Guo, Y.; Zhang, M. Entry of Challenge Virus Standard (CVS) -11 into N2a Cells Via a Clathrin-Mediated, Cholesterol-, Dynamin-, pH-Dependent Endocytic Pathway. Virol. J. 2019, 16, 80. [Google Scholar] [CrossRef]
- Mockel, M.; Rahn, E.; de la Cruz, N.; Wirtz, L.; van Lent, J.W.M.; Pijlman, G.P.; Knebel-Morsdorf, D. Herpes Simplex Virus 1 can Enter Dynamin 1 and 2 Double-Knockout Fibroblasts. J. Virol. 2019, 93, e00704-19. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.J.; Liu, C.C.; Zhou, J.; Wang, S.Q.; Gao, Z.C.; Zhang, X.M.; Zhou, B.; Chen, P.Y. Entry of Classical Swine Fever Virus into PK-15 Cells Via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway that Requires Rab5 and Rab7. J. Virol. 2016, 90, 9194–9208. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Schmid, S.L. The Dynamin Superfamily. Curr. Biol. 2018, 28, R411–R416. [Google Scholar] [CrossRef]
- Sonnino, S.; Prinetti, A. Membrane Domains and the "Lipid Raft" Concept. Curr. Med. Chem. 2013, 20, 4–21. [Google Scholar]
- Garcin, P.O.; Pante, N. The Minute Virus of Mice Exploits Different Endocytic Pathways for Cellular Uptake. Virology 2015, 482, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, P.; Nabi, I.R. Lipid Rafts, Caveolae, and their Endocytosis. Int. Rev. Cell. Mol. Biol. 2010, 282, 135–163. [Google Scholar] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Post, G.R.; Dawson, G. Characterization of a Cell Line Derived from a Human Oligodendroglioma. Mol. Chem. Neuropathol. 1992, 16, 303–317. [Google Scholar] [CrossRef]
- Desai, P.; Person, S. Incorporation of the Green Fluorescent Protein into the Herpes Simplex Virus Type 1 Capsid. J. Virol. 1998, 72, 7563–7568. [Google Scholar] [CrossRef]
- Montgomery, R.I.; Warner, M.S.; Lum, B.J.; Spear, P.G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 1996, 87, 427–436. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Crespillo, A.J.; Praena, B.; Tabares, E.; Revilla, Y.; Garcia, E.; Fraile-Ramos, A.; Baron, W.; Krummenacher, C.; Lopez-Guerrero, J.A. Role of Proteolipid Protein in HSV-1 Entry in Oligodendrocytic Cells. PLoS ONE 2016, 11, e0147885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bello-Morales, R.; López-Guerrero, J.A. Extracellular Vesicles in Herpes Viral Spread and Immune Evasion. Front. Microbiol. 2018, 9, 2572. [Google Scholar] [CrossRef] [PubMed]
- Praena, B.; Bello-Morales, R.; de Castro, F.; López-Guerrero, J.A. Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibit HSV-1 infection in oligodendrocytes. Antiviral Res. 2019, 168, 91–99. [Google Scholar] [CrossRef]
- Crespillo, A.J.; Praena, B.; Bello-Morales, R.; Lerma, L.; Vázquez-Calvo, A.; Martín-Acebes, M.A.; Tabarés, E.; Sobrino, F.; López-Guerrero, J.A. Inhibition of herpes virus infection in oligodendrocyte cultured cells by valproic acid. Virus Res. 2016, 214, 71–79. [Google Scholar] [CrossRef]
- Hanover, J.A.; Beguinot, L.; Willingham, M.C.; Pastan, I.H. Transit of Receptors for Epidermal Growth Factor and Transferrin through Clathrin-Coated Pits. Analysis of the Kinetics of Receptor Entry. J. Biol. Chem. 1985, 260, 15938–15945. [Google Scholar]
- Montesano, R.; Roth, J.; Robert, A.; Orci, L. Non-Coated Membrane Invaginations are Involved in Binding and Internalization of Cholera and Tetanus Toxins. Nature 1982, 296, 651–653. [Google Scholar] [CrossRef]
- Chen, S.; He, H.; Yang, H.; Tan, B.; Liu, E.; Zhao, X.; Zhao, Y. The Role of Lipid Rafts in Cell Entry of Human Metapneumovirus. J. Med. Virol. 2019, 91, 949–957. [Google Scholar] [CrossRef]
- Zhao, T.; Cui, L.; Yu, X.; Zhang, Z.; Shen, X.; Hua, X. Entry of Sapelovirus into IPEC-J2 Cells is Dependent on Caveolae-Mediated Endocytosis. Virol. J. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Matute, C. Pío del Río Hortega and the discovery of the oligodendrocytes. Front Neuroanat. 2015, 9, 92. [Google Scholar] [CrossRef]
- Snaidero, N.; Simons, M. The logistics of myelin biogenesis in the central nervous system. Glia 2017, 65, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Tognatta, R.; Miller, R.H. Contribution of the oligodendrocyte lineage to CNS repair and neurodegenerative pathologies. Neuropharmacology 2016, 110 Pt B, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Fedetz, M.; Alcina, A.; Tabarés, E.; López-Guerrero, J.A. High susceptibility of a human oligodendroglial cell line to herpes simplex type 1 infection. J. Neurovirol. 2005, 11, 190–198. [Google Scholar] [CrossRef] [PubMed]
- López-Guerrero, J.A.; de la Nuez, C.; Praena, B.; Sánchez-León, E.; Krummenacher, C.; Bello-Morales, R. Herpes Simplex Virus 1 Spread in Oligodendrocytic Cells Is Highly Dependent on MAL Proteolipid. J. Virol. 2020, 94, e01739-19. [Google Scholar]
- Pérez-Núñez, I.; Karaky, M.; Fedetz, M.; Barrionuevo, C.; Izquierdo, G.; Matesanz, F.; Alcina, A. Splice-site variant in ACSL5: A marker promoting opposing effect on cell viability and protein expression. Eur. J. Hum. Genet. 2019, 27, 1836–1844. [Google Scholar] [CrossRef]
- Podbielska, M.; Szulc, Z.M.; Kurowska, E.; Hogan, E.L.; Bielawski, J.; Bielawska, A.; Bhat, N.R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016, 57, 2028–2039. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Crespillo, A.J.; García, B.; Dorado, L.Á.; Martín, B.; Tabarés, E.; Krummenacher, C.; de Castro, F.; López-Guerrero, J.A. The effect of cellular differentiation on HSV-1 infection of oligodendrocytic cells. PLoS ONE 2014, 9, e89141. [Google Scholar] [CrossRef] [PubMed]
- Devadas, D.; Koithan, T.; Diestel, R.; Prank, U.; Sodeik, B.; Dohner, K. Herpes Simplex Virus Internalization into Epithelial Cells Requires Na+/H+ Exchangers and p21-Activated Kinases but neither Clathrin- nor Caveolin-Mediated Endocytosis. J. Virol. 2014, 88, 13378–13395. [Google Scholar] [CrossRef]
- Albecka, A.; Laine, R.F.; Janssen, A.F.; Kaminski, C.F.; Crump, C.M. HSV-1 Glycoproteins are Delivered to Virus Assembly Sites through Dynamin-Dependent Endocytosis. Traffic 2016, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.V.; Hou, J.; Major, E.O.; Straus, S.E. Herpes Simplex Virus Type 1 Enters Human Epidermal Keratinocytes, but Not Neurons, Via a pH-Dependent Endocytic Pathway. J. Virol. 2005, 79, 7609–7616. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T.; Macia, E.; Pelish, H.E. Use of Dynasore, the Small Molecule Inhibitor of Dynamin, in the Regulation of Endocytosis. Methods Enzymol. 2008, 438, 77–93. [Google Scholar] [PubMed]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, D.; Han, K.; Huang, X.; Liu, Y.; Liu, Q.; Yang, J.; Li, S.; Li, Y. Tembusu virus enters BHK-21 cells through a cholesterol-dependent and clathrin-mediated endocytosis pathway. Microb Pathog. 2020, 147, 104242. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.S.; Liu, C.; Liang, X.; Liu, Y.; Chen, J.; Cao, R.; Zhou, B. Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway. Viruses 2019, 11, 1112. [Google Scholar] [CrossRef]
- Inoue, Y.; Tanaka, N.; Tanaka, Y.; Inoue, S.; Morita, K.; Zhuang, M.; Hattori, T.; Sugamura, K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007, 81, 8722–8729. [Google Scholar] [CrossRef]
- Parton, R.G.; Hanzal-Bayer, M.; Hancock, J.F. Biogenesis of Caveolae: A Structural Model for Caveolin-Induced Domain Formation. J. Cell Sci. 2006, 119 Pt 5, 787–796. [Google Scholar] [CrossRef]
- Nicola, A.V. Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH. Traffic 2016, 17, 965–975. [Google Scholar] [CrossRef]
- Mayberry, C.L.; Soucy, A.N.; Lajoie, C.R.; DuShane, J.K.; Maginnis, M.S. JC Polyomavirus Entry by Clathrin-Mediated Endocytosis is Driven by Beta-Arrestin. J. Virol. 2019, 93, e01948-18. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Szczepanski, A.; Milewska, A.; Baster, Z.; Rajfur, Z.; Sarna, M.; Pyrc, K. Early Events during Human Coronavirus OC43 Entry to the Cell. Sci. Rep. 2018, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Petermann, P.; Rahn, E.; Thier, K.; Hsu, M.J.; Rixon, F.J.; Kopp, S.J.; Knebel-Morsdorf, D. Role of Nectin-1 and Herpesvirus Entry Mediator as Cellular Receptors for Herpes Simplex Virus 1 on Primary Murine Dermal Fibroblasts. J. Virol. 2015, 89, 9407–9416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahn, E.; Petermann, P.; Hsu, M.J.; Rixon, F.J.; Knebel-Morsdorf, D. Entry Pathways of Herpes Simplex Virus Type 1 into Human Keratinocytes are Dynamin- and Cholesterol-Dependent. PLoS ONE 2011, 6, e25464. [Google Scholar] [CrossRef] [PubMed]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-Independent Endocytosis: An Increasing Degree of Complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Konopka, C.A.; Schleede, J.B.; Skop, A.R.; Bednarek, S.Y. Dynamin and Cytokinesis. Traffic 2006, 7, 239–247. [Google Scholar] [CrossRef]
- Orth, J.D.; Krueger, E.W.; Cao, H.; McNiven, M.A. The Large GTPase Dynamin Regulates Actin Comet Formation and Movement in Living Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 167–172. [Google Scholar] [CrossRef]
- Xie, Y.; Miao, H.; Blankenship, J.T. Membrane Trafficking in Morphogenesis and Planar Polarity. Traffic 2018. [Google Scholar] [CrossRef]
- Cao, H.; Thompson, H.M.; Krueger, E.W.; McNiven, M.A. Disruption of Golgi Structure and Function in Mammalian Cells Expressing a Mutant Dynamin. J. Cell. Sci. 2000, 113 Pt 11, 1993–2002. [Google Scholar]
- Jones, S.M.; Howell, K.E.; Henley, J.R.; Cao, H.; McNiven, M.A. Role of Dynamin in the Formation of Transport Vesicles from the Trans-Golgi Network. Science 1998, 279, 573–577. [Google Scholar] [CrossRef]
- Wang, K.; Huang, S.; Kapoor-Munshi, A.; Nemerow, G. Adenovirus Internalization and Infection Require Dynamin. J. Virol. 1998, 72, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.M.; Leong, K.L.; Ng, M.M.; Chu, J.J. The Essential Role of Clathrin-Mediated Endocytosis in the Infectious Entry of Human Enterovirus 71. J. Biol. Chem. 2011, 286, 309–321. [Google Scholar] [CrossRef]
- Dudleenamjil, E.; Lin, C.Y.; Dredge, D.; Murray, B.K.; Robison, R.A.; Johnson, F.B. Bovine Parvovirus Uses Clathrin-Mediated Endocytosis for Cell Entry. J. Gen. Virol. 2010, 91, 3032–3041. [Google Scholar] [CrossRef]
- Cureton, D.K.; Massol, R.H.; Whelan, S.P.; Kirchhausen, T. The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis. PLoS Pathog. 2010, 6, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Cureton, D.K.; Massol, R.H.; Saffarian, S.; Kirchhausen, T.L.; Whelan, S.P. Vesicular Stomatitis Virus Enters Cells through Vesicles Incompletely Coated with Clathrin that Depend upon Actin for Internalization. PLoS Pathog. 2009, 5, e1000394. [Google Scholar] [CrossRef] [PubMed]
- Johannsdottir, H.K.; Mancini, R.; Kartenbeck, J.; Amato, L.; Helenius, A. Host Cell Factors and Functions Involved in Vesicular Stomatitis Virus Entry. J. Virol. 2009, 83, 440–453. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Liu, S.; Pang, D.W.; Xiao, G. Clathrin-Mediated Endocytosis in Living Host Cells Visualized through Quantum Dot Labeling of Infectious Hematopoietic Necrosis Virus. J. Virol. 2011, 85, 6252–6262. [Google Scholar] [CrossRef]
- Miller, S.E.; Mathiasen, S.; Bright, N.A.; Pierre, F.; Kelly, B.T.; Kladt, N.; Schauss, A.; Merrifield, C.J.; Stamou, D.; Honing, S.; et al. CALM Regulates Clathrin-Coated Vesicle Size and Maturation by Directly Sensing and Driving Membrane Curvature. Dev. Cell. 2015, 33, 163–175. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles Via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Sun, X.; Yau, V.K.; Briggs, B.J.; Whittaker, G.R. Role of Clathrin-Mediated Endocytosis during Vesicular Stomatitis Virus Entry into Host Cells. Virology 2005, 338, 53–60. [Google Scholar] [CrossRef]
- Miller, N.; Hutt-Fletcher, L.M. Epstein-Barr Virus Enters B Cells and Epithelial Cells by Different Routes. J. Virol. 1992, 66, 3409–3414. [Google Scholar] [CrossRef]
- Nanbo, A.; Kachi, K.; Yoshiyama, H.; Ohba, Y. Epstein-Barr Virus Exploits Host Endocytic Machinery for Cell-to-Cell Viral Transmission rather than a Virological Synapse. J. Gen. Virol. 2016, 97, 2989–3006. [Google Scholar] [CrossRef]
- Echarri, A.; Del Pozo, M.A. Caveolae. Curr. Biol. 2012, 22, R114–R116. [Google Scholar] [CrossRef]
- Ewers, H.; Helenius, A. Lipid-Mediated Endocytosis. Cold Spring Harb Perspect. Biol. 2011, 3, a004721. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Chen, Y.; Norkin, L.C. Bound Simian Virus 40 Translocates to Caveolin-Enriched Membrane Domains, and its Entry is Inhibited by Drugs that Selectively Disrupt Caveolae. Mol. Biol. Cell 1996, 7, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Gianni, T.; Gatta, V.; Campadelli-Fiume, G. αVβ3-Integrin Routes Herpes Simplex Virus to an Entry Pathway Dependent on Cholesterol-Rich Lipid Rafts and Dynamin2. Proc. Natl. Acad. Sci. USA 2010, 107, 22260–22265. [Google Scholar] [CrossRef] [PubMed]
- Shawli, G.T.; Adeyemi, O.O.; Stonehouse, N.J.; Herod, M.R. The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus. Viruses 2019, 11, 97. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praena, B.; Bello-Morales, R.; López-Guerrero, J.A. Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin. Viruses 2020, 12, 734. https://doi.org/10.3390/v12070734
Praena B, Bello-Morales R, López-Guerrero JA. Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin. Viruses. 2020; 12(7):734. https://doi.org/10.3390/v12070734
Chicago/Turabian StylePraena, Beatriz, Raquel Bello-Morales, and José Antonio López-Guerrero. 2020. "Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin" Viruses 12, no. 7: 734. https://doi.org/10.3390/v12070734
APA StylePraena, B., Bello-Morales, R., & López-Guerrero, J. A. (2020). Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin. Viruses, 12(7), 734. https://doi.org/10.3390/v12070734





