Abstract
With the frequent outbreaks of emerging infectious diseases in recent years, an effective broad-spectrum antiviral drug is becoming an urgent need for global public health. Cholesterol-25-hydroxylase (CH25H) and its enzymatic products 25-hydroxycholesterol (25HC), a well-known oxysterol that regulates lipid metabolism, have been reported to play multiple functions in modulating cholesterol homeostasis, inflammation, and immune responses. CH25H and 25HC were recently identified as exerting broadly antiviral activities, including upon a variety of highly pathogenic viruses such as human immunodeficiency virus (HIV), Ebola virus (EBOV), Nipah virus (NiV), Rift Valley fever virus (RVFV), and Zika virus (ZIKV). The underlying mechanisms for its antiviral activities are being extensively investigated but have not yet been fully clarified. In this study, we summarized the current findings on how CH25H and 25HC play multiple roles to modulate cholesterol metabolism, inflammation, immunity, and antiviral infections. Overall, 25HC should be further studied as a potential therapeutic agent to control emerging infectious diseases in the future.
1. Introduction
Frequent outbreaks of highly pathogenic viruses are becoming a severe challenge for global public health, and developing novel strategies to control viral infectious diseases is therefore a necessary scientific issue. Increasing studies have demonstrated that the signaling pathway in response to viral infections in immune cells is closely related to cellular metabolism, and there is an emerging field of so-called immune metabolism that has highlighted the dynamic interplay between immune responses and cellular metabolism. The intricacy of immune metabolism has been extensively investigated [1,2]. For example, during viral infections, the host’s metabolism is often hijacked to meet the needs of viral replication, and marked metabolic changes ensure an optimal environment for the generation of viral offspring. Both innate and adaptive immune responses in the host are induced to fight against viral infections [3].
A variety of lipid components play important roles in physiological processes. In addition to acting as structural composition and energy stores [4], lipids also play critical roles as signaling molecules in many physiological and pathophysiological activities. Among them, cholesterol-25-hydroxylase (CH25H) can catalyze cholesterol to produce a kind of oxysterol, 25-hydroxycholesterol (25HC). 25HC and its enzyme CH25H have been shown to coordinately regulate cellular cholesterol metabolism [5]. Interestingly, recent evidence showed that the CH25H gene belongs to the family of interferon-stimulating genes (ISGs), which play key roles in inflammation, innate immunity, and subsequent adaptive immune responses through interferon signaling [6]. Further studies have shown that CH25H and 25HC inhibit a variety of highly pathogenic viruses, such as human immunodeficiency virus (HIV), Ebola virus (EBOV), Nipah virus (NiV), Rift Valley fever virus (RVFV), and Zika virus (ZIKV) [7]. However, the mechanisms of its broadly antiviral activities have not been fully clarified. In this review, we summarize the current findings on how CH25H and 25HC play multifaceted functions that are involved in cellular cholesterol metabolism and immunomodulation and direct antiviral effects. This knowledge is expected to provide insight for developing potentially therapeutic drug candidates to control emerging and re-emerging infectious diseases.
2. CH25H and 25HC in Regulating Cholesterol Metabolism
Cellular cholesterol homeostasis is critical for cell survival and is mainly modulated by two key transcriptional regulators, sterol regulator-binding protein (SREBP) and liver X receptor (LXR). As shown in Figure 1, CH25H and 25HC are traditionally regarded as important regulators that maintain cholesterol homeostasis by inhibiting SREBP and activating LXR.
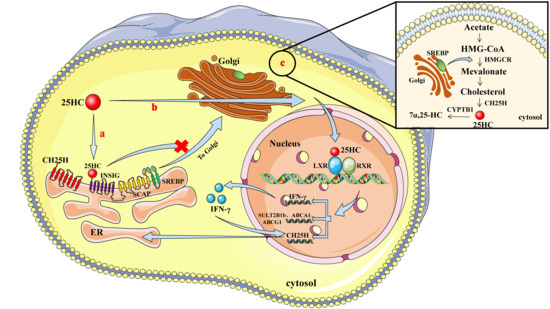
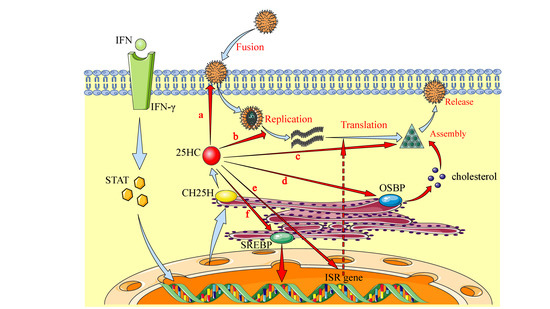
Figure 1.
Patterns illustrating how CH25H and 25HC are involved in cholesterol metabolism. a, Regulation of sterol regulatory element-binding proteins (SREBP) by 25HC. 25HC binds to insulin-induced gene 2 (INSIG2) proteins and forms SREBP/INSIG22/cleavage activator protein (SCAP) complexes that are retained on the endoplasmic reticulum (ER) and therefore block the transportation of SREBP-SCAP complexes to the Golgi apparatus. b, As the ligand of liver X receptor (LXR), 25HC can enter the nucleus to induce the expression of cholesterol-25-hydroxylase (CH25H), cholesterol sulfotransferase-2B1b (SULT2B1b), ATP-binding cassette transporter A1 (ABCA1), ATP-binding cassette transporter G1 (ABCG1), and interferon gamma (IFN-γ), and activated IFN-γ can enhance the expression of CH25H via feedback regulation. c, SREBP promotes the expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and subsequent cholesterol synthesis, while CH25H enzyme converts cholesterol to 25HC. 25HC 7α-hydroxylase (CYP7B1)-mediated hydroxylation contributes to converting 25HC to 7α,25-dihydroxycholesterol (7α,25HC). RXR, retinoid X receptor.
2.1. CH25H and 25HC
CH25H, also known as cholesterol-25-monooxygenase, belongs to the redox enzyme family and consists of 298 and 272 amino acids in human and mouse cells, respectively. CH25H is mainly localized in the endoplasmic reticulum (ER) and Golgi apparatus and catalyzes the oxidation of cholesterol to 25HC [8]. 25HC is a kind of endogenous hydroxysterol that is involved in a variety of metabolic pathways [9]. In normal physiological status, CH25H expression in most organs is low but stable, and reports showed that the level of 25HC in human and mice plasma is approximately 2–30 ng/mL [10]. Interestingly, the CH25H level is significantly upregulated when stimulated by viral infections or Toll-like receptor (TLR) agonists, and the concentration of 25HC in plasma increased to 200 ng/mL in TLR agonist-injected mice [11].
In general, CH25H and 25HC are thought to play critical roles to maintain cholesterol homeostasis. However, there are inconsistent observations between In Vitro and In Vivo studies. For example, CH25H-/- mice maintain intact cholesterol metabolism compared to wild-type mice [12], and patients with abnormally elevated 25HC levels demonstrate normal levels of intoxication and bile acid [13]. One explanation is the presence of an alternative pathway that compensates CH25H-mediated cholesterol metabolism In Vivo. 25HC can also be generated by 27-hydroxylase (CYP27A1), cholesterol 24-hydroxylase (CYP46A1), and Cytochrome P450 3A4 (CYP3A4), although a small proportion [14]. These data contribute to the debate regarding the role of CH25H and 25HC in cholesterol metabolism. Alternatively, recent evidence suggested that CH25H and 25HC might also act as important regulators of inflammation, immunity, and antiviral infections.
2.2. 25HC and SREBP
Cholesterol biosynthesis is regulated by a SREBP, which promotes the gene expression related to the mevalonate pathway, including cholesterol biosynthesis rate-limiting enzyme HMG-CoA reductase (HMGCR) [15].
In the absence of cholesterols, SREBPs bind to SREBP cleavage activator protein (SCAP) and form SREBP-SCAP complexes on ER. SREBP-SCAP complexes are then transported to Golgi apparatus, and cleaved into mature transcription factor forms by site 1 protease (S1P) and site 2 protease (S2P) in Golgi apparatus and then promote cholesterol biosynthesis [16]. 25HC regulates cholesterol metabolism by inhibiting the activities of SREBP [17].
In the presence of excessive cholesterols, 25HC binds to membrane-spanning ER protein—Insulin-induced gene 2 (INSIG2) protein to form SREBP/INSIG2/SCAP complexes, which are retained on ER and cannot transport to Golgi apparatus, leading to dysregulation of intracellular sterol metabolism [18].
2.3. 25HC and LXR
LXR is a transcriptional regulator of the nuclear receptor family and plays an important role in the regulation of cholesterol metabolism [19]. 25HC is reportedly one of the LXR natural ligands and can therefore regulate cholesterol metabolism by activating LXR [20].
The LXR pathway is activated when intracellular cholesterol is abundant to initiate gene expression related to the absorption, degradation, transportation, and excretion of cholesterol. 25HC or other oxysterols induce cholesterol metabolism-related gene expression in an LXR-dependent manner, such as CH25H, cholesterol sulfotransferase-2B1b (SULT2B1b), ATP-binding cassette transporter A1 (ABCA1), and ATP-binding cassette transporter G1 (ABCG1). In addition, 25HC also induces IFN-γ expression in an LXR-dependent manner, and then IFN-γ improves CH25H expression [21], and increased CH25H subsequently promotes the production of 25HC [22]. As a result, 25HC can regulate not only lipid metabolism homeostasis, but also IFN-γ-mediated immune responses through the LXR pathway [23].
3. The CH25H Gene Belongs to the ISG Family
Innate immunity provides the first line of defense against viral pathogens. Innate immune cells recognize pathogen-associated pattern molecules (PAMPs), such as viral double-stranded RNA or unmethylated CpG DNA motifs, through pattern recognition receptors, such as Toll-like receptors (TLRs) and RIG-like receptors (RLRs) [24]. The interferon (IFN) signaling pathways are then rapidly activated involving multiple kinases. IFNs can inhibit viral infections by activating a unique set of IFN-stimulated genes (ISGs) [25]. To date, hundreds of ISGs have been identified, but the antiviral mechanisms for most are not yet fully described [26].
Recent evidence showed that the CH25H gene is one of the ISGs, and many viral infections can induce the upregulation of CH25H in most mammal cells [27,28]. For example, CH25H can be induced by porcine reproductive and respiratory syndrome virus (PRRSV) in Marc-145 monkey kidney cells [29]. Avian leukosis virus subgroup J (ALV-J) upregulates the expression of chicken cholesterol 25-hydroxylase (chCH25H) in chicken peripheral blood mononuclear cells and embryo fibroblast cell lines (DF1) [30]. The level of CH25H in mice bone marrow-derived macrophages (BMDMs) and dendritic cells was upregulated when stimulated with lipopolysaccharide (LPS, TLR4 agonists) and poly I:C (TLR3 agonists) [31]. Our previous data also supported that CH25H expression is interferon-dependent in mice and rhesus monkeys [32]. This might be because both IFN active site and interferon response elements are located upstream of the CH25H open reading frame in the mouse genome, and the CH25H gene is upregulated in response to IFN stimulation [4]. Further studies have shown that TLR-induced CH25H expression in mice BMDMs is mediated by IFNR/JAK/STAT1 signal transduction [31].
However, there is some debate on whether human CH25H gene is an ISG. One study showed that both IFN-α and IFN-γ did not induce CH25H expression in primary human hepatocytes [33], but another report demonstrated that CH25H in primary human hepatocytes was significantly induced by type I interferon [34]. The exact reason for these inconsistent data is unknown and might be related to different concentrations or varying interferon stimulation times used in those studies.
4. Dual Roles of CH25H and 25HC in Augmenting Pro-Inflammation and Suppressing Inflammation
Persistent inflammation and abnormal immune activation are usually accompanied with chronically viral infections, and therefore an ideal antiviral drug should modulate inflammatory responses for immune reconstitution. Based on current literature, 25HC might play a dual role to repress or augment the production of inflammatory cytokines (Figure 2).
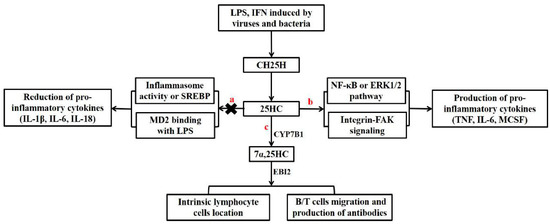
Figure 2.
Regulation of inflammation, innate immunity, and adaptive immunity by CH25H and 25HC. The expression of CH25H, which encodes cholesterol 25-hydroxylase, and the generation of 25HC were enhanced by pathogen-induced interferon (IFN) or lipopolysaccharide (LPS). a, 25HC has anti-inflammatory effects by suppressing SREBP or inflammasome activity and interacting with myeloid differentiation protein 2 (MD2) to prevent the LPS-induced activation of Akt and the NF-κB signaling pathway. b, 25HC acts as an inflammatory amplifier to promote the production of pro-inflammatory cytokines by activating the NF-κB and ERK1/2 pathways. c, Both innate immune cells and adaptive immune cells can be regulated by 7α-25HC through its ligand-G protein coupling receptor Epstein-Barr virus-induced gene 2 (EBI2).
4.1. Augmenting Pro-Inflammation by CH25H and 25HC
Innate immune sensors, such as STING, RIG-I/MDA5, and TLRs, have evolved to detect microbial infections by identifying PAMPs and then trigger the transcription of numerous host defense-related proteins, including pro-inflammatory cytokines [35,36]. Some studies supported that 25HC induced pro-inflammatory cytokines. For example, 25HC induced the secretion of pro-inflammatory cytokines and chemokines in monocytes/macrophages from human atherosclerotic plaque and epithelial cells, such as IL-1β, IL-6, IL-8, CCL5, and macrophage colony-stimulating factor (M-CSF) [37,38]. 25HC incubation also triggered IL-1β production in co-cultures of human monocytes and vascular smooth muscle cells [39]. In addition, 25HC was reported to play a role in foam cell formation in atherosclerosis [40]. CH25H expression was increased in alveolar macrophages and alveolar cells in patients with chronic obstructive pulmonary disease (COPD), and the concentration of 25HC and IL-8 in patients’ sputum was correlated with the number of neutrophils in the lung tissue [41]. Inflammatory cytokines in human keratinocytes were induced after 25HC incubation by promoting granzyme B release, which in turn mediated the maturation of IL-1α in cutaneous inflammatory responses [42].
The mechanism for 25HC promoting the release of inflammatory mediators, including cytokines, chemokines, and growth factors, is under investigation. Previous reports showed that 25HC-induced pro-inflammation was dependent on nuclear factor kappa-B (NF-κB) signaling, and it was observed that 25HC bound to α5β1/αvβ3 integrin as a lipid ligand to activate NF-κB signaling following nucleotide-binding oligomerization domain containing 2 (Nod2) activation [43]. 25HC-induced inflammatory signals were also related to the recruitment of transcription factor activator protein-1 (AP-1) components, FBJ osteosarcoma oncogene (FOS) and Jun proto-oncogene (JUN), and to the promoters of a subset of Toll-like receptor-responsive genes [44]. Further studies showed that 25HC-induced IL-8 secretion was calcium-dependent and associated with the ERK1/2 pathway [38]. Nevertheless, 25HC contributed to the cerebral inflammation of X-linked adrenoleukodystrophy (X-ALD) via activation of the NOD-like receptor protein 3 (NLRP3) inflammasome pathway, and the inflammasome was activated by caspase-1 following the production of key pro-inflammatory cytokines IL-1β and IL-18 [45].
4.2. Suppressing Inflammation by CH25H and 25HC
In contrast to the previously described observations, some studies demonstrated that 25HC played a role in suppressing inflammasome activity by inducing IFN, suppressing SREBP, antagonizing inflammasomes, and inhibiting the Akt/NF-κB signaling pathway.
Type I interferon (IFN) not only has potent antiviral activity, but also has an inhibitory effect on immunity to prevent uncontrolled inflammation, which can cause significant tissue damage in some acute viral infections [46,47] and autoimmune diseases [48,49]. A major aspect of IFN-mediated inhibition is the downregulation of inflammasome activity and IL-1β production [50,51]. The IFN-stimulated gene CH25H promotes the production of 25HC, and 25HC can promote the production of IFN and inhibit inflammation [21,23].
Some studies showed that 25HC inhibited IL-1β production and inflammasome activity by suppressing SREBP [52,53]. Infections with some kinds of pathogens, such as Listeria monocytogenes and mycobacteria tuberculosis, promoted 25HC production, while increased 25HC blocked the SREBP pathway activation and inflammasome activity via NLRP3 to inhibit IL-1β production, and therefore CH25H-overexpressed macrophages or mice were more susceptible to Listeria bacteria or mycobacteria tuberculosis infections [54,55]. 25HC also decreased the production of the interleukin-1 family by antagonizing the activities of NLRP- and AIM2-containing inflammasomes [56,57]. A study showed that 25HC treatment attenuated the pathological changes in LPS-induced acute lung injury in mice and reduced the overwhelming release of TLR4-mediated inflammatory cytokines by binding to myeloid differentiation protein 2 (MD-2) and subsequently suppressing the LPS-activated Akt/NF-κB signaling pathway [56]. Interestingly, 25HC’s anti-inflammation ability was also tested to treat mevalonate kinase deficiency (MKD), a hereditary auto-inflammatory disorder [58].
Therefore, 25HC seems to play opposite roles to regulate inflammation responses through a complex mechanism as reported in different studies (Figure 2). The exact reason for this mechanism is unknown, but it might be related to the various concentrations of 25HC. Our published study and other data support this hypothesis. One study showed that 25HC with nanomolar concentrations had an anti-inflammation effect, while 25HC with micromolar concentrations promoted pro-inflammation [45]. Our previous study also demonstrated that 300 ng/mL of 25HC dramatically downregulated LPS-induced inflammatory responses in primary mice splenocytes and monkey PBMCs, but this anti-inflammatory effect decreased at a higher 25HC concentration [32]. In addition to the concentration differences, we posit that the treatment time differences might be another reason, but there are no experimental data to support this hypothesis, and we will test it in our next project. Further studies are also needed to clarify how 25HC precisely modulates inflammation, especially in patients with chronic infectious diseases and autoimmune-related diseases.
5. Regulation of Immune Responses by CH25H and 25HC
Both innate and adaptive immune responses are important to fight against viral infections, and recent studies demonstrated that CH25H and 25HC play important roles not only in regulating cholesterol metabolism, but also in modulating innate and adaptive immune responses by regulating SREBP and LXR [59,60] (Figure 2).
5.1. Innate Immunity
As previously mentioned, the CH25H gene belongs to the ISG family, and therefore it is not surprising that CH25H is extensively involved in innate immunity in response to viral infections. CH25H expression increases when Toll-like receptors (TLRs) are activated, which leads to increased production of 25HC [11,22,31].
Studies have shown that 25HC acts by antagonizing SREBP processing to reduce IL-1β transcription and broadly repress IL-1-activating inflammasomes. Mouse macrophages lacking CH25H and that are unable to produce oxysterol 25HC will lead to the overproduction of IL-1 family cytokines [52].
As previously discussed in the section on 25HC and liver X receptor, LXRs are activated by 25HC as endogenous ligands. LXRs are related to the innate immune response of pathogens [61,62], and the lack of LXRα/β is highly sensitive to pathogen infections [63]. 25HC seems to elicit innate immune responses via LXR.
5.2. Adaptive Immunity
Adaptive immunity has a very close relationship with innate immunity and can be divided into two types: B cell-mediated humoral immune responses and T cell-mediated cellular immune responses. 25HC can affect both of these types of immune responses.
Humoral immune responses depend on the migration of highly activated B lymphocytes, and the migration of B and T lymphocytes is regulated by the G protein-coupled receptor Epstein-Barr virus-induced gene 2 (EBI2) [64]. 7α-25HC, which is produced from catalyzed 25HC by oxysterol 7α-hydroxylase (CYP7B1), is the EBI2 receptor’s lipid ligand [54]. In addition to B lymphocytes, bone marrow-derived dendritic cells and the group 3 innate lymphoid cells (ILC3s) have been reported to have 7α-25HC tropism. Therefore, as a chemokine for immune cells expressing EBI2 molecules, 25HC and its metabolite 7α-25HC are involved in the regulation of innate immunity and adaptive immune responses [65]. Further research reported that when B lymphocytes were treated with nanomolar concentrations of 25HC, they inhibited the IL-2-mediated activation of B cell differentiation and reduced the production of IgA antibodies. Nevertheless, compared to wild-type mice, there were significantly higher titers of IgA and IgG antibodies in different tissues of CH25H-/- mice [11]. Human patients with CYP7B1 gene deficiency cannot catalyze 25HC to 7α-25HC, and therefore their concentration of 25HC is usually 100 times that of healthy people [13]. Consistent with this observation, 25HC concentrations were increased in CYP7B1-/- mice, and the levels of IgA antibodies in these mice were significantly lower than in wild-type mice [66]. These data indicate that 25HC negatively regulates the secretion of IgA antibodies in B lymphocytes, suggesting the regulatory role of 25HC in the humoral immune system.
25HC also affects the transformation of CD4+ T cells from effector (IFN-γ+) to anti-inflammatory (IL-10+) phenotypes by controlling cholesterol flux. Moreover, the physiological level of 25HC in human CD4+ T cells significantly inhibits the production of IL-10 for cholesterol homeostasis by reducing c-Maf, which is the master transcriptional regulator of IL-10 cytokines [67]. Our previous studies showed that 25HC selectively suppressed pro-inflammatory CD4+ T lymphocytes secreting IL-2 and TNF-α cytokines in vaccinated mice, but had no significant immunosuppressive effects on cytotoxic CD8+ T lymphocytes or antibody-producing B lymphocytes [32]. The exact mechanism for regulating the adaptive immunity by 25HC is not fully clear, so further research should be conducted in this field.
7. Conclusions and Perspective
CH25H and 25HC are reported to exert multifaceted functions including lipid metabolism and immunomodulation, which are good examples to explain the essence of immunometabolism. More importantly, as a member of the ISG family, CH25H and its enzymatic product 25HC have been recently identified to have broadly antiviral activities. The underlying mechanisms are not fully clarified, but include the inhibition of virus adsorption, entry, and release by manipulating cholesterol metabolism; inhibition of virus replication through direct interactions with viral components; inhibition of virus infection by modulating innate immunity and virus-specific adaptive immunity; and the activation of the ISR, changing the endocytic transport patterns and other mechanisms.
Highly pathogenic virus outbreaks have recently become more frequent, at least partly due to crossing the species barrier from natural wildlife to humans, including avian influenza H5N1, H7N9, SARS-CoV, MERS-CoV, ZIKV, EBOV, and the coronavirus disease 2019 (COVID-19) pandemic, which is still rapidly spreading worldwide [93,94,95]. As civilization has progressed, numerous disharmonies have occurred between humans and nature. As a result, the next outbreak of severely infectious diseases is not “if,” but is the inevitable challenge of “when, where, and what diseases,” and therefore developing broad-spectrum and safe drug candidates against emerging pathogens has become a public health priority.
Considering the broadly antiviral activities of 25HC in recent publications, this compound should be further exploited as a potential therapeutic agent in preclinical and clinical studies. Of course, many scientific issues remain to be solved. What is the exact mechanism contributing to 25HC’s broad-spectrum antiviral activity? Why does 25HC rather than other hydroxy cholesterols improve antiviral activity? How are the dual roles of 25HC in augmenting pro-inflammation and suppressing inflammation modulated through a complex mechanism under different conditions? In-depth research into the molecular mechanisms can provide new methods of screening antiviral drugs and help clinicians understand the relationship between cell metabolism, immunity, and antiviral activity. Further studies also should be conducted on the safety and pharmacokinetics of 25HC. Taken together, further research into this compound as a drug candidate for broad-spectrum antiviral infections to control emerging infectious diseases should be pursued in the future.
Author Contributions
Project design by C.S., J.Z.; literature collection, original draft preparation and re-writing by J.Z., J.C., M.L., M.C., C.S.; picture drawing, J.C., M.L., M.C.; the research was supervised by C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81971927), the National Science and Technology Major Project of China (2018ZX10731101-002), the Science and Technology Planning Project of Shenzhen City (20190804095916056), and the High Level Project of Medicine in Longhua, Shenzhen (HLPM201907020105).
Acknowledgments
We would like to thank other members in our group for their helpful discussion and suggestions on this project.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Schoggins, J.W.; Randall, G. Lipids in innate antiviral defense. Cell Host Microbe 2013, 14, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Kunisawa, J. Diversity of energy metabolism in immune responses regulated by microorganisms and dietary nutrition. Int. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Holthuis, J.C.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Kandutsch, A.A.; Chen, H.W. Regulation of sterol synthesis in cultured cells by oxygenated derivatives of cholesterol. J. Cell. Physiol. 1975, 85, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Gale, M., Jr. Sterol-izing innate immunity. Immunity 2013, 38, 3–5. [Google Scholar] [CrossRef][Green Version]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Holmes, R.S.; Vandeberg, J.L.; Cox, L.A. Genomics and proteomics of vertebrate cholesterol ester lipase (LIPA) and cholesterol 25-hydroxylase (CH25H). 3 BIOTECH 2011, 1, 99–109. [Google Scholar] [CrossRef]
- Karuna, R.; Christen, I.; Sailer, A.W.; Bitsch, F.; Zhang, J. Detection of dihydroxycholesterols in human plasma using HPLC-ESI-MS/MS. Steroids 2015, 99, 131–138. [Google Scholar] [CrossRef]
- Bauman, D.R.; Bitmansour, A.D.; McDonald, J.G.; Thompson, B.M.; Liang, G.; Russell, D.W. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA 2009, 106, 16764–16769. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.G.; Russell, D.W. Editorial: 25-Hydroxycholesterol: A new life in immunology. J. Leukoc. Biol. 2010, 88, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Schule, R.; Siddique, T.; Deng, H.X.; Yang, Y.; Donkervoort, S.; Hansson, M.; Madrid, R.E.; Siddique, N.; Schols, L.; Bjorkhem, I. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J. Lipid Res. 2010, 51, 819–823. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Maeda, T.; Hirayama, T.; Saito, Y.; Teramoto, T.; Matsuzaki, Y. Cholesterol 25-hydroxylation activity of CYP3A. J. Lipid Res. 2011, 52, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 1980, 21, 505–517. [Google Scholar] [PubMed]
- Adams, C.M.; Reitz, J.; De Brabander, J.K.; Feramisco, J.D.; Li, L.; Brown, M.S.; Goldstein, J.L. Cholesterol and 25-Hydroxycholesterol Inhibit Activation of SREBPs by Different Mechanisms, Both Involving SCAP and Insigs. J. Biol. Chem. 2004, 279, 52772–52780. [Google Scholar] [CrossRef]
- Brown, M.S.; Dana, S.E.; Goldstein, J.L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J. Biol. Chem. 1975, 250, 4025–4027. [Google Scholar]
- Radhakrishnan, A.; Sun, L.P.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol. Cell 2004, 15, 259–268. [Google Scholar] [CrossRef]
- Zelcer, N. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Zhang, Y.; Ma, X.; Chen, Y.; Yu, M.; Ma, C.; Li, X.; Cao, Y.; Liu, J.; et al. Activation of liver X receptor plays a central role in antiviral actions of 25-hydroxycholesterol. J. Lipid Res. 2018, 59, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Diczfalusy, U.; Olofsson, K.E.; Carlsson, A.M.; Gong, M.; Golenbock, D.T.; Rooyackers, O.; Flaring, U.; Bjorkbacka, H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 2009, 50, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 2010, 10, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Thomsen, A.R. Sensing of RNA viruses: A review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012, 86, 2900–2910. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Wang, X.; Zhang, X.; Tian, H.; Deng, H.; Zhang, L.; Gao, G. Identification of new type I interferonstimulated genes and investigation of their involvement in IFN-β activation. PROTEIN CELL 2018, 9, 799–807. [Google Scholar] [CrossRef]
- Clark, P.J.; Thompson, A.J.; Vock, D.M.; Kratz, L.E.; Tolun, A.A.; Muir, A.J.; McHutchison, J.G.; Subramanian, M.; Millington, D.M.; Kelley, R.I.; et al. Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner. Hepatology 2012, 56, 49–56. [Google Scholar] [CrossRef]
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Kropp, K.A.; Forster, T.; Shui, G.; Lacaze, P.; Watterson, S.; Griffiths, S.J.; Spann, N.J.; et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013, 38, 106–118. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Liu, X.; Bai, J.; Zhao, Y.; Wang, X.; Jiang, P. Cholesterol 25-Hydroxylase is an Interferon-inducible Factor that Protects against Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Microbiol. 2017, 210, 153–161. [Google Scholar] [CrossRef]
- Xie, T.; Feng, M.; Dai, M.; Mo, G.; Ruan, Z.; Wang, G.; Shi, M.; Zhang, X. Cholesterol-25-hydroxylase Is a Chicken ISG That Restricts ALV-J Infection by Producing 25-hydroxycholesterol. Viruses 2019, 11, 498. [Google Scholar] [CrossRef]
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010, 88, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ma, F.; Ma, X.; Jia, W.; Pan, E.; Cheng, G.; Chen, L.; Sun, C. Regulating Innate and Adaptive Immunity for Controlling SIV Infection by 25-Hydroxycholesterol. Front. Immunol. 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tang, J.J.; Tao, W.; Cao, X.; Zhong, J. Identification of Cholesterol 25-Hydroxylase as a Novel Host Restriction Factor and a Part of the Primary Innate Immune Responses against Hepatitis C Virus Infection. J. Virol. 2015, 89, 6805–6816. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brey, I.; Berger, C.; Colpitts, C.C.; Boldanova, T.; Engelmann, M.; Todt, D.; Perin, P.M.; Behrendt, P.; Vondran, F.W.R.; Xu, S.; et al. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology 2015, 62, 702–714. [Google Scholar] [CrossRef]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef]
- Pandey, S.; Kawai, T.; Akira, S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb. Perspect. Biol. 2014, 7, a016246. [Google Scholar] [CrossRef]
- Liu, Y.; Hultén, L.M.; Wiklund, O. Macrophages Isolated From Human Atherosclerotic Plaques Produce IL-8, and Oxysterols May Have a Regulatory Function for IL-8 Production. Arterioscl. Throm. Vas. 1997, 17, 317–323. [Google Scholar] [CrossRef]
- Lemaire-Ewing, S.; Berthier, A.; Royer, M.C.; Logette, E.; Corcos, L.; Bouchot, A.; Monier, S.; Prunet, C.; Raveneau, M.; Rebe, C.; et al. 7beta-Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells: Oxysterols-induced IL-8 secretion is calcium-dependent. Cell Biol. Toxicol. 2009, 25, 127–139. [Google Scholar] [CrossRef]
- Fu, H.; Spieler, F.; Grossmann, J.; Riemann, D.; Larisch, M.; Hiebl, B.; Schlecht, K.; Jaschke, C.; Bartling, B.; Hofmann, B.; et al. Interleukin-1 potently contributes to 25-hydroxycholesterol-induced synergistic cytokine production in smooth muscle cell-monocyte interactions. Atherosclerosis 2014, 237, 443–452. [Google Scholar] [CrossRef]
- Gold, E.S.; Ramsey, S.A.; Sartain, M.J.; Selinummi, J.; Podolsky, I.; Rodriguez, D.J.; Moritz, R.L.; Aderem, A. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med. 2012, 209, 807–817. [Google Scholar] [CrossRef]
- Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; Furusawa, M.; Uno, Y.; et al. Increased 25-hydroxycholesterol concentrations in the lungs of patients with chronic obstructive pulmonary disease. Respirology 2012, 17, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Laprevote, O.; Rat, P. 25-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: New insights into degenerative pathways. Chem. Phys. Lipids 2017, 207, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.M.; Shil, N.K.; Gc, J.B.; Colburn, Z.T.; Tsai, S.Y.; Segovia, J.A.; Chang, T.H.; Bandyopadhyay, S.; Natesan, S.; Jones, J.C.R.; et al. Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nat Commun 2019, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Jin, H.H.; Cho, H.J.; Hwang, I.; Pyo, K.Y.; Im, I.; Lee, H.; Lee, E.; Yang, W.; et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 2016, 7, 13129. [Google Scholar] [CrossRef]
- Kobasa, D.; Jones, S.M.; Shinya, K.; Kash, J.C.; Copps, J.; Ebihara, H.; Hatta, Y.; Kim, J.H.; Halfmann, P.; Hatta, M.; et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 2007, 445, 319–323. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. The 1918 influenza pandemic: Insights for the 21st century. J. Infect. Dis. 2007, 195, 1018–1028. [Google Scholar] [CrossRef]
- Trinchieri, G. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. The role of interferon-beta in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes. Immunology 2013, 139, 11–18. [Google Scholar] [CrossRef]
- Ludigs, K.; Parfenov, V.; Du Pasquier, R.A.; Guarda, G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cell. Mol. Life Sci. 2012, 69, 3395–3418. [Google Scholar] [CrossRef]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Forster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Dang, E.V.; McDonald, J.G.; Liang, G.; Russell, D.W.; Cyster, J.G. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 2014, 345, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Anna, S. Cholesterol metabolism and immunity. N. Engl. J. Med. 2014, 371, 1933–1935. [Google Scholar]
- Changlu Liu, X.V.Y.; Wu, J. Oxysterols direct B-cell migration through EBI2. Nature 2011, 475, 519–523. [Google Scholar]
- Zou, T.; Garifulin, O.; Berland, R.; Boyartchuk, V.L. Listeria monocytogenes infection induces prosurvival metabolic signaling in macrophages. Infect. Immun. 2011, 79, 1526–1535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouyang, W.; Zhou, H.; Liu, C.; Wang, S.; Han, Y.; Xia, J.; Xu, F. 25-Hydroxycholesterol protects against acute lung injury via targeting MD-2. J. Cell. Mol. Med. 2018, 22, 5494–5503. [Google Scholar] [CrossRef]
- Dang, E.V.; Mcdonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 2017, 171, 1057–1071. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Gratton, R.; Braga, L.; Celsi, F.; Crovella, S. 25-Hydroxycholesterol and inflammation in Lovastatin-deregulated mevalonate pathway. Int. J. Biochem. Cell Biol. 2017, 92, 26–33. [Google Scholar] [CrossRef]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Traversari, C.; Russo, V. Control of the immune system by oxysterols and cancer development. Curr. Opin. Pharmacol. 2012, 12, 729–735. [Google Scholar] [CrossRef]
- Joseph, S.B.; Bradley, M.N.; Castrillo, A.; Bruhn, K.W.; Mak, P.A.; Pei, L.; Hogenesch, J.; O’Connell, R.M.; Cheng, G.; Saez, E.; et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 2004, 119, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.; Vander Beken, S.; Romano, M.; Steffensen, K.R.; Stijlemans, B.; Gustafsson, J.A.; Grooten, J.; Huygen, K. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J. Clin. Invest. 2009, 119, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- A-Gonzalez, N.; Bensinger, S.J.; Hong, C.; Beceiro, S.; Bradley, M.N.; Zelcer, N.; Deniz, J.; Ramirez, C.; Diaz, M.; Gallardo, G.; et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 2009, 31, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.P.; Kelly, L.M.; Cyster, J.G. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol. 2010, 22, 413–419. [Google Scholar] [CrossRef]
- Emgård, J.; Kammoun, H.; García-Cassani, B.; Chesné, J.; Parigi, S.M.; Jacob, J.-M.; Cheng, H.-W.; Evren, E.; Das, S.; Czarnewski, P.; et al. Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 2018, 48, 120–132. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Perucha, E.; Melchiotti, R.; Bibby, J.A.; Wu, W.; Frederiksen, K.S.; Roberts, C.A.; Hall, Z.; LeFriec, G.; Robertson, K.A.; Lavender, P.; et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat Commun 2019, 10, 498. [Google Scholar] [CrossRef]
- Lange, Y.; Ye, J.; Strebel, F. Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J. Lipid Res. 1995, 36, 1092–1097. [Google Scholar]
- Yuan, Y.; Wang, Z.; Tian, B.; Zhou, M.; Fu, Z.F.; Zhao, L. Cholesterol 25-hydroxylase suppresses rabies virus infection by inhibiting viral entry. Arch. Virol. 2019, 164, 2963–2974. [Google Scholar] [CrossRef]
- Shrivastava-Ranjan, P.; Bergeron, É.; Chakrabarti, A.K.; Albariño, C.G.; Flint, M.; Nichol, S.T.; Spiropoulou, C.F. 25-Hydroxycholesterol Inhibition of Lassa Virus Infection through Aberrant GP1 Glycosylation. Mbio 2016, 7, e01808-16. [Google Scholar] [CrossRef]
- Teissier, É.; Pécheur, E.-I. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur. Biophys. J. 2007, 36, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Antiviral effect of 25-hydroxycholesterol against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2018, 23, 395–404. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Q.; Tan, J.; Feng, W.-H. Lipid rafts both in cellular membrane and viral envelope are critical for PRRSV efficient infection. Virology 2015, 484, 170–180. [Google Scholar] [CrossRef]
- Olsen, B.N.; Schlesinger, P.H.; Ory, D.S.; Baker, N.A. 25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes. Biophys. J. 2011, 100, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, L.; Zhang, L.; Guo, Z.Z.; Chu, B.B. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017, 98, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Bigay, J.; Moser von Filseck, J.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef]
- Barajas, D.; Xu, K.; de Castro Martin, I.F.; Sasvari, Z.; Brandizzi, F.; Risco, C.; Nagy, P.D. Co-opted oxysterol-binding ORP and VAP proteins channel sterols to RNA virus replication sites via membrane contact sites. PLoS Pathog. 2014, 10, e1004388. [Google Scholar] [CrossRef]
- Roulin, P.S.; Lötzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.M.; Wenk, M.R.; Greber, U.F. Rhinovirus Uses a Phosphatidylinositol 4-Phosphate/Cholesterol Counter-Current for the Formation of Replication Compartments at the ER-Golgi Interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef]
- Ridgway, N.D.; Dawson, P.A.; Ho, Y.K.; Brown, M.S.; Goldstein, J.L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J. Cell Biol. 1992, 116, 307–319. [Google Scholar] [CrossRef]
- Wang, H.; Perry, J.W.; Lauring, A.S.; Neddermann, P.; De Francesco, R.; Tai, A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology 2014, 146, 1373–1385. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Bordier, B.B.; Ohkanda, J.; Liu, P.; Lee, S.Y.; Salazar, F.H.; Marion, P.L.; Ohashi, K.; Meuse, L.; Kay, M.A.; Casey, J.L.; et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J. Clin. Investig. 2003, 112, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gale, M.; Keller, B.C.; Huang, H.; Ye, J. Identification of FBL2 As a Geranylgeranylated Cellular Protein Required for Hepatitis C Virus RNA Replication. Mol. Cell 2005, 18, 425–434. [Google Scholar] [CrossRef]
- Ke, W.; Fang, L.; Jing, H.; Tao, R.; Wang, T.; Li, Y.; Long, S.; Wang, D.; Xiao, S. Cholesterol 25-Hydroxylase Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication through Enzyme Activity-Dependent and -Independent Mechanisms. J. Virol. 2017, 91, e00827-17. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhao, G.; Wang, H.; He, H. Cholesterol 25-Hydroxylase inhibits bovine parainfluenza virus type 3 replication through enzyme activity-dependent and -independent ways. Vet. Microbiol. 2019, 239, 108456. [Google Scholar] [CrossRef]
- Song, Z.; Bai, J.; Nauwynck, H.; Lin, L.; Liu, X.; Yu, J.; Jiang, P. 25-Hydroxycholesterol provides antiviral protection against highly pathogenic porcine reproductive and respiratory syndrome virus in swine. Vet. Microbiol. 2019, 231, 63–70. [Google Scholar] [CrossRef]
- Shibata, N.; Carlin, A.F.; Spann, N.J.; Saijo, K.; Morello, C.S.; McDonald, J.G.; Romanoski, C.E.; Maurya, M.R.; Kaikkonen, M.U.; Lam, M.T.; et al. 25-Hydroxycholesterol activates the integrated stress response to reprogram transcription and translation in macrophages. J. Biol. Chem. 2013, 288, 35812–35823. [Google Scholar] [CrossRef]
- Civra, A.; Cagno, V.; Donalisio, M.; Biasi, F.; Leonarduzzi, G.; Poli, G.; Lembo, D. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Sci. Rep. 2014, 4, 7487. [Google Scholar] [CrossRef]
- Civra, A.; Francese, R.; Gamba, P.; Testa, G.; Cagno, V.; Poli, G.; Lembo, D. 25-Hydroxycholesterol and 27-hydroxycholesterol inhibit human rotavirus infection by sequestering viral particles into late endosomes. Redox Biol. 2018, 19, 318–330. [Google Scholar] [CrossRef]
- You, H.; Yuan, H.; Fu, W.; Su, C.; Wang, W.; Cheng, T.; Zheng, C. Herpes simplex virus type 1 abrogates the antiviral activity of Ch25h via its virion host shutoff protein. Antiviral Res. 2017, 143, 69–73. [Google Scholar] [CrossRef]
- Bouabid, B.; Teresa, R.; Anna, A.; Merijn, V.; Hans, N.; Elisabetta, G.; Sara, B.; Moldawer, L.L. RNA-Sequence Analysis of Primary Alveolar Macrophages after In Vitro Infection with Porcine Reproductive and Respiratory Syndrome Virus Strains of Differing Virulence. PLoS ONE 2014, 9, e91918. [Google Scholar]
- Serquina, A.K.P.; Kambach, D.M.; Sarker, O.; Ziegelbauer, J.M. Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection. mBio 2017, 8, e00576-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, H.; Guo, R.; Li, Y.; Shen, B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect 2015, 4, e28. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).